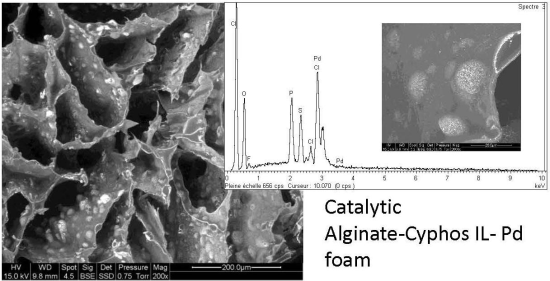
Study of Alginate-Supported Ionic Liquid and Pd Catalysts
Abstract
:1. Introduction
2. Results and Discussion
| Ref. | IL | (%) | type | (w/w) | Coagulating agent * | T (°C) | Cellulose |
|---|---|---|---|---|---|---|---|
| A1 | 111 | 2 | A | 4 | HCl | −78 °C | No |
| A2 | 111 | 2 | A | 4 | HCl | −20 °C | No |
| A3 (a) | 111 | 2 | A | 4 | CaCl2 | −78 °C | No |
| A4 (a) | 101 | 2 | LF-240D | 4 | CaCl2 | −78 °C | No |
| A5 (a) | 101 | 2 | LF-200S | 4 | CaCl2 | −78 °C | No |
| A6 (b) | 111 | 3 | A | 4 | HCl | −78 °C | No |
| A7 (b) | 111 | 3 | A | 8 | HCl | −78 °C | No |
| A8 (b) | 111 | 2 | A | 8 | HCl | −78 °C | No |
| A9 | 111 | 2 | A | 2 | HCl | −78 °C | No |
| A10 | 111 | 2 | A | 4 | HCl | −78 °C | Yes |
2.1. Characteristics of Materials
2.1.1. Materials with Low Stability in Water
2.1.2. Pd(II) Sorption Properties
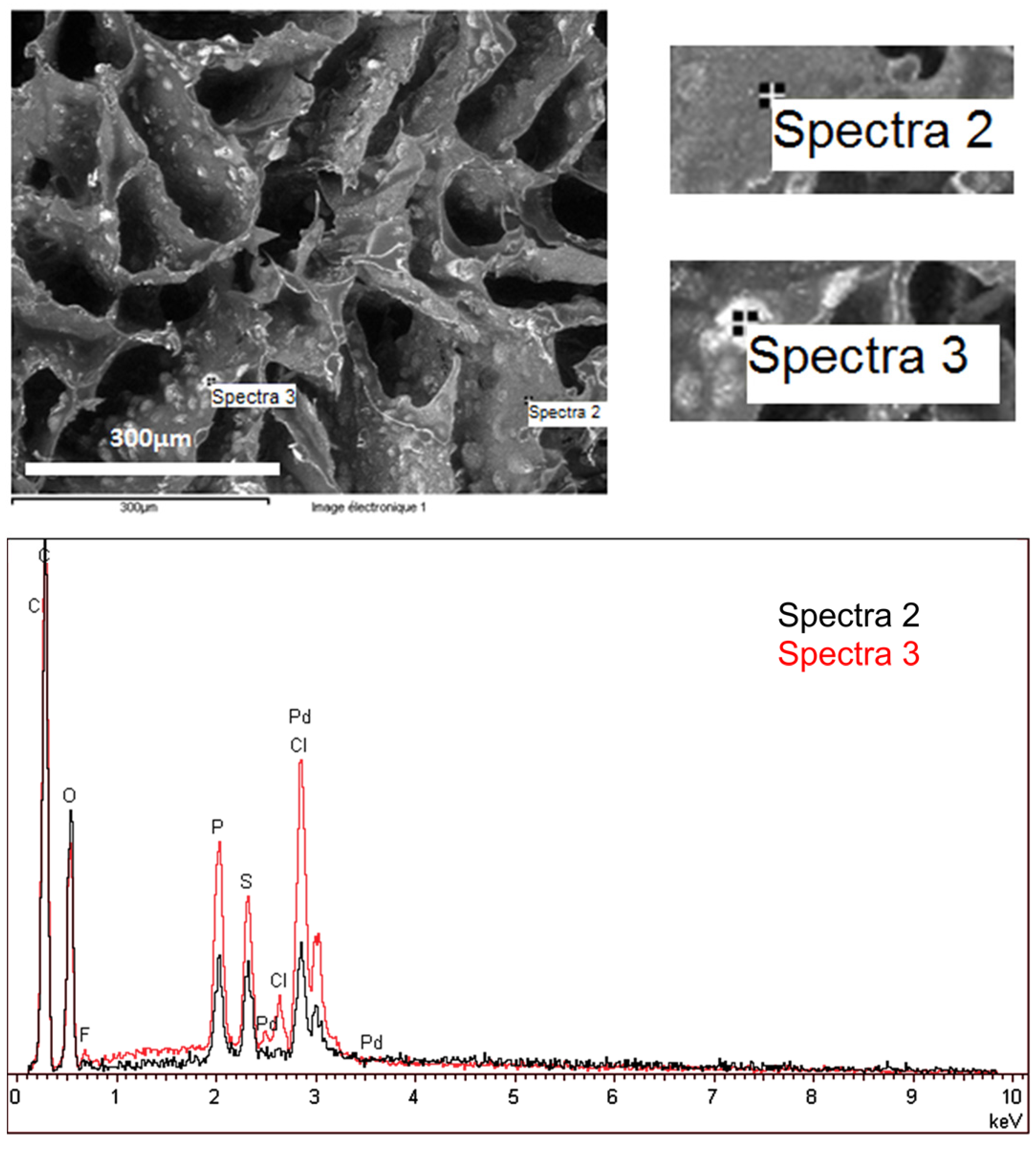
2.1.3. Element Analysis of the Catalytic Materials
| Material | (mg Pd g−1) | (mg LI g−1) | (mol/mol) |
|---|---|---|---|
| A1 | 62.8 ± 2.2 | 27.8 ± 2.2 | 0.66 |
| A2 | 54.5 ± 4.3 | 30.8 ± 2.0 | 0.51 |
| A3 | 46.9 ± 2.4 | 22.7 ± 1.5 | 0.6 |
| A4 | 20.7 ± 1.0 | 25.0 ± 0.7 | 0.24 |
| A5 | 15.3 ± 1.1 | 24.5 ± 1.6 | 0.18 |
| A9 | 22.0 ± 1.5 | 29.5 ± 0.9 | 0.22 |
| A10 | 18.9 ± 1.3 | 24.7 ± 0.5 | 0.22 |
2.1.4. Morphology of the Catalytic Materials
2.1.5. Distribution of Elements

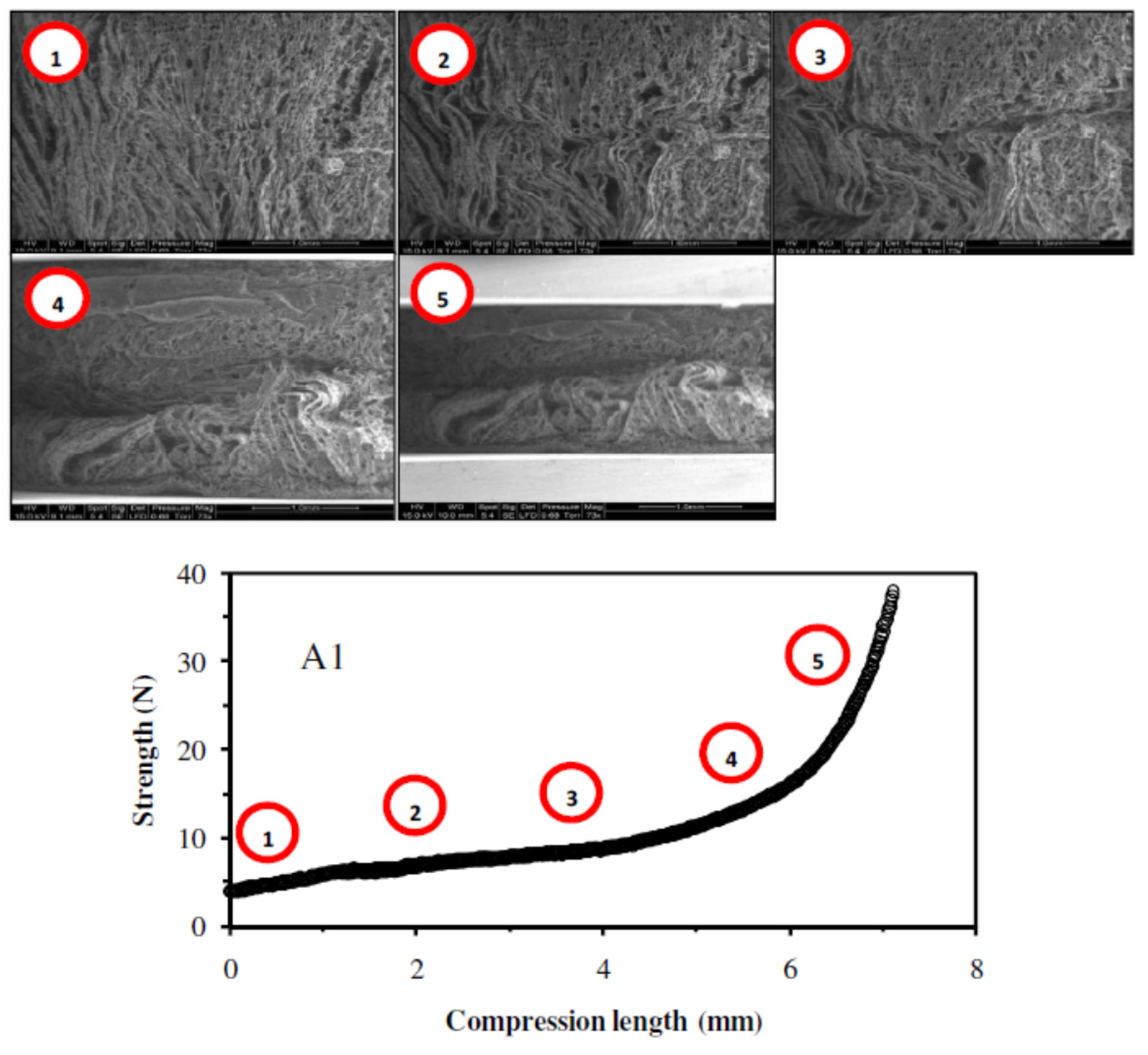
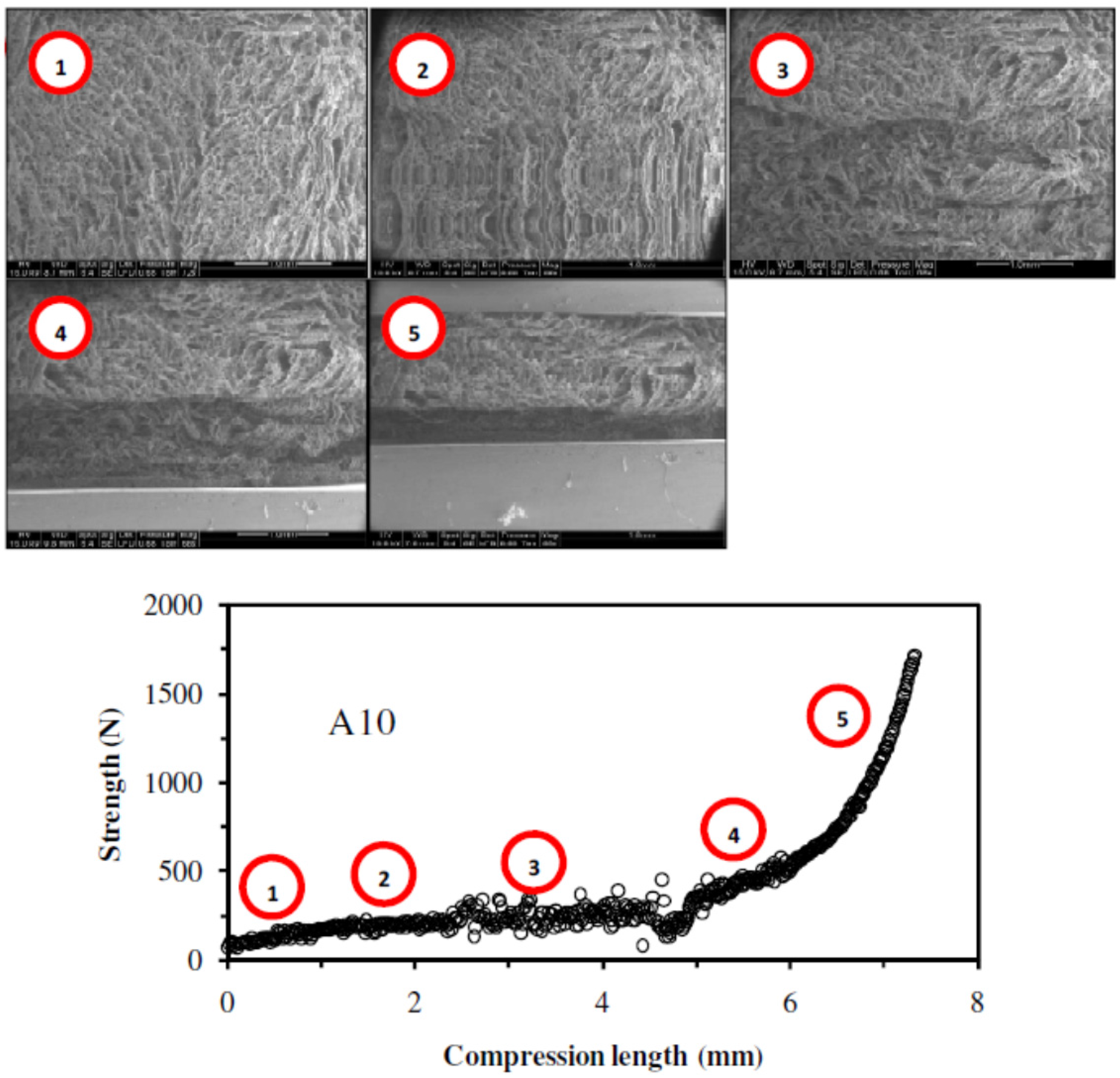
2.1.6. Mechanical Properties
2.2. Catalytic Properties of the Materials
| Material | Conversion yield (%) (a) | time (min) | Initial rate (min−1) | Half-reaction time (min) | (mol 4-NA mol−1 Pd min−1) |
|---|---|---|---|---|---|
| (a) After a maximum reaction time of 60 min. | |||||
| A1 | 100 | 5 | 0.54 | 1.3 | 0.12 |
| A2 | 100 | 6 | 0.51 | 1.5 | 0.14 |
| A3 | 100 | 7 | 0.10 | 9 | 0.13 |
| A4 | 90 | 3 | 0.11 | 14 | 0.28 |
| A5 | 90 | 4 | 0.07 | 16 | 0.43 |
| A9 | 70 | 7 | 0.04 | 26 | 0.29 |
| A10 | 100 | 7 | 0.46 | 1.5 | 0.32 |
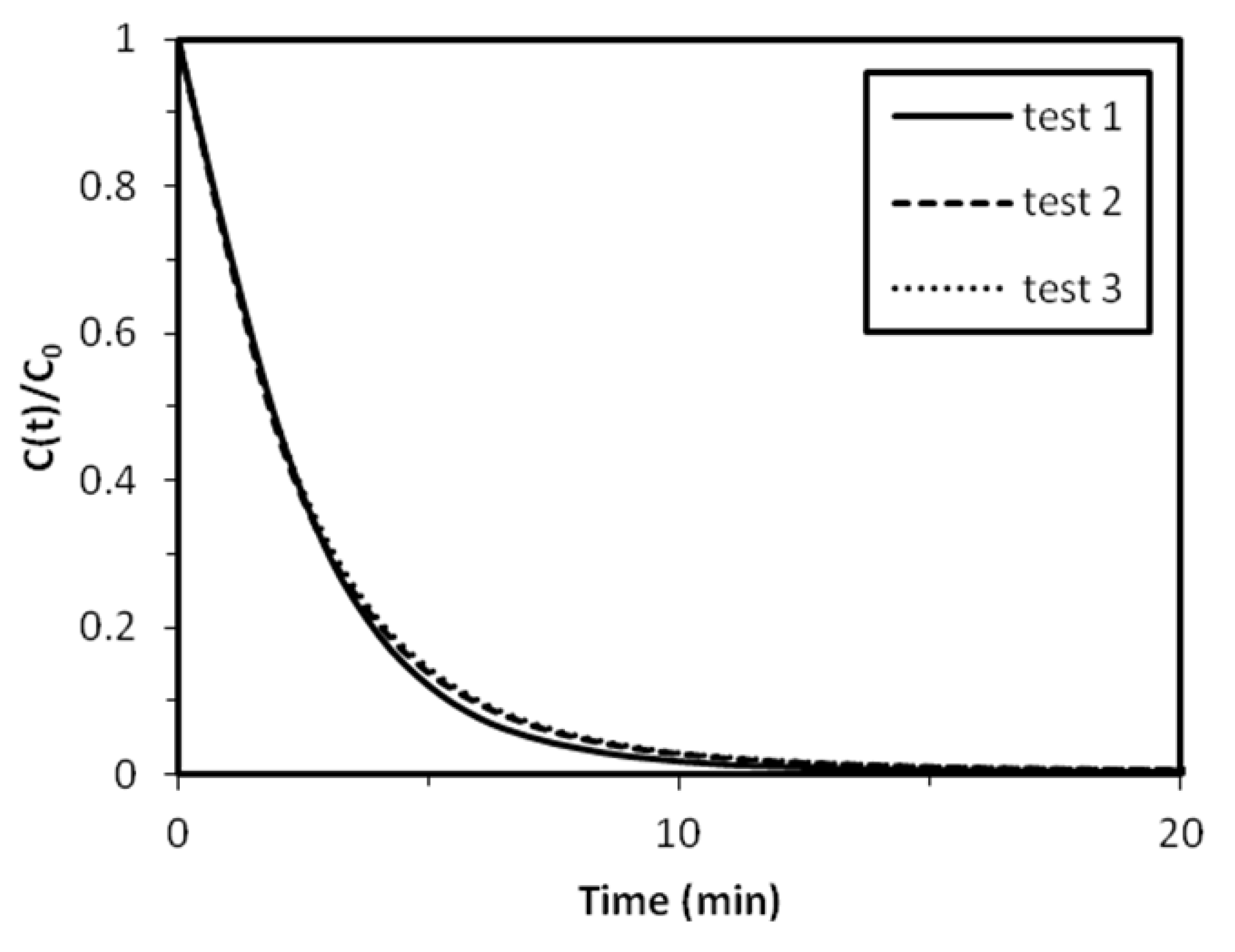
2.2.1. Reproducibility of Experiments
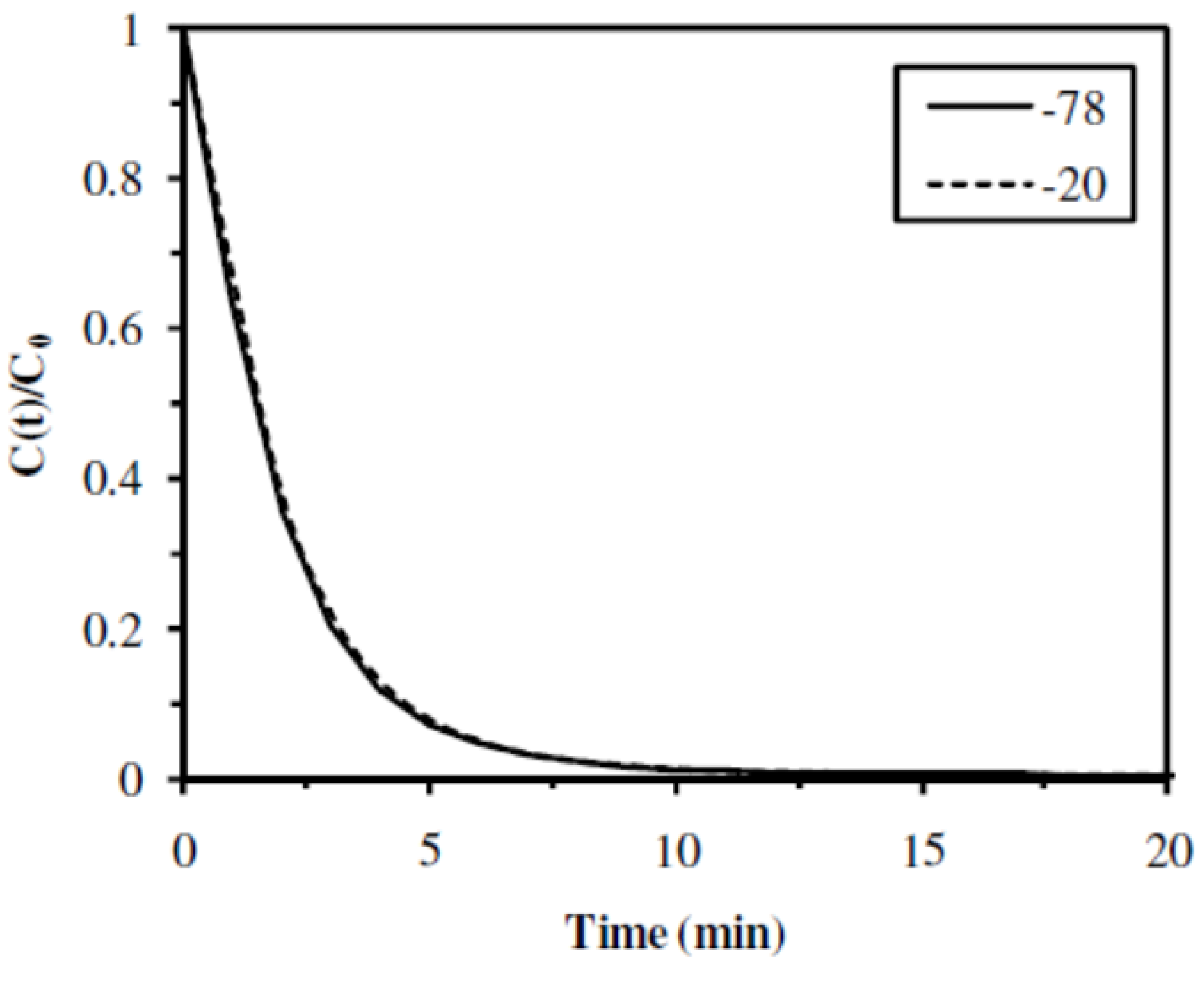
2.2.2. Influence of the Freezing Temperature
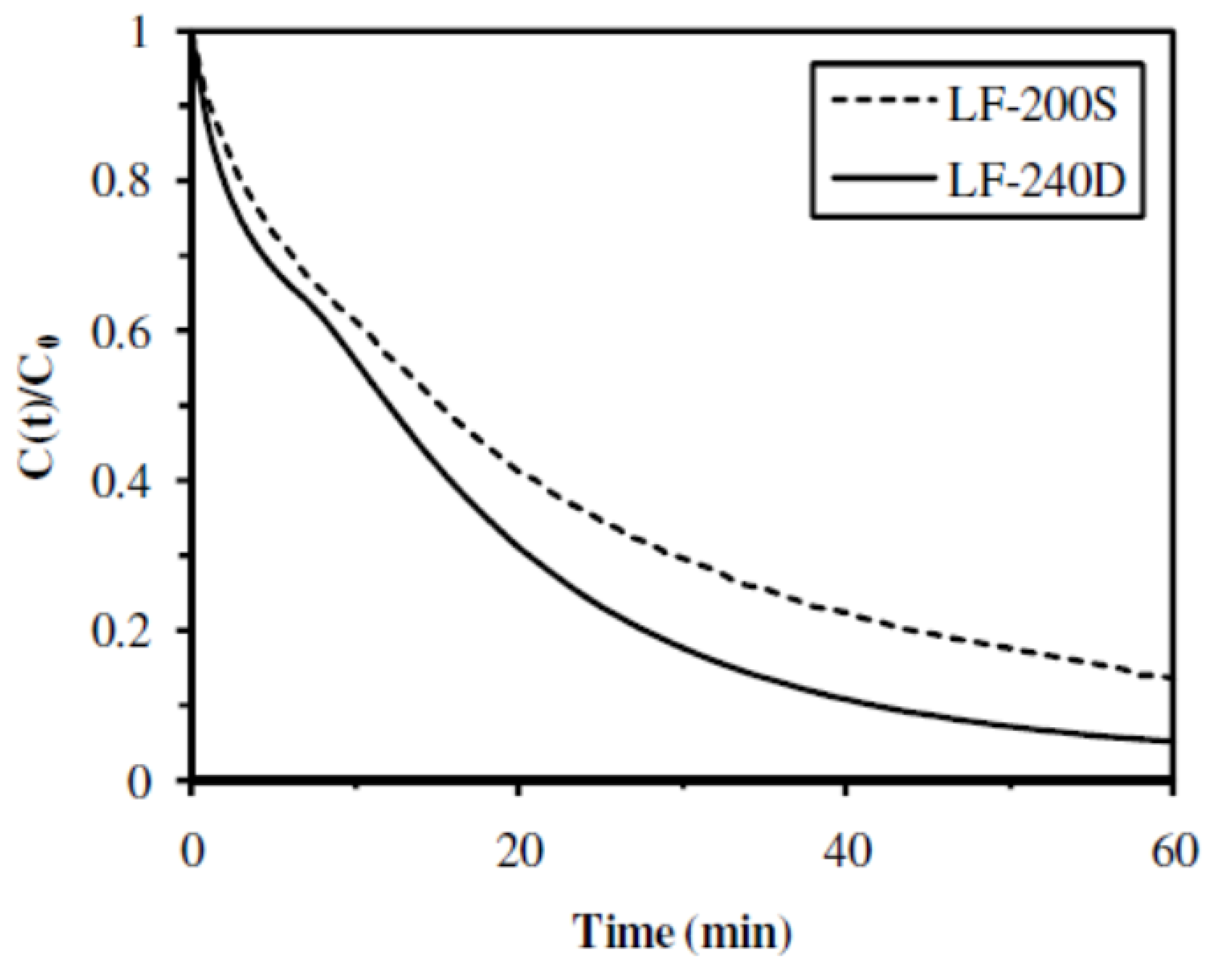
2.2.3. Influence of the M/G Molar Ratio
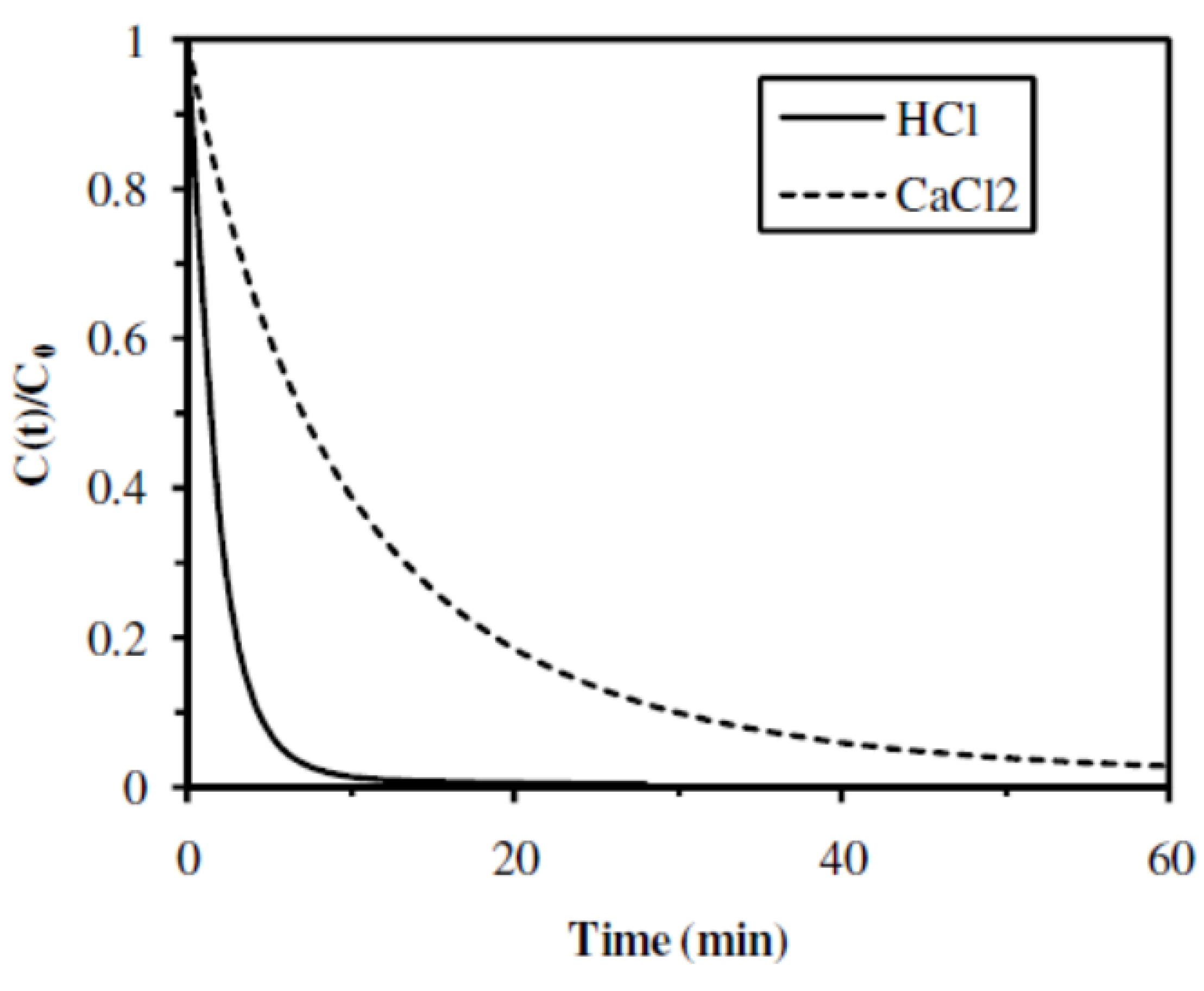
2.2.4. Influence of the Gelation Mode
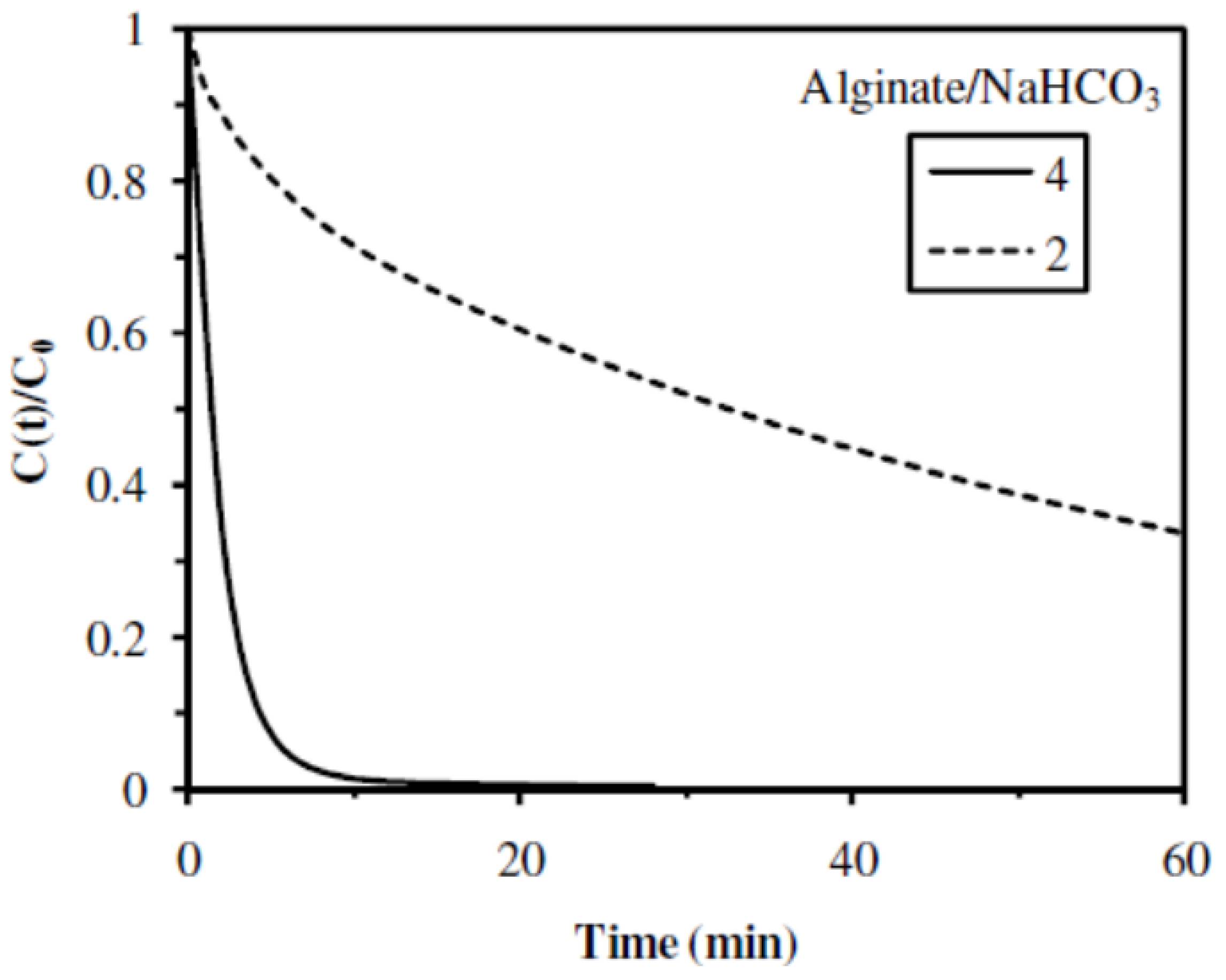
2.2.5. Influence of the Alginate/NaHCO3 Ratio
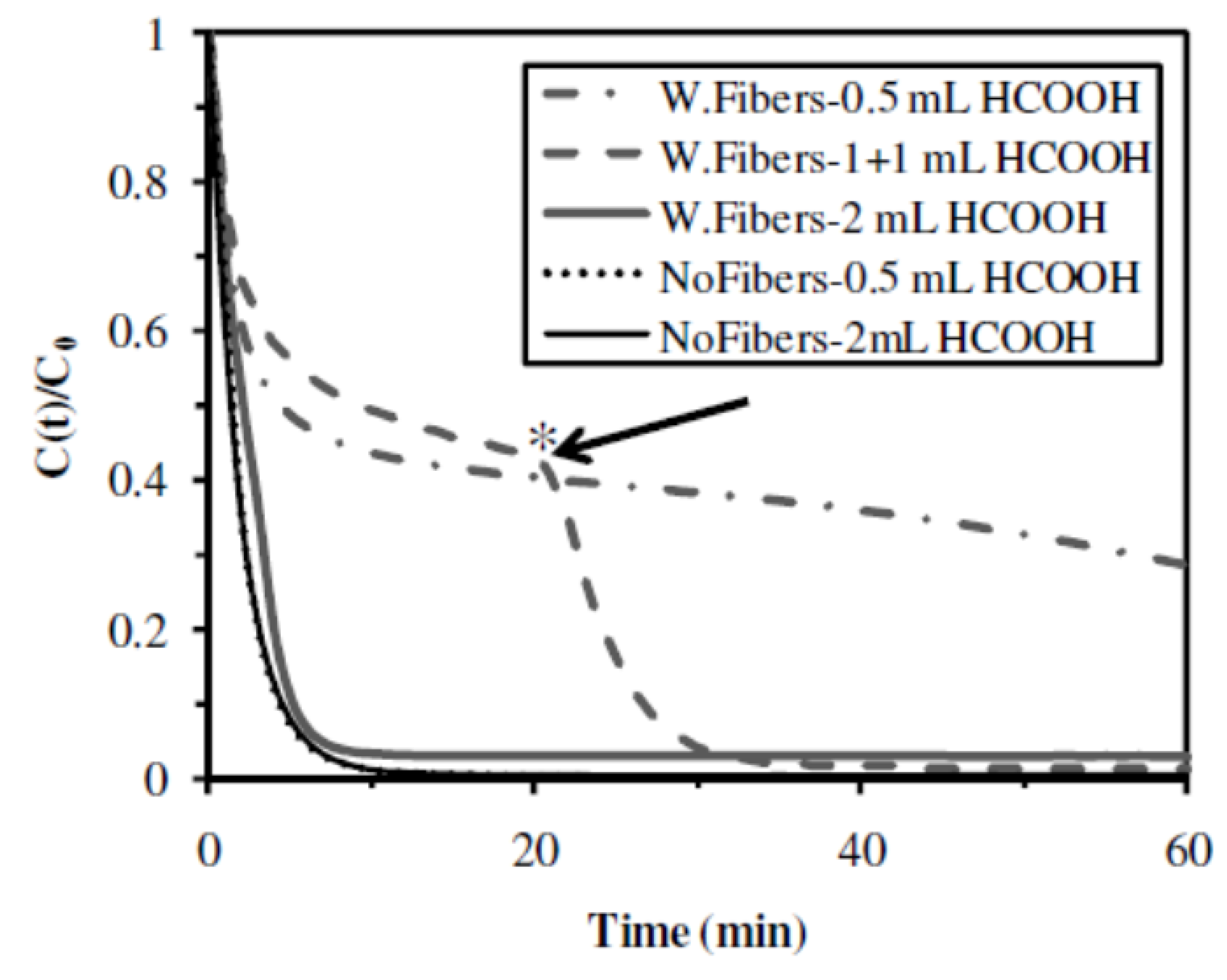
2.2.6. Influence of the Presence of Cellulose Fibers
2.3. Elaboration of Catalytic Materials for Specific Applications
| Desired properties | Parameters for the elaboration of the materials |
|---|---|
| High porosity | No cellulose fibers |
| Good mechanical resistance to compression | Inclusion of cellulose fibers |
| Good stability in water | Alginate/NaHCO3 mass ratio of 2 or 4 |
| High IL immobilization | No cellulose fibers |
| High reaction kinetics | HCl gelation |
| High catalytic activity | Inclusion of cellulose fibers (but increased amount of hydrogen donor) |
3. Experimental Section
3.1. Materials
3.2. Elaboration of the Catalytic Materials
3.2.1. Elaboration of Highly Porous Monoliths (HPMs)
3.2.2. Metal Immobilization
3.3. Characterization of Catalytic Materials
3.4. Catalytic Reaction
4. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgements
References
- Mayer, H.A.; Auer, F.; Schneller, T.; Lindner, E. Chemistry in interphases—A new approach to organometallic syntheses and catalysis. Angew. Chem. Int. Ed. 1999, 38, 2155–2174. [Google Scholar]
- Sheldon, R.A. Green solvents for sustainable organic synthesis: State of the art. Green Chem. 2005, 7, 267–278. [Google Scholar] [CrossRef]
- Reddy, V.P.; Sinn, E.; Afrasiabi, Z.; Perambuduru, M.; Oh, W.S.; Alleti, R. Gadolinium triflate immobilized in imidazolium based ionic liquids: A recyclable catalyst and green solvent for acetylation of alcohols and amines. Green Chem. 2005, 7, 203–206. [Google Scholar] [CrossRef]
- Wasserscheid, P. Continuous reactions using ionic liquids as catalytic phase. J. Ind. Eng. Chem. 2007, 13, 325–338. [Google Scholar]
- Wasserscheid, P.; Haumann, M.; Fehrmann, R.; Riisager, A. Supported Ionic Liquid Phase (SILP) catalysis: An innovative concept for homogeneous catalysis in continuous fixed-bed reactors. Eur. J. Inorg. Chem. 2006, 695–706. [Google Scholar]
- Wasserscheid, P.; Haumann, M.; Harter, P.; Schneider, M.J.; Bittermann, A.; Szesni, N.; Werner, S. Screening of Supported Ionic Liquid Phase (SILP) catalysts for the very low temperature water-gas-shift reaction. Appl. Catal. A 2010, 377, 70–75. [Google Scholar] [CrossRef]
- de Vos, D.E.; Jacobs, P.A.; Wahlen, J.; Alaerts, L. Recent progress in the immobilization of catalysts for selective oxidation in the liquid phase. Chem. Commun. 2008, 1727–1737. [Google Scholar]
- Yokoyama, C.; Tomida, D.; Qiao, K.; Kume, Y. Selective hydrogenation of cinnamaldehyde catalyzed by palladium nanoparticles immobilized on ionic liquids modified-silica gel. Catal. Commun. 2008, 9, 369–375. [Google Scholar] [CrossRef]
- Dupont, J.; dos Santos, J.H.Z.; Pavan, F.A.; Chiaro, S.S.X.; Gelesky, M.A. Supported ionic liquid phase rhodium nanoparticle hydrogenation catalysts. Dalton Trans. 2007, 5549–5553. [Google Scholar]
- Dez, I.; Gaumont, A.C.; Madec, P.J.; Perrigaud, K.; Baudoux, J. Development of new SILP catalysts using chitosan as support. Green Chem. 2007, 9, 1346–1351. [Google Scholar] [CrossRef]
- Dez, I.; Gaumont, A.C.; Guibal, E.; Marinel, S.; Madec, P.J.; Goupil, J.M.; Perrigaud, K.; Moucel, R. Importance of the conditioning of the chitosan support in a catalyst-containing ionic liquid phase immobilised on chitosan: The palladium-catalysed allylation reaction case. Adv. Synth. Catal. 2010, 352, 433–439. [Google Scholar] [CrossRef]
- Dupont, J.; Dias, S.L.P.; Pavan, F.A.; Foppa, L.; Scheeren, C.W.; Gelesky, M.A. Metal nanoparticle/ionic liquid/cellulose: New catalytically active membrane materials for hydrogenation reactions. Biomacromolecules 2009, 10, 1888–1893. [Google Scholar] [CrossRef] [PubMed]
- Guibal, E.; di Renzo, F.; Quignard, F. From natural polysaccharides to materials for catalysis, adsorption, and remediation. Top. Curr. Chem. 2010, 294, 165–197. [Google Scholar] [PubMed]
- Guibal, E. Heterogeneous catalysis on chitosan-based materials: A review. Prog. Polym. Sci. 2005, 30, 71–109. [Google Scholar] [CrossRef]
- Hardy, J.J.E.; Macquarrie, D.J. Applications of functionalized chitosan in catalysis. Ind. Eng. Chem. Res. 2005, 44, 8499–8520. [Google Scholar] [CrossRef]
- Guibal, E.; Vincent, T. Chitosan-supported palladium catalyst. 3. Influence of experimental parameters on nitrophenol degradation. Langmuir 2003, 19, 8475–8483. [Google Scholar] [CrossRef]
- Guibal, E.; Vincent, T.; Blondet, F.P. Hydrogenation of nitrotoluene using palladium supported on chitosan hollow fiber: Catalyst characterization and influence of operative parameters studied by experimental design methodology. Int. J. Biol. Macromol. 2008, 43, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, F.; Valentin, R.; Quignard, F. Aerogel materials from marine polysaccharides. New J. Chem. 2008, 32, 1300–1310. [Google Scholar] [CrossRef]
- Quignard, F.; Domard, A.; Viton, C.; Molvinger, K.; Valentin, R. From hydrocolloids to high specific surface area porous supports for catalysis. Biomacromolecules 2005, 6, 2785–2792. [Google Scholar] [CrossRef] [PubMed]
- Brunel, D.; Quignard, F.; Molvinger, K.; Valentin, R. Supercritical CO2 dried chitosan: An efficient intrinsic heterogeneous catalyst in fine chemistry. New J. Chem. 2003, 27, 1690–1692. [Google Scholar] [CrossRef]
- Choplin, A.; Quignard, F. Cellulose: A new bio-support for aqueous phase catalysts. Chem. Commun. 2001, 21–22. [Google Scholar]
- Jouannin, C.; Vincent, T.; Guibal, E. Immobilization of extractants in biopolymer capsules for the synthesis of new resins: A focus on the encapsulation of tetraalkyl phosphonium ionic liquids. J. Mater. Chem. 2009, 19, 8515–8527. [Google Scholar] [CrossRef]
- Guibal, E.; Parodi, A.; Vincent, T. Immobilization of Cyphos IL-101 in biopolymer capsules for the synthesis of Pd sorbents. React. Funct. Polym. 2008, 68, 1159–1169. [Google Scholar] [CrossRef]
- Guibal, E.; Vincent, T.; Taulemesse, J.M.; Gaumont, A.C.; Dez, I.; Jouannin, C. Palladium supported on alginate/ionic liquid highly porous monoliths: Application to 4-nitroaniline hydrogenation. Appl. Catal. B 2011, 103, 444–452. [Google Scholar] [CrossRef]
- Wang, D.M.; Lai, J.Y.; Hou, L.T.; Hsien, T.Y.; Hsieh, H.J.; Kuo, P.Y.; Ho, M.H. Preparation of porous scaffolds by using freeze-extraction and freeze-gelation methods. Biomaterials 2004, 25, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.J.; Lai, J.Y.; Wang, D.M.; Ho, M.H.; Lin, Y.A.; Yuan, N.Y. Effects of the cooling mode on the structure and strength of porous scaffolds made of chitosan, alginate, and carboxymethyl cellulose by the freeze-gelation method. Carbohydr. Polym. 2009, 78, 349–356. [Google Scholar] [CrossRef]
- Guo, Y.B.; Parks, W.M. A casting based process to fabricate 3D alginate scaffolds and to investigate the influence of heat transfer on pore architecture during fabrication. Mater. Sci. Eng. C 2008, 28, 1435–1440. [Google Scholar]
- Cohen, S.; Glicklis, R.; Zmora, S. Tailoring the pore architecture in 3-D alginate scaffolds by controlling the freezing regime during fabrication. Biomaterials 2002, 23, 4087–4094. [Google Scholar] [PubMed]
- Quignard, F.; di Renzo, F.; Garrone, E.; Bonelli, B.; Horga, R.; Valentin, R. FTIR spectroscopy of NH3 on acidic and ionotropic alginate aerogels. Biomacromolecules 2006, 7, 877–882. [Google Scholar] [CrossRef] [PubMed]
© 2012 by the authors. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jouannin, C.; Vincent, C.; Dez, I.; Gaumont, A.-C.; Vincent, T.; Guibal, E. Study of Alginate-Supported Ionic Liquid and Pd Catalysts. Nanomaterials 2012, 2, 31-53. https://doi.org/10.3390/nano2010031
Jouannin C, Vincent C, Dez I, Gaumont A-C, Vincent T, Guibal E. Study of Alginate-Supported Ionic Liquid and Pd Catalysts. Nanomaterials. 2012; 2(1):31-53. https://doi.org/10.3390/nano2010031
Chicago/Turabian StyleJouannin, Claire, Chloë Vincent, Isabelle Dez, Annie-Claude Gaumont, Thierry Vincent, and Eric Guibal. 2012. "Study of Alginate-Supported Ionic Liquid and Pd Catalysts" Nanomaterials 2, no. 1: 31-53. https://doi.org/10.3390/nano2010031
APA StyleJouannin, C., Vincent, C., Dez, I., Gaumont, A.-C., Vincent, T., & Guibal, E. (2012). Study of Alginate-Supported Ionic Liquid and Pd Catalysts. Nanomaterials, 2(1), 31-53. https://doi.org/10.3390/nano2010031