RETRACTED: Potential Impact of Multi-Walled Carbon Nanotubes Exposure to the Seedling Stage of Selected Plant Species
Abstract
:1. Introduction
2. Results and Discussion
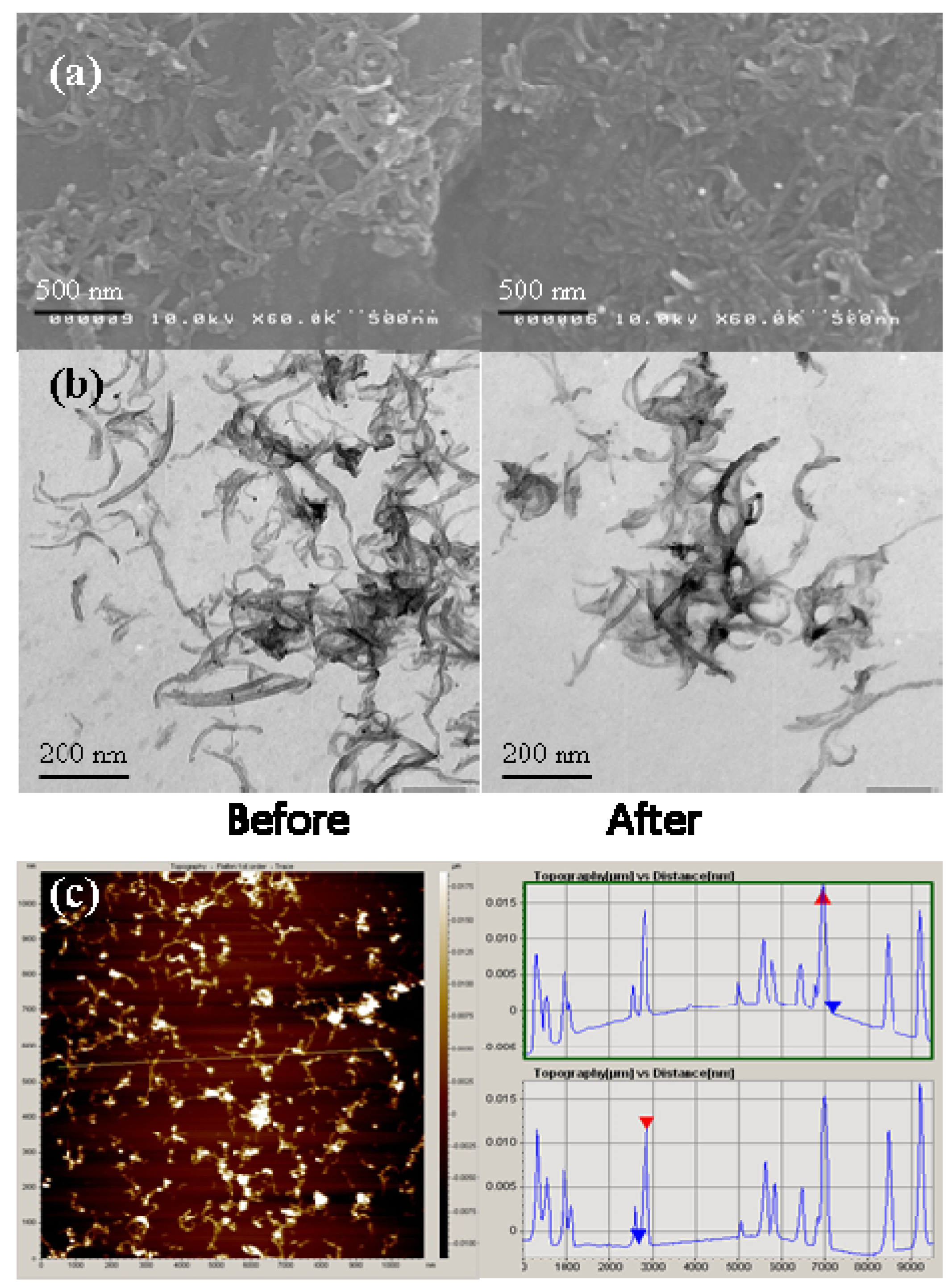
2.1. MWNTs Analysis
2.2. MWNTs Induced Morphological Changes in Plants

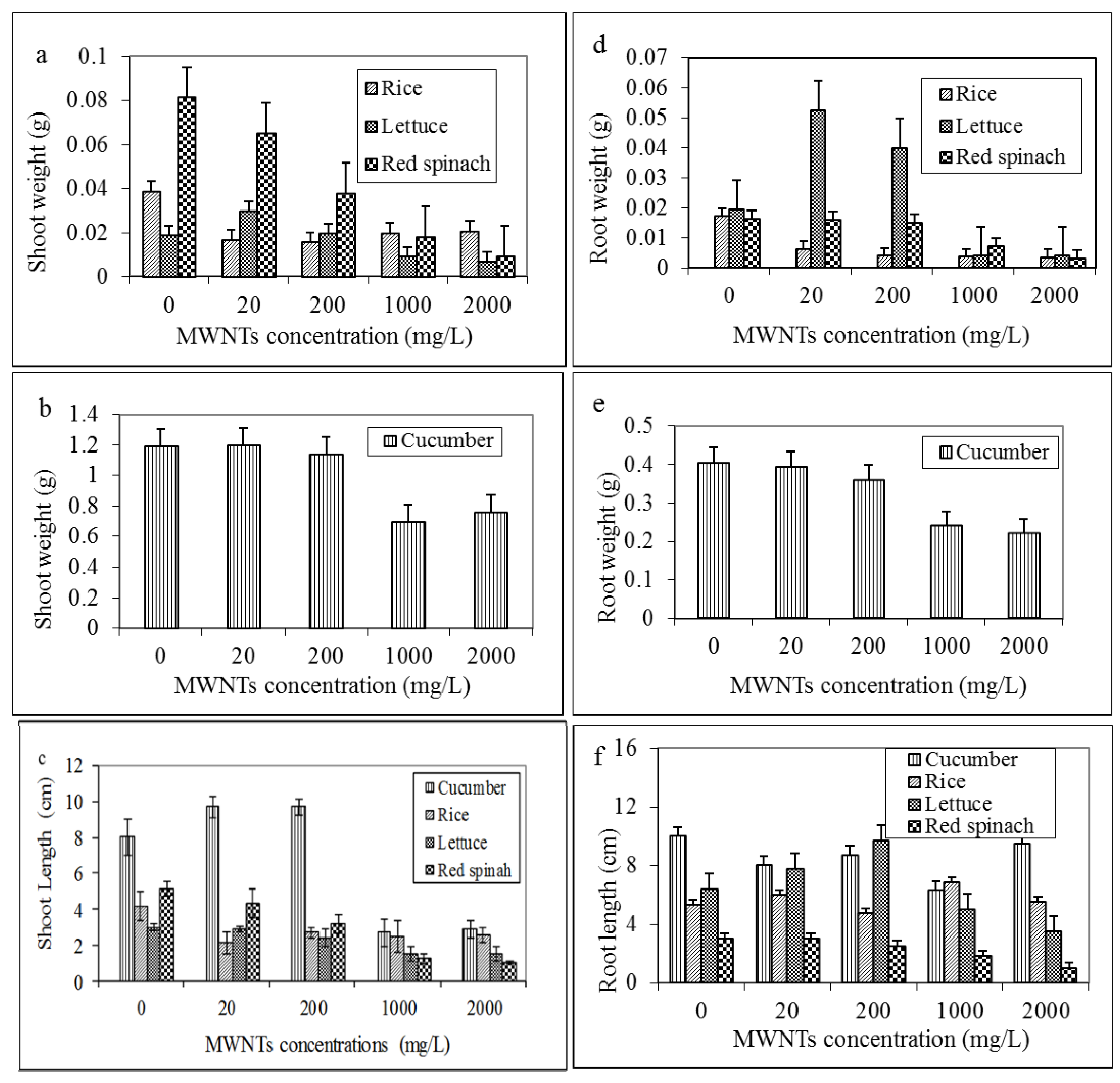
2.3. Effects of MWNTs on the Growth of Plants
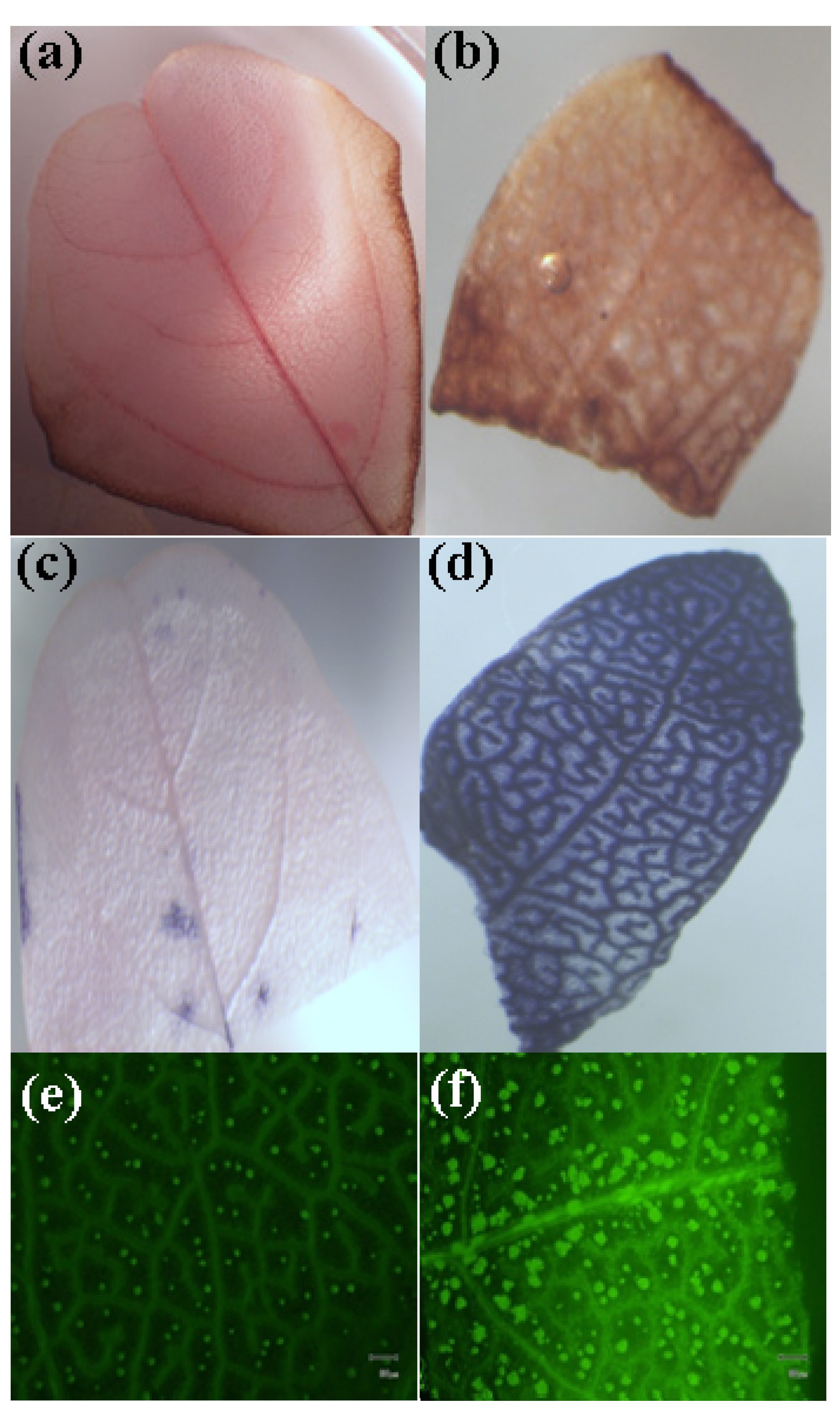
2.4. MWNTs Induces Cell Death and Membrane Damage in Plants

2.5. MWNTs Induces ROS Generation in Red Spinach
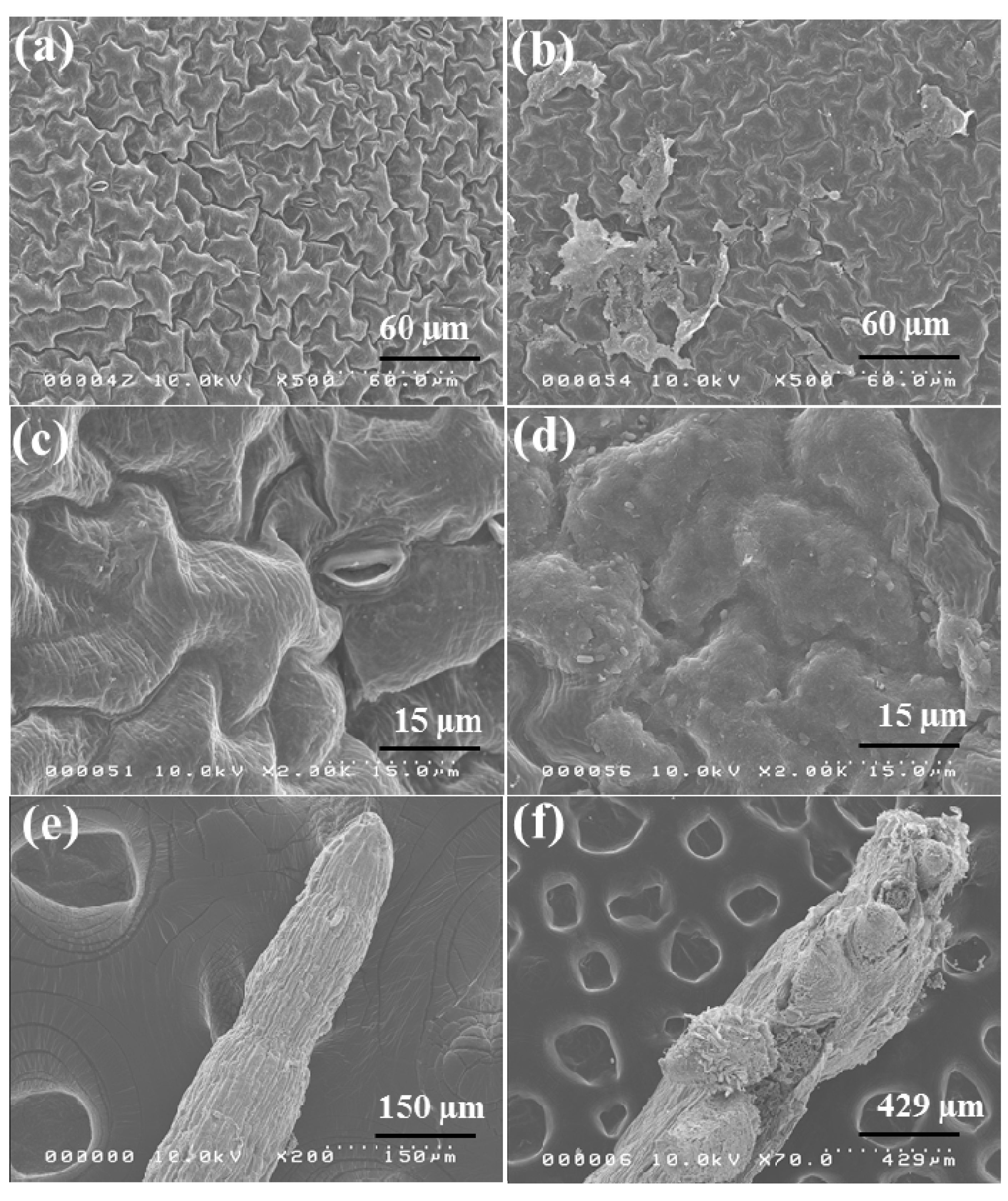
2.6. Morphological Observation of Red Spinach Roots and Leaves Using SEM
3. Experimental Section
3.1. Nanomaterials, Chemicals, and Seeds
3.2. Atomic Force Microscopy (AFM), Scanning Electron Microscope (SEM) and Transmission Electron Microscope (TEM)
3.3. Hydroponic Culture and Effects of MWNTs on the Growth of the Plants
3.4. Effect of MWNTs on Cell Death (Evans Blue and Electrolyte Leakage)
3.5. Detection of ROS (Hydrogen Peroxide, Hydroperoxides, and Superoxide)
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nel, A.; Xia, T.; Meng, H.; Wang, X.; Lin, S.; Ji, Z.; Zhang, H. Nanomaterial toxicity testing in the 21st century: Use of a predictive toxicological approach and high-throughput screening. Acc. Chem. Res. 2013, 46, 607–621. [Google Scholar]
- Wang, Y.; Kempa, K.; Kimball, B.; Carlson, J.B.; Benham, G.; Li, W.Z.; Kempa, T.; Rybczynski, J.; Herczynski, A.; Ren, Z.F. Receiving and transmitting light-like radio waves: Antenna effect in arrays of aligned carbon nanotubes. Appl. Phys. Lett. 2004, 85, 2607–2609. [Google Scholar]
- Milne, W.I.; Teo, K.B.K.; Amaratunga, G.A.J.; Legagneux, P.; Gangloff, L.; Schnell, J.P.; Semet, V.; Binh, V.T.; Groening, O. Carbon nanotubes as field emission sources. J. Mater. Chem. 2004, 14, 933–943. [Google Scholar]
- Joseph, T.; Morrison, M. Nanotechnology in Agriculture and Food. 2006. Available online: http://www.nanoforum.org (accessed on 17 January 2008).
- Moore, M.N. Do nanoparticles present ecotoxicological risks for the health of the aquatic environment? Environ. Int. 2006, 32, 967–976. [Google Scholar] [CrossRef]
- Nowack, B.; Bucheli, T.D. Occurrence, behavior and effects of nanoparticles in the environment. Environ. Pollut. 2007, 150, 5–22. [Google Scholar] [CrossRef]
- Zhang, W.X.; Karn, B. Nanoscale environmental science and technology-challenges and opportunities. Environ. Sci. Technol. 2005, 39, 94A–95A. [Google Scholar] [CrossRef]
- Handy, R.D.; Owen, R.; Vlsami-Jones, E. The ecotoxicology of nanoparticles and nanomaterials: Current status, knowledge gaps, challenges, and future needs. Ecotoxicology 2008, 17, 315–325. [Google Scholar] [CrossRef]
- Barrena, R.; Casals, E.; Colon, J.; Font, X.; Sanchez, A.; Puntes, V. Evaluation of the ecotoxicity of model nanoparticles. Chemosphere 2009, 75, 850–857. [Google Scholar] [CrossRef]
- Sharifi, S.; Behzadi, S.; Laurent, S.; Forrest, M.L.; Stroeve, P.; Mahmoudi, M. Toxicity of nanomaterials. Chem. Soc. Rev. 2012, 41, 2323–2343. [Google Scholar]
- Nair, R.; Varghese, S.H.; Nair, B.G.; Maekawa, T.; Yoshida, Y.; Kumar, D.S. Nanoparticulate material delivery to plants. Plant Sci. 2010, 179, 154–163. [Google Scholar] [CrossRef]
- Ma, X.; Geiser-Lee, J.; Deng, Y.; Kolmakov, A. Interactions between engineered nanoparticles (ENPs) and plants: Phytotoxicity, uptake and accumulation. Sci. Total Environ. 2010, 408, 3053–3061. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Madler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef]
- Canas, J.E.; Long, M.Q.; Nations, S.; Vandan, R.; Dai, L.; Ambikapathi, R.; Lee, E.H.; Olszyk, D. Effects of functionalized and nonfunctionalized single-walled carbon nanotubes on root elongation of select crop species. Environ. Toxicol. Chem. 2008, 27, 1922–1931. [Google Scholar] [CrossRef]
- Ghodake, G.; Seo, Y.D.; Park, D.; Lee, D.S. Phytotoxicity of carbon nanotubes assessed by Brassica juncea and Phaseolus mungo. J. Nanoelectron. Optoelectron. 2010, 5, 157–160. [Google Scholar] [CrossRef]
- Begum, P.; Ikhtiari, R.; Fugetsu, B.; Matsuoka, M.; Akasaka, T.; Watari, F. Phytotoxicity of multi-walled carbon nanotubes assessed by selected plant species in the seedling stage. Appl. Surf. Sci. 2012, 262, 120–124. [Google Scholar] [CrossRef]
- Kawai-Yamada, M.; Ohori, Y.; Uchimiya, H. Dissection of Arabidopsis Bax inhibitor-1 suppressing Bax-, hydrogen peroxide-, and salicylic acid-induced cell death. Plant Cell 2004, 16, 21–32. [Google Scholar] [CrossRef]
- Pennacchio, M.; Jefferson, L.V.; Havens, K. Arabidopsis thaliana: A new test species for phytotoxicity bioassays. J. Chem. Ecol. 2005, 31, 1877–1885. [Google Scholar] [CrossRef]
- Stampoulis, D.; Sinha, S.K.; White, J.C. Assay-dependent phytotoxicity of nanoparticles to plants. Environ. Sci. Technol. 2009, 43, 9473–9479. [Google Scholar] [CrossRef]
- Lee, C.W.; Mahendra, S.; Zodrow, K.; Li, D.; Tsai, Y.C.; Braam, J.; Alvarez, P.J.J. Developmental phytotoxicity of metal oxide nanoparticles to Arabidopsis thaliana. Environ. Toxicol. Chem. 2010, 29, 669–675. [Google Scholar] [CrossRef]
- Lin, D.H.; Xing, B.S. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007, 50, 243–250. [Google Scholar]
- Lahiani, M.H.; Dervishi, E.; Chen, J.; Nima, Z.; Gaume, A.; Biris, A.S.; Khodakovskaya, M.V. Impact of carbon nanotube exposure to seeds of valuable crops. Appl. Mater. Interfaces 2013, 5, 7965–7973. [Google Scholar] [CrossRef]
- Begum, P.; Ikhtiari, R.; Fugetsu, B. Graphene phytotoxicity in the seedling stage of cabbage, tomato, red spinach, and lettuce. Carbon 2011, 49, 3907–3919. [Google Scholar] [CrossRef]
- Lin, C.; Fugetsu, B.; Su, Y.; Watari, F. Studies on toxicity of multi-walled carbon nanotubes on Arabidopsis T87 suspension cells. J. Hazard. Mater. 2009, 170, 578–583. [Google Scholar] [CrossRef]
- Campbell, N.A. Biology, 2nd ed.; The Benjamin/Cummings Publishing Company: Redwood City, CA, USA, 1990. [Google Scholar]
- Begum, P.; Fugetsu, B. Phytotoxicity of multi-walled carbon nanotubes on red spinach and role of ascorbic acid as an antioxidant. J. Hazard. Mater. 2012, 243, 212–222. [Google Scholar] [CrossRef]
- Mur, L.A.J.; Kenton, P.; Lioyd, A.J.; Ougham, H.; Prats, E. The hypersensitive response; The centenary is upon us but how much do we know? J. Exp. Bot. 2008, 59, 501–520. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Tan, X.M.; Lin, C.; Fugetsu, B. Studies on toxicity of multi-walled carbon nanotubes on suspension rice cells. Carbon 2009, 47, 3479–3487. [Google Scholar] [CrossRef]
- Beckman, K.B.; Ames, B.N. Oxidative decay of DNA. J. Biol. Chem. 1997, 272, 19633–19636. [Google Scholar] [CrossRef]
- Berlett, B.S.; Stadtman, E.R. Protein oxidation in aging, disease, and oxidative stress. J. Biol. Chem. 1997, 272, 20313–20316. [Google Scholar] [CrossRef]
- Landsiedel, R.; Kapp, M.D.; Schulz, M.; Wiench, K.; Oesch, F. Genotoxicity investigations on nanomaterials: Methods, preparation and characterization of test material, potential artifacts and limitations—Many questions, some answers. Mutat. Res. 2009, 681, 241–258. [Google Scholar] [CrossRef]
- Kovacic, P.; Somanathan, R. Biomechanisms of nanoparticles (toxicants, antioxidants and therapeutics): Electron transfer and reactive oxygen species. J. Nanosci. Nanotechnol. 2010, 10, 7919–7930. [Google Scholar] [CrossRef]
- Zimmermann, U.; Schneider, H.; Wegner, L.; Wagner, H.J.; Szimtenings, M.; Haase, A.; Bentrup, F.W. What are the driving forces for water lifting in the xylem conduit? Plant Physiol. 2002, 114, 327–335. [Google Scholar] [CrossRef]
- Lee, S.; Choi, H.; Suh, S.; Doo, I.S.; Oh, K.Y.; Choi, E.J.; Taylor, A.T.S.; Low, P.S.; Lee, Y. Oligogalacturonic acid and chitosan reduce stomatal aperture by inducing the evolution of reactive oxygen species from guard cells of tomato and Commelina communis. Plant Physiol. 1999, 121, 147–152. [Google Scholar] [CrossRef]
- Levine, A.; Tenhaken, R.; Dixon, R.A.; Lamb, C. H2O2 from the oxidative burst orchestrates the plant hypersensitive response. Cell 1994, 79, 583–593. [Google Scholar]
- McAinsh, M.R.; Clayton, H.; Mansfield, T.A.; Hetherington, A.M. Changes in stomatal behavior and cytosolic free calcium in response to oxidative stress. Plant Physiol. 1996, 111, 1031–1042. [Google Scholar]
- Agarwal, M.; Murugan, M.S.; Sharma, A.; Rai, R.; Kamboj, A.; Sharma, H.; Roy, S.K. Nanoparticles and its toxic effects: A review. Int. J. Curr. Microbiol. Appl. Sci. 2013, 2, 76–82. [Google Scholar]
- Sayes, C.M.; Liang, F.; Hudson, J.L.; Mendez, J.; Guo, W.; Beach, J.M.; Moore, V.C.; Doyle, C.D.; West, J.L.; Billups, W.E.; et al. Functionalization density dependence of single-walled carbon nanotubes cytotoxicity in vitro. Toxicol. Lett. 2006, 16, 135–142. [Google Scholar]
- Lin, C.; Su, Y.; Takahiro, M.; Fugetsu, B. Multi-walled carbon nanotubes induce oxidative stress and vacuolar structure changes to Arabidopsis T87 suspension cells. Nano Biomed. 2010, 2, 170–181. [Google Scholar]
- Miralles, P.; Johnson, E.; Church, T.L.; Harris, A.T. Multiwalled carbon nanotubes in alfalfa and wheat: Toxicology and uptake. J. R. Soc. Interface 2012, 9, 3514–3527. [Google Scholar] [CrossRef]
- Khodakovskaya, M.; Dervishi, E.; Mahmood, M.; Xu, Y.; Li, Z.; Watanabe, F.; Alexandru, S.B. Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth. ACS Nano 2009, 3, 3221–3227. [Google Scholar] [CrossRef]
- Keller, A.A.; Wang, H.; Zhou, D.; Lenihan, H.S.; Cherr, G.; Cardinale, B.J.; Miller, R.; Ji, Z. Stability and aggregation of metal oxide nanoparticles in natural aqueous matrices. Environ. Sci. Technol. 2010, 44, 1962–1967. [Google Scholar] [CrossRef]
- Lee, W.; An, Y.; Yoon, H. Toxicity and bioavailability of copper nanoparticles to the terrestrials plants mung bean (Phaseolus radiatus) and wheat (Triticum awstivum): Plant uptake for water insoluble nanoparticles. Environ. Toxicol. Chem. 2008, 27, 1915–1921. [Google Scholar] [CrossRef]
- Guthrie, R.L. Xylem structure and ecological dominance in a forest community. Am. J. Bot. 1989, 76, 1216–1228. [Google Scholar] [CrossRef]
- Rico, C.M.; Majumdar, S.; Duarte-Gardea, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Interaction of nanoparticles with edible plants and their possible implications in the food chain. J. Agric. Food Chem. 2011, 59, 3485–3498. [Google Scholar] [CrossRef]
- Lin, S.; Reppert, J.; Hu, Q.; Hudson, J.A.S.; Reid, M.L.; Ratnikova, T.A.; Rao, A.M.; Luo, H.; Ke, P.C. Uptake, translocation, and transmission of carbon nanomaterials in rice plants. Small 2009, 5, 1128–1132. [Google Scholar]
- Lockshin, R.A.; Zakeri, Z. Apoptosis, autophagy, and more. Int. J. Biochem. Cell Biol. 2004, 36, 2405–2419. [Google Scholar] [CrossRef]
- Gadjev, I.; Stone, J.M.; Gechev, T.S. Programmed cell death in plants: New insights into redox regulation and the role of hydrogen peroxide. Int. Rev. Cell Mol. Biol. 2008, 270, 87–144. [Google Scholar] [CrossRef]
- Lamb, C.; Dixon, R.A. The oxidative burst in plant disease resistance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 251–275. [Google Scholar] [CrossRef]
- Gechev, T.S.; van Breusegem, F.; Stone, J.M.; Denev, I.; Laloi, C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays 2006, 28, 1091–1101. [Google Scholar] [CrossRef]
- Moller, I.M.; Jensen, P.E; Hansson, A. Oxidative modifications to cellular components in plants. Annu. Rev. Plant Biol. 2007, 58, 459–481. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Laloi, C.; Przybyla, D.; Apel, K. A genetic approach towards elucidating the biological activity of different reactive oxygen species in Arabidopsis thaliana. J. Exp. Bot. 2006, 57, 1719–1724. [Google Scholar] [CrossRef]
- Van Breusegem, F.; Dat, J. Reactive oxygen species in plant cell death. Plant Physiol. 2006, 141, 384–390. [Google Scholar] [CrossRef] [Green Version]
- Hoagland, D.R.; Arnon, D.I. The Water Culture Method for Growing Plants without Soil; College of Agriculture, University of California: Berkeley, CA, USA, 1950; pp. 1–39. [Google Scholar]
- US EPA-Ecological Effects Test Guidelines (OPPTS 850.4200) Seed Germination/Root Elongation Toxicity Test. 1996. 1996. Available online: http://www.epa.gov/ocspp/pubs/frs/publications/OPPTS_Harmonized/850_Ecological_Effects_Test_Guidelines/Drafts/850-4200.pdf (accessed on 3 November 2009).
- Lopez-Moreno, M.L.; De La Rosa, G.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. X-ray absorption spectroscopy (XAS) corroboration of the uptake and storage of CeO2 nanoparticles and assessment of their differential toxicity in four edible plant species. J. Agric. Food. Chem. 2010, 58, 3689–3693. [Google Scholar] [CrossRef]
- Baker, C.J.; Mock, N.M. An improved method for monitoring cell death in cell suspension and leaf disc assays using Evans Blue. Plant Cell Tissue Organ Cult. 1994, 39, 7–12. [Google Scholar]
- Lutts, S.; Kinet, J.M.; Bouharmont, J. NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivar differing in salinity resistance. Ann. Bot. 1996, 78, 389–398. [Google Scholar] [CrossRef]
- Graham, R.C.; Karnowsky, M.J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: Ultrastructural cytochemistry by a new technique. J. Histochem. Cytochem. 1966, 14, 291–302. [Google Scholar] [CrossRef]
- Naton, B.; Hahlbrock, K.; Schmelzer, E. Correlation of rapid cell death with metabolic changes in fungus-infected, cultured parsley cells. Plant Physiol. 1996, 112, 433–444. [Google Scholar]
- Doke, N. Involvement of superoxide anion generation in hypersensitive response of potato tuber tissues to infection with an incompatible race of Phytophtora infestans. Physiol. Plant Pathol. 1983, 23, 345–357. [Google Scholar] [CrossRef]
- Khodakovskayaa, M.V.; Silvaa, K.de.S.; Nedosekinb, D.A.; Dervishic, E.; Birisa, A.S.; Shashkovb, E.V.; Galanzhab, E.I.; Zharovb, V.P. Complex genetic, photothermal, and photoacoustic analysis of nanoparticle-plant interactions. Proc. Natl. Acad. Sci. USA 2011, 108, 1028–1033. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Begum, P.; Ikhtiari, R.; Fugetsu, B. RETRACTED: Potential Impact of Multi-Walled Carbon Nanotubes Exposure to the Seedling Stage of Selected Plant Species. Nanomaterials 2014, 4, 203-221. https://doi.org/10.3390/nano4020203
Begum P, Ikhtiari R, Fugetsu B. RETRACTED: Potential Impact of Multi-Walled Carbon Nanotubes Exposure to the Seedling Stage of Selected Plant Species. Nanomaterials. 2014; 4(2):203-221. https://doi.org/10.3390/nano4020203
Chicago/Turabian StyleBegum, Parvin, Refi Ikhtiari, and Bunshi Fugetsu. 2014. "RETRACTED: Potential Impact of Multi-Walled Carbon Nanotubes Exposure to the Seedling Stage of Selected Plant Species" Nanomaterials 4, no. 2: 203-221. https://doi.org/10.3390/nano4020203
APA StyleBegum, P., Ikhtiari, R., & Fugetsu, B. (2014). RETRACTED: Potential Impact of Multi-Walled Carbon Nanotubes Exposure to the Seedling Stage of Selected Plant Species. Nanomaterials, 4(2), 203-221. https://doi.org/10.3390/nano4020203



