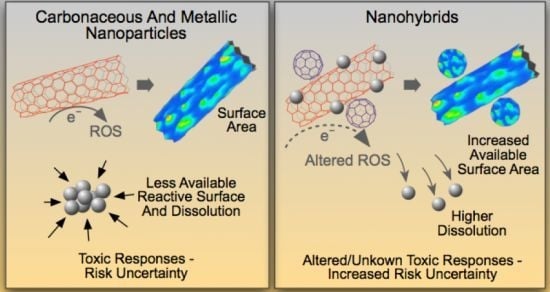
Emergent Properties and Toxicological Considerations for Nanohybrid Materials in Aquatic Systems
Abstract
:1. Introduction
2. Classification, Applications and Characterization of Nanohybrids
| Broad Application Areas | Specific Applications | NH Class | Specific Types | Citation | Environmental Exposure Pathway |
|---|---|---|---|---|---|
| Electronics and energy | Field effect transistors | CCNH | Fullerene-CNT peapods | [46,74,75] | Leachate; surface water |
| Graphene-CNT hybrid | [76] | ||||
| CMNH | Graphene-ZnO hybrid | [77] | |||
| Graphene nanosheet/metal nitride hybrid | [78] | ||||
| OMCNH | Graphene-organic molecule hybrid | [79,80] | |||
| Poly(3-hexylthiophene)-fullerene hybrid | [81] | ||||
| Energy storage/supercapacitors | CCNH | Graphene oxide-CNT peapods | [82] | ||
| CMNH | MnO2/CNT hybrid | [83] | |||
| CNT/RuO2 hybrid | [84] | ||||
| Graphene-Mn3O4 | [85] | ||||
| Lithium ion batteries/storage | CCNH | Fullerene-CNT peapods | [86] | ||
| Graphene-CNT hybrid | [87,88,89] | ||||
| Carbon nano-onions | [90] | ||||
| CMNH | Graphene-TiO2 hybrid | [91] | |||
| MMNH | ZnO-Au hybrid | [92] | |||
| Transparent conductive films | CCNH | CNT-graphene exohedral hybrid | [76,93,94] | ||
| Fullerene/CNT/graphene-oxide hybrid | [95] | ||||
| CMNH | SWNT-Au | [96] | |||
| MMNH OMCNH | Ag/TiO2 nanowire | [97] | |||
| Graphene-Ag nanowire | [98] | ||||
| Photovoltaics | CCNH | Graphene-fullerene hybrid | [99,100,101,102] | ||
| Optical limiting devices | CMNH | CNT-fullerene | [103] | ||
| ZnO-graphene quantum dots | [104] | ||||
| Graphene/TiO2 | [105] | ||||
| MMNH | Ag/TiO2 nanowire | [106] | |||
| OMCNH | Fullerene/CNT with porphyrins/phthalocyanines | [107] | |||
| dihydronaphthyl-fullerene | [108] | ||||
| CCNH CMNH | Graphene-fullerene hybrid | [109] | |||
| Fullerene-CNT | [110] | ||||
| MWNT-ZnO NH | [111] | ||||
| MMNH | Au@TiO2, Au@ZrO2, Ag@TiO2, and Ag@ZrO2 core-shell NHs | [112] | |||
| Fuel Cell | OMCNH | Oligothiophene-graphene, porphyrin-graphene | [13,113] | ||
| MMNH | Pt-Pd | [114] | |||
| CCNH | Graphene-CNT exohedral hybrid | [115] | |||
| CMNH | CNT/TiO2-Pt | [116] | |||
| Pt-reduced graphene oxide | [21] | ||||
| MMNH | Pd-Cu | [117] | |||
| Biomedical | Bioimaging and cancer therapy | CMNH | Quantum dot-Fe3O4-CNT | [118] | Atmosphere |
| MMNH | Au-Fe shell-core | [119,120] | |||
| MRI agents | CMNH | Gadofullerene | [121,122,123] | ||
| Drug delivery | CCNH | Fullerene-CNT | [124] | ||
| CMNH | Quantum dot-Fe3O4-CNT | [118] | |||
| MMNH | Au-Fe3O4 | [125] | |||
| OMCNH | Pluronic F-127/graphene | [126] | |||
| Parclitaxel-Au | [127] | ||||
| Environmental monitoring and remediation | Chemical sensing | CCNH | Carbon nanotube-graphene nanosheet hybrid | [128] | Leachate |
| CMNH | Pt-graphene | [129] | |||
| MWNT-zerovalent iron | [130] | ||||
| Graphene-iron | [131] | ||||
| Graphene-ZnO | [132] | ||||
| MMNH | Au-Ag | [133] | |||
| Pt/TiO2 nanotube | [134] | ||||
| OMCNH | Hematoporphyrin-ZnO | [135] | |||
| Biosensors | CCNH | Reduced graphene oxide-MWNT | [136] | ||
| Gas sensors | CCNH | Graphene-CNT hybrid | [137] | ||
| Contaminant degradation | CMNH | CNT-TiO2 | [138] | ||
| ZnO-reduced graphene oxide | [139] | ||||
| Pathogen detection | MMNH | Fe3O4-Au-Fe3O4 nanodumbbelland Fe3O4-AuNR nanonecklace | [140] | ||
| Au-Ag | [141] | ||||
| Antimicrobial | CMNH | CdSe-Au | [142] | ||
| Graphene-ZnO | [132] | ||||
| Ag-graphene oxide | [143] | ||||
| Heavy metal removal | CCNH | Carbon nano-onions | [144] | ||
| Bio-imaging | CCNH | Carbon nano-onions | [145] | ||
| Catalysis | Catalyst support/catalyst | OMCNH | CNT-enzyme | [146] | Atmosphere; leachate |
| CCNH | N-doped CNT-graphene peapods | [147] | |||
| CMNH | CNT/Pd | [148] | |||
| Graphene-Au | [149] | ||||
| MMNH | Au-Pd core-shell structure | [150] | |||
| Construction industry | Nano-reinforcement in composites | Pt/Pd-Fe/TiO2 | [114] | leachate | |
| CCNH | CNT-Graphene nanoplatelet hybrid | [151] | |||
| Structural health monitoring | CCNH | CNT-graphene nanoplatelet hybrid | [152] | ||
| Miscellaneous | Antimicrobial coating/paint | CCNH | Carbon nano-onions | [153] | Leachate |
| Temperature sensor | CCNH | Azafullerene-CNT peapods | [154] | - | |
| Heat transfer | CCNH | Graphene wrapped MWNT | [155] | - |
3. Key Properties Relevant to Toxicity
3.1. Alteration of Well-Known NM Properties Relevant to Toxicity
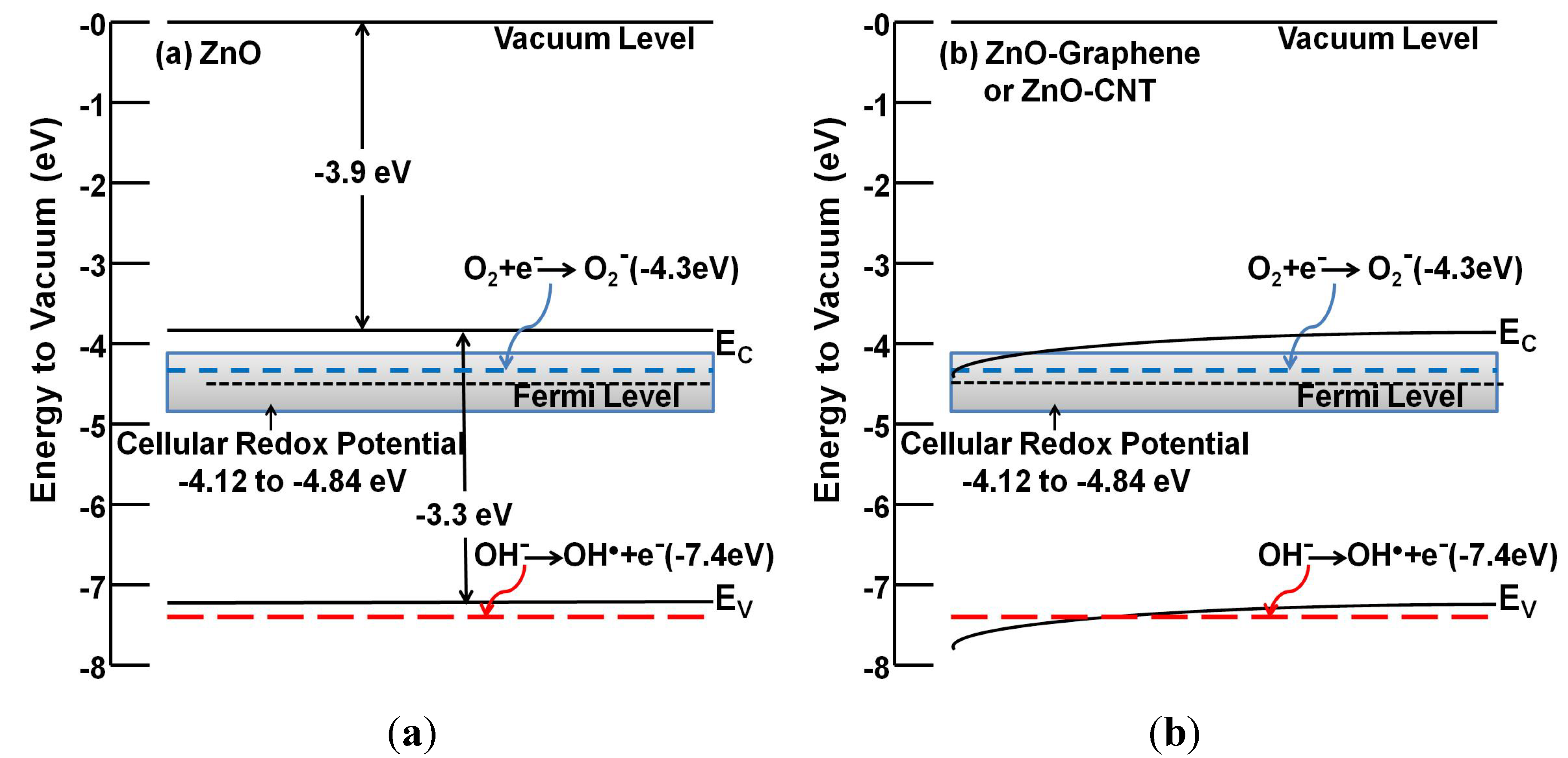
3.1.1. Bandgap Energetics, Photocatalytic Activity and ROS Generation Potential
3.1.2. Dissolution Characteristics
3.1.3. Surface Chemistry
3.2. Emergence of Novel Toxicological Properties for NHs
3.2.1. Dimensionality and Surface Morphology
3.2.2. Mechanical Properties
3.2.3. Synergistic Properties
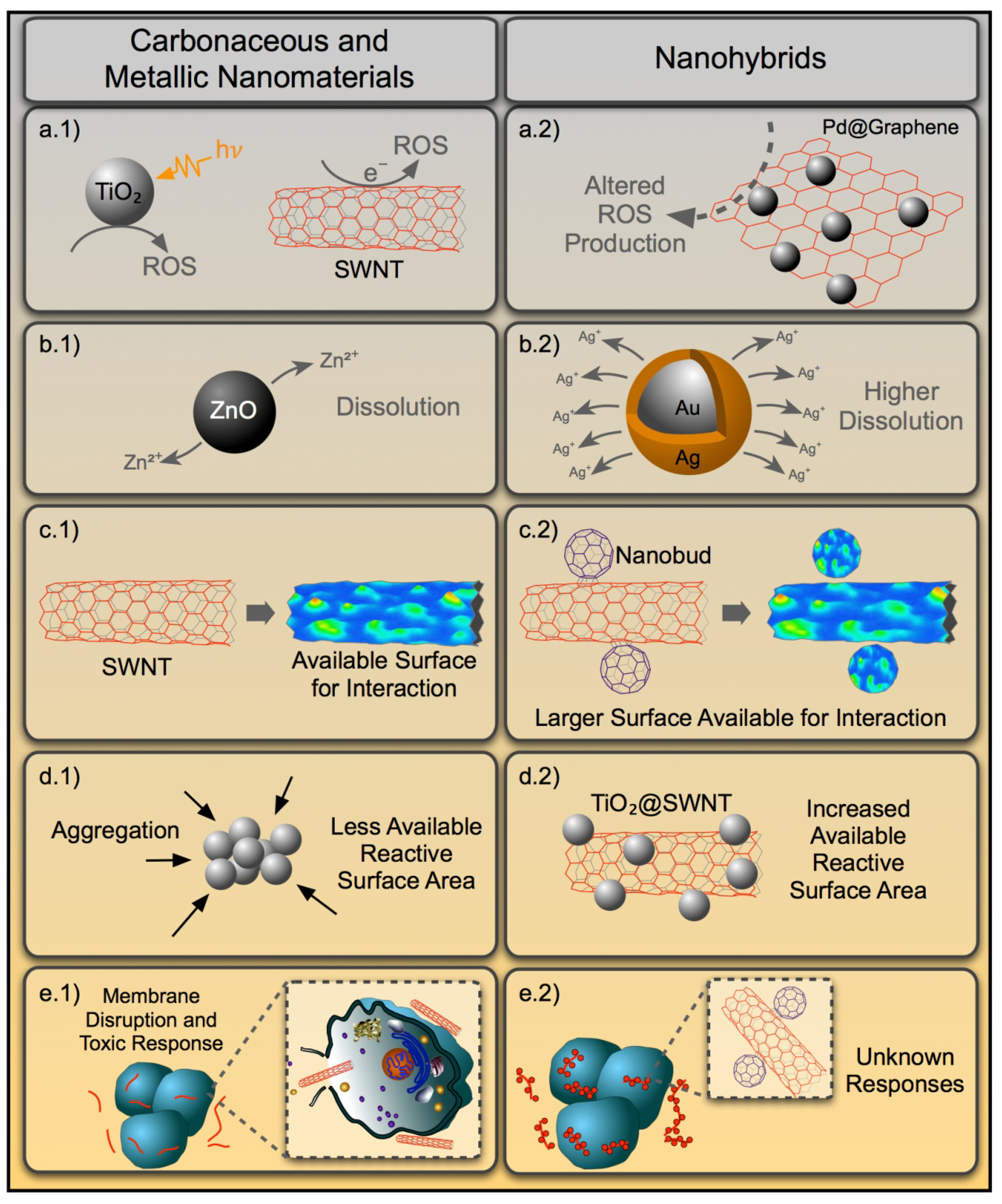
4. Toxicological Implications for NHs Based on Current Biological Effects and Mechanisms of Action
4.1. Aquatic Nanoparticle Toxicity Testing Strategies
4.2. Biological Mechanisms of Metal/Metal Oxide Nanoparticle Toxicity
4.3. Biological Mechanisms of Carbon Nanoparticle Toxicity
5. Application of Biological Effects of Constituent NMs to Understanding NH Toxicity
6. Conclusions
Author Contributions
Conflicts of Interest
References
- Feynman, R.P. There’s plenty of room at the bottom. Eng. Sci. 1960, 23, 22–36. [Google Scholar]
- Li, C.Y.; Li, L.Y.; Cai, W.W.; Kodjie, S.L.; Tenneti, K.K. Nanohybrid shish-kebabs: Periodically functionalized carbon nanotubes. Adv. Mater. 2005, 17, 1198–1202. [Google Scholar] [CrossRef]
- Muszynski, R.; Seger, B.; Kamat, P.V. Decorating graphene sheets with gold nanoparticles. J. Phys. Chem. C 2008, 112, 5263–5266. [Google Scholar] [CrossRef]
- Pielichowski, K.; Njuguna, J.; Janowski, B.; Pielichowski, J. Polyhedral oligomeric silsesquioxanes (poss)-containing nanohybrid polymers. In Supramolecular Polymers/Polymeric Betains/Oligomers; Donnio, B., Guillon, D., Harada, A., Hashidzume, A., Jaeger, W., Janowski, B., Kudaibergenov, S., Laschewsky, A., Njuguna, J., Pielichowski, J., et al., Eds.; Springer-Verlag: New York, NY, USA, 2006; Volume 201, pp. 225–296. [Google Scholar]
- Akasaka, T.; Nagase, S. Endofullerenes: A New Family of Carbon Clusters; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; Volume 3. [Google Scholar]
- Carril, M.; Fernández, I.; Rodríguez, J.; García, I.; Penadés, S. Gold-coated iron oxide glyconanoparticles for MRI, CT, and US multimodal imaging. Particle Particle Syst. Charact. 2013, 31, 81–87. [Google Scholar]
- Guo, S.J.; Dong, S.J.; Wang, E. Gold/platinum hybrid nanoparticles supported on multiwalled carbon nanotube/silica coaxial nanocables: Preparation and application as electrocatalysts for oxygen reduction. J. Phys. Chem. C 2008, 112, 2389–2393. [Google Scholar] [CrossRef]
- Logothetidis, S. Flexible organic electronic devices: Materials, process and applications. Mater. Sci. Eng. B Adv. Funct. Solid State Mater. 2008, 152, 96–104. [Google Scholar] [CrossRef]
- Sanchez, C.; Belleville, P.; Popall, M.; Nicole, L. Applications of advanced hybrid organic-inorganic nanomaterials: From laboratory to market. Chem. Soc. Rev. 2011, 40, 696–753. [Google Scholar] [CrossRef]
- Popov, A.A.; Yang, S.; Dunsch, L. Endohedral fullerenes. Chem. Rev. 2013, 113, 5989–6113. [Google Scholar] [CrossRef]
- Prakash, S.; Malhotra, M.; Shao, W.; Tomaro-Duchesneau, C.; Abbasi, S. Polymeric nanohybrids and functionalized carbon nanotubes as drug delivery carriers for cancer therapy. Adv. Drug Deliv. Rev. 2011, 63, 1340–1351. [Google Scholar] [CrossRef]
- Bhaskar, S.; Tian, F.R.; Stoeger, T.; Kreyling, W.; de la Fuente, J.M.; Grazu, V.; Borm, P.; Estrada, G.; Ntziachristos, V.; Razansky, D. Multifunctional nanocarriers for diagnostics, drug delivery and targeted treatment across blood-brain barrier: Perspectives on tracking and neuroimaging. Part. Fibre Toxicol. 2010, 7. [Google Scholar] [CrossRef]
- Guo, Z.; Du, F.; Ren, D.M.; Chen, Y.S.; Zheng, J.Y.; Liu, Z.B.; Tian, J.G. Covalently porphyrin-functionalized single-walled carbon nanotubes: A novel photoactive and optical limiting donor-acceptor nanohybrid. J. Mater. Chem. 2006, 16, 3021–3030. [Google Scholar] [CrossRef]
- Li, J.H.; Zhang, J.Z. Optical properties and applications of hybrid semiconductor nanomaterials. Coord. Chem. Rev. 2009, 253, 3015–3041. [Google Scholar] [CrossRef]
- McDowell, M.; Wright, A.E.; Hammer, N.I. Semiconductor nanocrystals hybridized with functional ligands: New composite materials with tunable properties. Materials 2010, 3, 614–637. [Google Scholar] [CrossRef]
- Watcharotone, S.; Dikin, D.A.; Stankovich, S.; Piner, R.; Jung, I.; Dommett, G.H.B.; Evmenenko, G.; Wu, S.E.; Chen, S.F.; Liu, C.P.; et al. Graphene-silica composite thin films as transparent conductors. Nano Lett. 2007, 7, 1888–1892. [Google Scholar] [CrossRef]
- Wang, D.F.; Zhao, H.G.; Wu, N.Q.; El Khakani, M.A.; Ma, D.L. Tuning the charge-transfer property of PbS-quantum dot/TiO2-nanobelt nanohybrids via quantum confinement. J. Phys. Chem. Lett. 2010, 1, 1030–1035. [Google Scholar] [CrossRef]
- El-Bashir, S.M. Photophysical properties of fluorescent pmma/SiO2 nanohybrids for solar energy applications. J. Lumines. 2012, 132, 1786–1791. [Google Scholar] [CrossRef]
- Schulz-Drost, C.; Sgobba, V.; Gerhards, C.; Leubner, S.; Calderon, R.M.K.; Ruland, A.; Guldi, D.M. Innovative inorganic-organic nanohybrid materials: Coupling quantum dots to carbon nanotubes. Angew. Chem. Int. Edit. 2010, 49, 6425–6429. [Google Scholar] [CrossRef]
- Mishra, A.K.; Bose, S.; Kuila, T.; Kim, N.H.; Lee, J.H. Silicate-based polymer-nanocomposite membranes for polymer electrolyte membrane fuel cells. Prog. Polym. Sci. 2012, 37, 842–869. [Google Scholar] [CrossRef]
- Park, D.-H.; Jeon, Y.; Ok, J.; Park, J.; Yoon, S.-H.; Choy, J.-H.; Shul, Y.-G. Pt nanoparticle-reduced graphene oxide nanohybrid for proton exchange membrane fuel cells. J. Nanosci. Nanotechnol. 2012, 12, 5669–5672. [Google Scholar] [CrossRef]
- Feng, L.L.; Gao, G.; Huang, P.; Wang, X.S.; Zhang, C.L.; Zhang, J.L.; Guo, S.W.; Cui, D.X. Preparation of Pt Ag alloy nanoisland/graphene hybrid composites and its high stability and catalytic activity in methanol electro-oxidation. Nanoscale Res. Lett. 2011, 6, 551–560. [Google Scholar] [CrossRef]
- Jung, J.H.; Park, M.; Shinkai, S. Fabrication of silica nanotubes by using self-assembled gels and their applications in environmental and biological fields. Chem. Soc. Rev. 2010, 39, 4286–4302. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E.; Aranda, P.; Darder, M.; Rytwo, G. Hybrid materials based on clays for environmental and biomedical applications. J. Mater. Chem. 2010, 20, 9306–9321. [Google Scholar] [CrossRef]
- Lin, B.Z.; Li, X.L.; Xu, B.H.; Chen, Y.L.; Gao, B.F.; Fan, X.R. Improved photocatalytic activity of anatase TiO2-pillared HTaWO6 for degradation of methylene blue. Microporous Mesoporous Mat. 2012, 155, 16–23. [Google Scholar] [CrossRef]
- Xia, T.; Kovochich, M.; Brant, J.; Hotze, M.; Sempf, J.; Oberley, T.; Sioutas, C.; Yeh, J.I.; Wiesner, M.R.; Nel, A.E. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 2006, 6, 1794–1807. [Google Scholar] [CrossRef]
- Oberdörster, G.; Oberdörster, E.; Oberdorster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef]
- Oberdorster, G.; Stone, V.; Donaldson, K. Toxicology of nanoparticles: A historical perspective. Nanotoxicology 2007, 1, 2–25. [Google Scholar] [CrossRef]
- Warheit, D.B.; Sayes, C.M.; Reed, K.L. Nanoscale and fine zinc oxide particles: Can in vitro assays accurately forecast lung hazards following inhalation exposures? Environ. Sci. Technol. 2009, 43, 7939–7945. [Google Scholar] [CrossRef]
- Lovern, S.B.; Strickler, J.R.; Klaper, R. Behavioral and physiological changes in Daphnia magna when exposed to nanoparticle suspensions (titanium dioxide, nano-C-60, and C(60)HxC(70)Hx). Environ. Sci. Technol. 2007, 41, 4465–4470. [Google Scholar] [CrossRef]
- Wiench, K.; Wohlleben, W.; Hisgen, V.; Radke, K.; Salinas, E.; Zok, S.; Landsiedel, R. Acute and chronic effects of nano- and non-nano-scale TiO2 and ZnO particles on mobility and reproduction of the freshwater invertebrate Daphnia magna. Chemosphere 2009, 76, 1356–1365. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, G.; Chen, C.; Yu, H.; Wang, T.; Ma, Y.; Jia, G.; Gao, Y.; Li, B.; Sun, J.; et al. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol. Lett. 2007, 168, 176–185. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Jiao, F.; Lao, F.; Li, W.; Gu, Y.; Li, Y.; Ge, C.; Zhou, G.; Li, B.; et al. Time-dependent translocation and potential impairment on central nervous system by intranasally instilled TiO2 nanoparticles. Toxicology 2008, 254, 82–90. [Google Scholar] [CrossRef]
- Love, S.A.; Maurer-Jones, M.A.; Thompson, J.W.; Lin, Y.-S.; Haynes, C.L. Assessing nanoparticle toxicity. Annu. Rev. Anal. Chem. Palo Alto Calif. 2012, 5, 181–205. [Google Scholar] [CrossRef]
- Khlebtsov, N.; Dykman, L. Biodistribution and toxicity of engineered gold nanoparticles: A review of in vitro and in vivo studies. Chem. Soc. Rev. 2011, 40, 1647–1671. [Google Scholar] [CrossRef]
- Choi, O.; Hu, Z. Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environ. Sci. Technol. 2008, 42, 4583–4588. [Google Scholar] [CrossRef]
- El Badawy, A.M.; Silva, R.G.; Morris, B.; Scheckel, K.G.; Suidan, M.T.; Tolaymat, T.M. Surface charge-dependent toxicity of silver nanoparticles. Environ. Sci. Technol. 2010, 45, 283–287. [Google Scholar]
- Franklin, N.M.; Rogers, N.J.; Apte, S.C.; Batley, G.E.; Gadd, G.E.; Casey, P.S. Comparative toxicity of nanoparticulate ZnO, bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): The importance of particle solubility. Environ. Sci. Technol. 2007, 41, 8484–8490. [Google Scholar] [CrossRef]
- Peng, X.; Palma, S.; Fisher, N.S.; Wong, S.S. Effect of morphology of ZnO nanostructures on their toxicity to marine algae. Aquat. Toxicol. 2011, 102, 186–196. [Google Scholar] [CrossRef]
- Cho, S.-J.; Idrobo, J.-C.; Olamit, J.; Liu, K.; Browning, N.D.; Kauzlarich, S.M. Growth mechanisms and oxidation resistance of gold-coated iron nanoparticles. Chem. Mater. 2005, 17, 3181–3186. [Google Scholar] [CrossRef]
- Tsuji, M.; Miyamae, N.; Lim, S.; Kimura, K.; Zhang, X.; Hikino, S.; Nishio, M. Crystal structures and growth mechanisms of Au@Ag core-shell nanoparticles prepared by the microwave-polyol method. Cryst. Growth Design 2006, 6, 1801–1807. [Google Scholar] [CrossRef]
- Tsuji, M.; Matsuo, R.; Jiang, P.; Miyamae, N.; Ueyama, D.; Nishio, M.; Hikino, S.; Kumagae, H.; Kamarudin, K.S.N.; Tang, X.-L. Shape-dependent evolution of Au@Ag core-shell nanocrystals by PVP-assisted N,N-dimethylformamide reduction. Cryst. Growth Design 2008, 8, 2528–2536. [Google Scholar] [CrossRef]
- Banerjee, M.; Sharma, S.; Chattopadhyay, A.; Ghosh, S.S. Enhanced antibacterial activity of bimetallic gold-silver core-shell nanoparticles at low silver concentration. Nanoscale 2011, 3, 5120–5125. [Google Scholar] [CrossRef]
- Li, T.; Albee, B.; Alemayehu, M.; Diaz, R.; Ingham, L.; Kamal, S.; Rodriguez, M.; Bishnoi, S.W. Comparative toxicity study of Ag, Au, and Ag-Au bimetallic nanoparticles on Daphnia magna. Analyt. Bioanalyt. Chem. 2010, 398, 689–700. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Yang, J.-W. Self-assembled flower-like TiO2 on exfoliated graphite oxide for heavy metal removal. J. Ind. Eng. Chem. 2012, 18, 1178–1185. [Google Scholar] [CrossRef]
- Shimada, T.; Ohno, Y.; Okazaki, T.; Sugai, T.; Suenaga, K.; Kishimoto, S.; Mizutani, T.; Inoue, T.; Taniguchi, R.; Fukui, N.; et al. Transport properties of C-78, C-90 and Dy@C-82 fullerenes-nanopeapods by field effect transistors. Physica E 2004, 21, 1089–1092. [Google Scholar] [CrossRef]
- Alam, M.J.; Tsuji, M.; Matsunaga, M.; Yamaguchi, D. Shape changes in Au-Ag bimetallic systems involving polygonal Au nanocrystals to spherical Au/Ag alloy and excentered Au core Ag/Au alloy shell particles under oil-bath heating. CrystEngComm 2011, 13, 2984–2993. [Google Scholar] [CrossRef]
- Rai, A.; Chaudhary, M.; Ahmad, A.; Bhargava, S.; Sastry, M. Synthesis of triangular Au core-Ag shell nanoparticles. Mater. Res. Bulletin 2007, 42, 1212–1220. [Google Scholar] [CrossRef]
- Sánchez-Iglesias, A.; Carbó-Argibay, E.; Glaria, A.; Rodríguez-González, B.; Pérez-Juste, J.; Pastoriza-Santos, I.; Liz-Marzán, L.M. Rapid epitaxial growth of Ag on Au nanoparticles: From Au nanorods to core-shell Au@Ag octahedrons. Chem. A Eur. J. 2010, 16, 5558–5563. [Google Scholar] [CrossRef]
- Zhang, X.; Tsuji, M.; Lim, S.; Miyamae, N.; Nishio, M.; Hikino, S.; Umezu, M. Synthesis and growth mechanism of pentagonal bipyramid-shaped gold-rich Au/Ag alloy nanoparticles. Langmuir 2007, 23, 6372–6376. [Google Scholar] [CrossRef]
- Huang, C.-C.; Yang, Z.; Chang, H.-T. Synthesis of dumbbell-shaped Au-Ag core-shell nanorods by seed-mediated growth under alkaline conditions. Langmuir 2004, 20, 6089–6092. [Google Scholar] [CrossRef]
- Nasibulin, A.G.; Pikhitsa, P.V.; Jiang, H.; Brown, D.P.; Krasheninnikov, A.V.; Anisimov, A.S.; Queipo, P.; Moisala, A.; Gonzalez, D.; Lientschnig, G.; et al. A novel hybrid carbon material. Nat. Nanotechnol. 2007, 2, 156–161. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, L.; Zhang, C.G.; Casillas, G.; Sun, Z.Z.; Yan, Z.; Ruan, G.D.; Peng, Z.W.; Raji, A.R.O.; Kittrell, C. A seamless three-dimensional carbon nanotube graphene hybrid material. Nat. Commun. 2012, 3. [Google Scholar] [CrossRef]
- Shahabi, A.; Ghassemi, M.; Mirnouri Langroudi, S.M.; Rezaei Nejad, H.; Hamedi, M.H. Effect of defect and C60s density variation on tensile and compressive properties of peapod. Comput. Mater. Sci. 2010, 50, 586–594. [Google Scholar] [CrossRef]
- Tauster, S.J.; Fung, S.C.; Baker, R.T.K.; Horsley, J.A. Strong interactions in supported-metal catalysts. Science 1981, 211, 1121–1125. [Google Scholar]
- Akalework, N.G.; Pan, C.-J.; Su, W.-N.; Rick, J.; Tsai, M.-C.; Lee, J.-F.; Lin, J.-M.; Tsai, L.-D.; Hwang, B.-J. Ultrathin TiO2-coated MWCNTs with excellent conductivity and SMSI nature as Pt catalyst support for oxygen reduction reaction in PEMFCs. J. Mater. Chem. 2012, 22, 20977–20985. [Google Scholar] [CrossRef]
- Valodkar, M.; Modi, S.; Pal, A.; Thakore, S. Synthesis and anti-bacterial activity of Cu, Ag and Cu-Ag alloy nanoparticles: A green approach. Mater. Res. Bulletin 2011, 46, 384–389. [Google Scholar] [CrossRef]
- Nel, A.E.; Maedler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Verma, A.; Stellacci, F. Effect of surface properties on nanoparticle-cell interactions. Small 2010, 6, 12–21. [Google Scholar] [CrossRef]
- Smith, B.W.; Monthioux, M.; Luzzi, D.E. Encapsulated C-60 in carbon nanotubes. Nature 1998, 396, 323–324. [Google Scholar]
- Rahman, G.M.A.; Guldi, D.M.; Zambon, E.; Pasquato, L.; Tagmatarchis, N.; Prato, M. Dispersable carbon nanotube/gold nanohybrids: Evidence for strong electronic interactions. Small 2005, 1, 527–530. [Google Scholar] [CrossRef]
- Tsoufis, T.; Tomou, A.; Gournis, D.; Douvalis, A.P.; Panagiotopoulos, I.; Kooi, B.; Georgakilas, V.; Arfaoui, I.; Bakas, T. Novel nanohybrids derived from the attachment of fept nanoparticles on carbon nanotubes. J. Nanosci. Nanotechnol. 2008, 8, 5942–5951. [Google Scholar] [CrossRef]
- Fu, D.Y.; Han, G.Y.; Chang, Y.Z.; Dong, J.H. The synthesis and properties of ZnO-graphene nano hybrid for photodegradation of organic pollutant in water. Mater. Chem. Phys. 2012, 132, 673–681. [Google Scholar] [CrossRef]
- Zhao, X.J.; Mai, Z.B.; Kang, X.H.; Dai, Z.; Zou, X.Y. Clay-chitosan-gold nanoparticle nanohybrid: Preparation and application for assembly and direct electrochemistry of myoglobin. Electrochim. Acta 2008, 53, 4732–4739. [Google Scholar] [CrossRef]
- Kumar, A.; Chaudhary, V. Time resolved emission studies of Ag-adenine-templated Cds (Ag/Cds) nanohybrids. Nanotechnology 2009, 20. [Google Scholar] [CrossRef]
- Huang, J.; Sun, Y.; Huang, S.; Yu, K.; Zhao, Q.; Peng, F.; Yu, H.; Wang, H.; Yang, J. Crystal engineering and SERS properties of Ag-Fe3O4 nanohybrids: From heterodimer to core-shell nanostructures. J. Mater. Chem. 2011, 21, 17930–17937. [Google Scholar] [CrossRef]
- Elim, H.I.; Cai, B.; Kurata, Y.; Sugihara, O.; Kaino, T.; Adschiri, T.; Chu, A.-L.; Kambe, N. Refractive index control and rayleigh scattering properties of transparent TiO2 nanohybrid polymer. J. Phys. Chem. B 2009, 113, 10143–10148. [Google Scholar]
- Ohno, T.; Tagawa, S.; Itoh, H.; Suzuki, H.; Matsuda, T. Size effect of TiO-SiO2 nano-hybrid particle. Mater. Chem. Phys. 2009, 113, 119–123. [Google Scholar] [CrossRef]
- Stassinopoulos, A.; Das, R.N.; Anastasiadis, S.H.; Giannelis, E.P.; Anglos, D. Random lasing action from ZnO-silica nanohybrids. J. Opt. 2010, 12. [Google Scholar] [CrossRef]
- Ghosh, S.; Goudar, V.S.; Padmalekha, K.G.; Bhat, S.V.; Indi, S.S.; Vasan, H.N. ZnO/Ag nanohybrid: Synthesis, characterization, synergistic antibacterial activity and its mechanism. RSC Adv. 2012, 2, 930–940. [Google Scholar] [CrossRef]
- Acierno, D.; Filippone, G.; Romeo, G.; Russo, P. Dynamics of stress bearing particle networks in poly(propylene)/alumina nanohybrids. Macromol. Mater. Eng. 2007, 292, 347–353. [Google Scholar] [CrossRef]
- Chen, C.; Gunawan, P.; Xu, R. Self-assembled Fe3O4-layered double hydroxide colloidal nanohybrids with excellent performance for treatment of organic dyes in water. J. Mater. Chem. 2011, 21, 1218–1225. [Google Scholar] [CrossRef]
- Cui, Y.; Ren, B.; Yao, J.-L.; Gu, R.-A.; Tian, Z.-Q. Synthesis of agcoreaushell bimetallic nanoparticles for immunoassay based on surface-enhanced raman spectroscopy. J. Phys. Chem. B 2006, 110, 4002–4006. [Google Scholar]
- Li, Y.; Kaneko, T.; Hatakeyama, R. Photoresponse of Fullerene and Azafullerene Peapod Field Effect Transistors. In Proceedings of the 9th IEEE Conference on Nanotechnology (IEEE-NANO 2009), Genoa, Italy, 26–30 July 2009; pp. 86–89.
- Li, Y.F.; Kaneko, T.; Hatakeyama, R. Electrical transport properties of fullerene peapods interacting with light. Nanotechnology 2008, 19. [Google Scholar] [CrossRef]
- Bol, A.A.; Chandra, B.; Kasry, A.; Maarouf, A.; Martyna, G.J.; Tulevski, G.S. Carbon nanotube-graphene hybrid transparent conductor and field effect transistor. U.S. Patent 20130130037 A1, 23 May 2013. [Google Scholar]
- Song, W.; Kwon, S.Y.; Myung, S.; Jung, M.W.; Kim, S.J.; Min, B.K.; Kang, M.-A.; Kim, S.H.; Lim, J.; An, K.-S. High-mobility ambipolar ZnO-graphene hybrid thin film transistors. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef]
- Chen, J.; Mao, S.; Lu, G. Graphene-based field-effect transistor biosensors. U.S. Patent 20120214172 A1, 23 August 2012. [Google Scholar]
- Ha, T.-J.; Akinwande, D.; Dodabalapur, A. Hybrid graphene/organic semiconductor field-effect transistors. Appl. Phys. Lett. 2012, 101. [Google Scholar] [CrossRef]
- Huang, J.; Hines, D.R.; Jung, B.J.; Bronsgeest, M.S.; Tunnell, A.; Ballarotto, V.; Katz, H.E.; Fuhrer, M.S.; Williams, E.D.; Cumings, J. Polymeric semiconductor/graphene hybrid field-effect transistors. Organ. Electron. 2011, 12, 1471–1476. [Google Scholar] [CrossRef]
- Takeomi, M.; Vipul, S.; Shinya, O.; Shuichi, N.; Wataru, T.; Shuzi, H.; Keiichi, K. Ambipolar transport in bilayer organic field-effect transistor based on poly(3-hexylthiophene) and fullerene derivatives. Jpn. J. Appl. Phys. 2010, 49. [Google Scholar] [CrossRef]
- Wu, C.; Huang, X.Y.; Wu, X.F.; Xie, L.Y.; Yang, K.; Jiang, P.K. Graphene oxide-encapsulated carbon nanotube hybrids for high dielectric performance nanocomposites with enhanced energy storage density. Nanoscale 2013, 5, 3847–3855. [Google Scholar] [CrossRef]
- Raymundo-Pinero, E.; Khomenko, V.; Frackowiak, E.; Beguin, F. Performance of manganese oxide/cnts composites as electrode materials for electrochemical capacitors. J. Electrochem. Soc. 2005, 152, A229–A235. [Google Scholar]
- Chen, P.; Chen, H.; Qiu, J.; Zhou, C. Inkjet printing of single-walled carbon nanotube/RuO2 nanowire supercapacitors on cloth fabrics and flexible substrates. Nano Res. 2010, 3, 594–603. [Google Scholar] [CrossRef]
- Chen, J.H.; Mao, S.; Wen, Z.H. One-Pot Fabrication of Crumpled Graphene-Based Nanohybrids for Supercapacitors; UWM Research Foundation: Milwaukee, WI, USA, 2013. [Google Scholar]
- Kawasaki, S.; Iwai, Y.; Hirose, M. Electrochemical lithium ion storage property of C60 encapsulated single-walled carbon nanotubes. Mater. Res. Bulletin 2009, 44, 415–417. [Google Scholar] [CrossRef]
- Zhou, M.; Wu, F.; Wang, Y. Graphene derivative-carbon nanotube composite material and preparation methods thereof. U.S. Patent 20130252499 A1, 26 September 2013. [Google Scholar]
- Chen, S.; Yeoh, W.; Liu, Q.; Wang, G. Chemical-free synthesis of graphene-carbon nanotube hybrid materials for reversible lithium storage in lithium-ion batteries. Carbon 2012, 50, 4557–4565. [Google Scholar] [CrossRef]
- Chen, S.Q.; Chen, P.; Wang, Y. Carbon nanotubes grown in situ on graphene nanosheets as superior anodes for li-ion batteries. Nanoscale 2011, 3, 4323–4329. [Google Scholar] [CrossRef]
- Han, F.D.; Yao, B.; Bai, Y.J. Preparation of carbon nano-onions and their application as anode materials for rechargeable lithium-ion batteries. J. Phys. Chem. C 2011, 115, 8923–8927. [Google Scholar] [CrossRef]
- Ding, S.; Chen, J.S.; Luan, D.; Boey, F.Y.C.; Madhavi, S.; Lou, X.W. Graphene-supported anatase TiO2 nanosheets for fast lithium storage. Chem. Commun. 2011, 47, 5780–5782. [Google Scholar] [CrossRef]
- Ahmad, M.; Shi, Y.Y.; Nisar, A.; Sun, H.Y.; Shen, W.C.; Wei, M.; Zhu, J. Synthesis of hierarchical flower-like ZnO nanostructures and their functionalization by Au nanoparticles for improved photocatalytic and high performance li-ion battery anodes. J. Mater. Chem. 2011, 21, 7723–7729. [Google Scholar] [CrossRef]
- Hong, T.-K.; Lee, D.W.; Choi, H.J.; Shin, H.S.; Kim, B.-S. Transparent, flexible conducting hybrid multilayer thin films of multiwalled carbon nanotubes with graphene nanosheets. ACS Nano 2010, 4, 3861–3868. [Google Scholar] [CrossRef]
- Tung, V.C.; Chen, L.M.; Allen, M.J.; Wassei, J.K.; Nelson, K.; Kaner, R.B.; Yang, Y. Low-temperature solution processing of graphene-carbon nanotube hybrid materials for high-performance transparent conductors. Nano Lett. 2009, 9, 1949–1955. [Google Scholar] [CrossRef]
- Tung, V.C.; Huang, J.H.; Tevis, I.; Kim, F.; Kim, J.; Chu, C.W.; Stupp, S.I.; Huang, J.X. Surfactant-free water-processable photoconductive all-carbon composite. J. Am. Chem. Soc. 2011, 133, 4940–4947. [Google Scholar]
- Kong, B.S.; Jung, D.H.; Oh, S.K.; Han, C.S.; Jung, H.T. Single-walled carbon nanotube gold nanohybrids: Application in highly effective transparent and conductive films. J. Phys. Chem. C 2007, 111, 8377–8382. [Google Scholar] [CrossRef]
- Kholmanov, I.N.; Stoller, M.D.; Edgeworth, J.; Lee, W.H.; Li, H.; Lee, J.; Barnhart, C.; Potts, J.R.; Piner, R.; Akinwande, D.; et al. Nanostructured hybrid transparent conductive films with antibacterial properties. ACS Nano 2012, 6, 5157–5163. [Google Scholar] [CrossRef]
- Liu, Y.; Chang, Q.; Huang, L. Transparent, flexible conducting graphene hybrid films with a subpercolating network of silver nanowires. J. Mater. Chem. C 2013, 1, 2970–2974. [Google Scholar]
- Qu, S.; Li, M.; Xie, L.; Huang, X.; Yang, J.; Wang, N.; Yang, S. Noncovalent functionalization of graphene attaching [6,6]-phenyl-C61-butyric acid methyl ester (PCBM) and application as electron extraction layer of polymer solar cells. ACS Nano 2013, 7, 4070–4081. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; He, D.; Wu, H.; Wang, H.; Zhou, P.; Fu, M. Influence of polymer/fullerene-graphene structure on organic polymer solar devices. Integr. Ferroelectr. 2012, 137, 1–9. [Google Scholar] [CrossRef]
- Yang, J.; Heo, M.; Lee, H.J.; Park, S.M.; Kim, J.Y.; Shin, H.S. Reduced graphene oxide (rGO)-wrapped fullerene (C-60) wires. ACS Nano 2011, 5, 8365–8371. [Google Scholar] [CrossRef]
- Yu, D.S.; Park, K.; Durstock, M.; Dai, L.M. Fullerene-grafted graphene for efficient bulk heterojunction polymer photovoltaic devices. J. Phys. Chem. Lett. 2011, 2, 1113–1118. [Google Scholar] [CrossRef]
- Wu, W.; Zhu, H.R.; Fan, L.Z.; Yang, S.H. Synthesis and characterization of a grapevine nanostructure consisting of single-walled carbon nanotubes with covalently attached 60 fullerene balls. Chem. Eur. J. 2008, 14, 5981–5987. [Google Scholar] [CrossRef]
- Son, D.I.; Kwon, B.W.; Park, D.H.; Seo, W.S.; Yi, Y.; Angadi, B.; Lee, C.L.; Choi, W.K. Emissive ZnO-graphene quantum dots for white-light-emitting diodes. Nat. Nanotechnol. 2012, 7, 465–471. [Google Scholar] [CrossRef]
- Kim, H.-I.; Moon, G.-H.; Monllor-Satoca, D.; Park, Y.; Choi, W. Solar photoconversion using graphene/TiO2 composites: Nanographene shell on TiO2 core versus TiO2 nanoparticles on graphene sheet. J. Phys. Chem. C 2012, 116, 1535–1543. [Google Scholar]
- Sun, M.; Fu, W.; Yang, H.; Sui, Y.; Zhao, B.; Yin, G.; Li, Q.; Zhao, H.; Zou, G. One-step synthesis of coaxial Ag/TiO2 nanowire arrays on transparent conducting substrates: Enhanced electron collection in dye-sensitized solar cells. Electrochem. Commun. 2011, 13, 1324–1327. [Google Scholar] [CrossRef]
- D’Souza, F.; Chitta, R.; Sandanayaka, A.S.D.; Subbaiyan, N.K.; D’Souza, L.; Araki, Y.; Ito, O. Supramolecular carbon nanotube-fullerene donor-acceptor hybrids for photoinduced electron transfer. J. Am. Chem. Soc. 2007, 129, 15865–15871. [Google Scholar]
- Deng, L.-L.; Feng, J.; Sun, L.-C.; Wang, S.; Xie, S.-L.; Xie, S.-Y.; Huang, R.-B.; Zheng, L.-S. Functionalized dihydronaphthyl-C60 derivatives as acceptors for efficient polymer solar cells with tunable photovoltaic properties. Sol. Energy Mater. Sol. Cells 2012, 104, 113–120. [Google Scholar] [CrossRef]
- Liu, Z.B.; Xu, Y.F.; Zhang, X.Y.; Zhang, X.L.; Chen, Y.S.; Tian, J.G. Porphyrin and fullerene covalently functionalized graphene hybrid materials with large nonlinear optical properties. J. Phys. Chem. B 2009, 113, 9681–9686. [Google Scholar] [CrossRef]
- Mackiewicz, N.; Bark, T.; Cao, B.; Delaire, J.A.; Riehl, D.; Ling, W.L.; Foillard, S.; Doris, E. Fullerene-functionalized carbon nanotubes as improved optical limiting devices. Carbon 2011, 49, 3998–4003. [Google Scholar] [CrossRef]
- Zhu, Y.W.; Elim, H.I.; Foo, Y.L.; Yu, T.; Liu, Y.J.; Ji, W.; Lee, J.Y.; Shen, Z.X.; Wee, A.T.S.; Thong, J.T.L.; et al. Multiwalled carbon nanotubes beaded with ZnO nanoparticles for ultrafast nonlinear optical switching. Adv. Mater. 2006, 18, 587–592. [Google Scholar] [CrossRef]
- Tom, R.T.; Nair, A.S.; Singh, N.; Aslam, M.; Nagendra, C.L.; Philip, R.; Vijayamohanan, K.; Pradeep, T. Freely dispersible Au@TiO2, Au@ZrO2, Ag@TiO2, and Ag@ZrO2 core-shell nanoparticles: One-step synthesis, characterization, spectroscopy, and optical limiting properties. Langmuir 2003, 19, 3439–3445. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, J.; Zhang, X.; Liu, Z.; Wan, X.; Tian, J.; Wang, T.; Chen, Y. Synthesis, characterization and optical limiting property of covalently oligothiophene-functionalized graphene material. Carbon 2009, 47, 3113–3121. [Google Scholar] [CrossRef]
- Shinde, V.M.; Madras, G. Low temperature co oxidation and water gas shift reaction over Pt/Pd substituted in Fe/TiO2 catalysts. Int. J. Hydrog. Energy 2012, 37, 18798–18814. [Google Scholar] [CrossRef]
- Jha, N.; Jafri, R.I.; Rajalakshmi, N.; Ramaprabhu, S. Graphene-multi walled carbon nanotube hybrid electrocatalyst support material for direct methanol fuel cell. Int. J. Hydrog. Energy 2011, 36, 7284–7290. [Google Scholar] [CrossRef]
- Rigdon, W.A.; Sightler, J.J.; Larrabee, D.; McPherson, E.; Huang, X. Titania and carbon nanotube composite catalyst supports for durable electrocatalyst performance. ECS Trans. 2013, 50, 1681–1692. [Google Scholar] [CrossRef]
- Xiong, L.; Huang, Y.X.; Liu, X.W.; Sheng, G.P.; Li, W.W.; Yu, H.Q. Three-dimensional bimetallic Pd-Cu nanodendrites with superior electrochemical performance for oxygen reduction reaction. Electrochim. Acta 2013, 89, 24–28. [Google Scholar] [CrossRef]
- Chen, M.-L.; He, Y.-J.; Chen, X.-W.; Wang, J.-H. Quantum dots conjugated with Fe3O4-filled carbon nanotubes for cancer-targeted imaging and magnetically guided drug delivery. Langmuir 2012, 28, 16469–16476. [Google Scholar] [CrossRef]
- Timothy, A.L.; James, B.; Jesse, A.; Konstantin, S. Hybrid plasmonic magnetic nanoparticles as molecular specific agents for MRI/optical imaging and photothermal therapy of cancer cells. Nanotechnology 2007, 18. [Google Scholar] [CrossRef]
- Fan, Z.; Shelton, M.; Singh, A.K.; Senapati, D.; Khan, S.A.; Ray, P.C. Multifunctional plasmonic shell-magnetic core nanoparticles for targeted diagnostics, isolation, and photothermal destruction of tumor cells. ACS Nano 2012, 6, 1065–1073. [Google Scholar] [CrossRef]
- Bolskar, R.D. Gadofullerene MRI contrast agents. Nanomedicine 2008, 3, 201–213. [Google Scholar] [CrossRef]
- Laus, S.; Sitharaman, B.; Tóth, É.; Bolskar, R.D.; Helm, L.; Asokan, S.; Wong, M.S.; Wilson, L.J.; Merbach, A.E. Destroying gadofullerene aggregates by salt addition in aqueous solution of Gd@C60(OH)x and Gd@C60[C(COOH2)]10. J. Am. Chem. Soc. 2005, 127, 9368–9369. [Google Scholar] [CrossRef]
- Toth, E.; Bolskar, R.D.; Borel, A.; Gonzalez, G.; Helm, L.; Merbach, A.E.; Sitharaman, B.; Wilson, L.J. Water-soluble gadofullerenes: Toward high-relaxivity, Ph-responsive MRI contrast agents. J. Am. Chem. Soc. 2005, 127, 799–805. [Google Scholar] [CrossRef]
- Simon, F.; Peterlik, H.; Pfeiffer, R.; Bernardi, J.; Kuzmany, H. Fullerene release from the inside of carbon nanotubes: A possible route toward drug delivery. Chem. Phys. Lett. 2007, 445, 288–292. [Google Scholar] [CrossRef]
- Chen, W.; Xu, N.F.; Xu, L.G.; Wang, L.B.; Li, Z.K.; Ma, W.; Zhu, Y.Y.; Xu, C.L.; Kotov, N.A. Multifunctional magnetoplasmonic nanoparticle assemblies for cancer therapy and diagnostics (theranostics). Macromol. Rapid Commun. 2010, 31, 228–236. [Google Scholar]
- Hu, H.Q.; Yu, J.H.; Li, Y.Y.; Zhao, J.; Dong, H.Q. Engineering of a novel pluronic F127/graphene nanohybrid for Ph responsive drug delivery. J. Biomed. Mater. Res. Part A 2012, 100, 141–148. [Google Scholar]
- Gibson, J.D.; Khanal, B.P.; Zubarev, E.R. Paclitaxel-functionalized gold nanoparticles. J. Am. Chem. Soc. 2007, 129, 11653–11661. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, J.; Xi, Q.; Yang, W. A high performance electrochemical sensor for acetaminophen based on single-walled carbon nanotube-graphene nanosheet hybrid films. Sens. Actuators B Chem. 2012, 161, 648–654. [Google Scholar] [CrossRef]
- Guo, S.; Wen, D.; Zhai, Y.; Dong, S.; Wang, E. Platinum nanoparticle ensemble-on-graphene hybrid nanosheet: One-pot, rapid synthesis, and used as new electrode material for electrochemical sensing. ACS Nano 2010, 4, 3959–3968. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, D.; Zhou, L.; Zhang, X.; Fan, P.; Quan, X. Intensified internal electrolysis for degradation of methylene blue as model compound induced by a novel hybrid material: Multi-walled carbon nanotubes immobilized on zero-valent iron plates (Fe0-CNTs). Chem. Eng. J. 2013, 217, 99–107. [Google Scholar] [CrossRef]
- Jabeen, H.; Chandra, V.; Jung, S.; Lee, J.W.; Kim, K.S.; Bin Kim, S. Enhanced Cr(VI) removal using iron nanoparticle decorated graphene. Nanoscale 2011, 3, 3583–3585. [Google Scholar] [CrossRef]
- Kavitha, T.; Gopalan, A.I.; Lee, K.-P.; Park, S.-Y. Glucose sensing, photocatalytic and antibacterial properties of graphene-ZnO nanoparticle hybrids. Carbon 2012, 50, 2994–3000. [Google Scholar] [CrossRef]
- Liu, J.M.; Wang, X.X.; Cui, M.L.; Lin, L.P.; Jiang, S.L.; Jiao, L.; Zhang, L.H. A promising non-aggregation colorimetric sensor of AuNRs-Ag+ for determination of dopamine. Sens. Actuators B Chem. 2013, 176, 97–102. [Google Scholar] [CrossRef]
- Han, X.A.; Zhu, Y.H.; Yang, X.L.; Li, C.Z. Electrocatalytic activity of Pt doped TiO2 nanotubes catalysts for glucose determination. J. Alloy. Compd. 2010, 500, 247–251. [Google Scholar] [CrossRef]
- Sarkar, S.; Makhal, A.; Bora, T.; Lakhsman, K.; Singha, A.; Dutta, J.; Pal, S.K. Hematoporphyrin-ZnO nanohybrids: Twin applications in efficient visible-light photocatalysis and dye-sensitized solar cells. ACS Appl. Mater. Interf. 2012, 4, 7027–7035. [Google Scholar] [CrossRef]
- Mani, V.; Devadas, B.; Chen, S.M. Direct electrochemistry of glucose oxidase at electrochemically reduced graphene oxide-multiwalled carbon nanotubes hybrid material modified electrode for glucose biosensor. Biosens. Bioelectron. 2013, 41, 309–315. [Google Scholar] [CrossRef]
- Ai, L.; Jiang, J. Removal of methylene blue from aqueous solution with self-assembled cylindrical graphene-carbon nanotube hybrid. Chem. Eng. J. 2012, 192, 156–163. [Google Scholar] [CrossRef]
- Zhao, D.; Yang, X.; Chen, C.; Wang, X. Enhanced photocatalytic degradation of methylene blue on multiwalled carbon nanotubes-TiO2. J. Colloid Interf. Sci. 2013, 398, 234–239. [Google Scholar] [CrossRef]
- Lv, T.; Pan, L.K.; Liu, X.J.; Lu, T.; Zhu, G.; Sun, Z. Enhanced photocatalytic degradation of methylene blue by ZnO-reduced graphene oxide composite synthesized via microwave-assisted reaction. J. Alloy. Compd. 2011, 509, 10086–10091. [Google Scholar] [CrossRef]
- Wang, C.; Irudayaraj, J. Multifunctional magnetic-optical nanoparticle probes for simultaneous detection, separation, and thermal ablation of multiple pathogens. Small 2010, 6, 283–289. [Google Scholar] [CrossRef]
- Zhu, S.; Du, C.; Fu, Y. Localized surface plasmon resonance-based hybrid Au-Ag nanoparticles for detection of Staphylococcus aureus enterotoxin B. Opt. Mater. 2009, 31, 1608–1613. [Google Scholar] [CrossRef] [Green Version]
- Costi, R.; Saunders, A.E.; Elmalem, E.; Salant, A.; Banin, U. Visible light-induced charge retention and photocatalysis with hybrid CdSe-Au nanodumbbells. Nano Lett. 2008, 8, 637–641. [Google Scholar] [CrossRef]
- Das, M.R.; Sarma, R.K.; Saikia, R.; Kale, V.S.; Shelke, M.V.; Sengupta, P. Synthesis of silver nanoparticles in an aqueous suspension of graphene oxide sheets and its antimicrobial activity. Colloids Surf. B Biointerf. 2011, 83, 16–22. [Google Scholar] [CrossRef]
- Seymour, M.B.; Su, C.; Gao, Y.; Lu, Y.; Li, Y. Characterization of carbon nano-onions for heavy metal ion remediation. J. Nanopart. Res. 2012, 14. [Google Scholar] [CrossRef]
- Sonkar, S.K.; Ghosh, M.; Roy, M.; Begum, A.; Sarkar, S. Carbon nano-onions as nontoxic and high-fluorescence bioimaging agent in food chain-an in vivo study from unicellular E. coli to multicellular C. elegans. Mater. Exp. 2012, 2, 105–114. [Google Scholar]
- Preethichandra, D.M.G.; Ekanayake, E.M.I.M. Nano-biosensor development for biomedical and environmental measurements. In New Developments and Applications in Sensing Technology; Mukhopadhyay, S., Lay-Ekuakille, A., Fuchs, A., Eds.; Springer: Berlin, Heidelberg, Germary, 2011; Volume 83, pp. 279–292. [Google Scholar]
- Lv, R.; Cui, T.; Jun, M.-S.; Zhang, Q.; Cao, A.; Su, D.S.; Zhang, Z.; Yoon, S.-H.; Miyawaki, J.; Mochida, I.; et al. Open-ended, n-doped carbon nanotube-graphene hybrid nanostructures as high-performance catalyst support. Adv. Funct. Mater. 2011, 21, 999–1006. [Google Scholar]
- Karousis, N.; Tsotsou, G.-E.; Evangelista, F.; Rudolf, P.; Ragoussis, N.; Tagmatarchis, N. Carbon nanotubes decorated with palladium nanoparticles: Synthesis, characterization, and catalytic activity. J. Phys. Chem. C 2008, 112, 13463–13469. [Google Scholar]
- Li, Y.; Fan, X.; Qi, J.; Ji, J.; Wang, S.; Zhang, G.; Zhang, F. Gold nanoparticles-graphene hybrids as active catalysts for suzuki reaction. Mater. Res. Bulletin 2010, 45, 1413–1418. [Google Scholar] [CrossRef]
- Han, J.; Zhou, Z.; Yin, Y.; Luo, X.; Li, J.; Zhang, H.; Yang, B. One-pot, seedless synthesis of flowerlike Au-Pd bimetallic nanoparticles with core-shell-like structure via sodium citrate coreduction of metal ions. CrystEngComm 2012, 14, 7036–7042. [Google Scholar] [CrossRef]
- Li, W.K.; Dichiara, A.; Bai, J.B. Carbon nanotube-graphene nanoplatelet hybrids as high-performance multifunctional reinforcements in epoxy composites. Compos. Sci. Technol. 2013, 74, 221–227. [Google Scholar] [CrossRef]
- Hwang, S.H.; Park, H.W.; Park, Y.B. Piezoresistive behavior and multi-directional strain sensing ability of carbon nanotube-graphene nanoplatelet hybrid sheets. Smart Mater. Struct. 2013, 22. [Google Scholar] [CrossRef]
- Joly-Pottuz, L.; Vacher, B.; Ohmae, N.; Martin, J.M.; Epicier, T. Anti-wear and friction reducing mechanisms of carbon nano-onions as lubricant additives. Tribol. Lett. 2008, 30, 69–80. [Google Scholar] [CrossRef]
- Li, Y.; Kaneko, T.; Hatakeyama, R. C59N peapods sensing the temperature. Sensors 2013, 13, 966–974. [Google Scholar] [CrossRef]
- Aravind, S.S.J.; Ramaprabhu, S. Graphene wrapped multiwalled carbon nanotubes dispersed nanofluids for heat transfer applications. J. Appl. Phys. 2012, 112. [Google Scholar] [CrossRef]
- Vizuete, M.; Barrejon, M.; Gomez-Escalonilla, M.J.; Langa, F. Endohedral and exohedral hybrids involving fullerenes and carbon nanotubes. Nanoscale 2012, 4, 4370–4381. [Google Scholar] [CrossRef]
- Griffitt, R.J.; Luo, J.; Gao, J.; Bonzongo, J.C.; Barber, D.S. Effects of particle composition and species on toxicity of metallic nanomaterials in aquatic organisms. Environ. Toxicol. Chem. 2008, 27, 1972–1978. [Google Scholar] [CrossRef]
- Baek, Y.-W.; An, Y.-J. Microbial toxicity of metal oxide nanoparticles (CuO, NiO, ZnO, and Sb2O3) to Escherichia coli, Bacillus subtilis, and Streptococcus aureus. Sci. Total Environ. 2011, 409, 1603–1608. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef]
- Sergio, M.; Behzadi, H.; Otto, A.; Spoel, D. Fullerenes toxicity and electronic properties. Environ. Chem. Lett. 2013, 11, 105–118. [Google Scholar] [CrossRef]
- Zhang, H.; Ji, Z.; Xia, T.; Meng, H.; Low-Kam, C.; Liu, R.; Pokhrel, S.; Lin, S.; Wang, X.; Liao, Y.-P.; et al. Use of metal oxide nanoparticle band gap to develop a predictive paradigm for oxidative stress and acute pulmonary inflammation. ACS Nano 2012, 6, 4349–4368. [Google Scholar]
- Burello, E.; Worth, A.P. A theoretical framework for predicting the oxidative stress potential of oxide nanoparticles. Nanotoxicology 2011, 5, 228–235. [Google Scholar] [CrossRef]
- Singh, L.T.; Sugavaneshwar, R.P.; Nanda, K.K. Carbon nanotube-ZnO nanowire hybrid architectures as multifunctional devices. AIP Adv. 2013, 3. [Google Scholar] [CrossRef]
- Hwang, J.O.; Lee, D.H.; Kim, J.Y.; Han, T.H.; Kim, B.H.; Park, M.; No, K.; Kim, S.O. Vertical ZnO nanowires/graphene hybrids for transparent and flexible field emission. J. Mater. Chem. 2011, 21, 3432–3437. [Google Scholar]
- Wang, Y.J.; Liu, J.C.; Liu, L.; Sun, D.D. Enhancing stability and photocatalytic activity of ZnO nanoparticles by surface modification of graphene oxide. J. Nanosci. Nanotechnol. 2012, 12, 3896–3902. [Google Scholar] [CrossRef]
- Chambers, B.A.; Afrooz, A.R.M.N.; Bae, S.; Aich, N.; Katz, L.; Saleh, N.B.; Kirisits, M.J. Effects of chloride and ionic strength on physical morphology, dissolution, and bacterial toxicity of silver nanoparticles. Environ. Sci. Technol. 2013, 48, 761–769. [Google Scholar]
- Shoults-Wilson, W.A.; Reinsch, B.C.; Tsyusko, O.V.; Bertsch, P.M.; Lowry, G.V.; Unrine, J.M. Role of particle size and soil type in toxicity of silver nanoparticles to earthworms. Soil Sci. Soc. Am. J. 2011, 75, 365–377. [Google Scholar] [CrossRef]
- Yu, L.-P.; Fang, T.; Xiong, D.-W.; Zhu, W.-T.; Sima, X.-F. Comparative toxicity of nano-ZnO and bulk ZnO suspensions to zebrafish and the effects of sedimentation, OH production and particle dissolution in distilled water. J. Environ. Monit. 2011, 13, 1975–1982. [Google Scholar] [CrossRef]
- Meng, J.; Ji, Y.L.; Liu, J.; Cheng, X.L.; Guo, H.; Zhang, W.Q.; Wu, X.C.; Xu, H.Y. Using gold nanorods core/silver shell nanostructures as model material to probe biodistribution and toxic effects of silver nanoparticles in mice. Nanotoxicology 2014, 8, 686–696. [Google Scholar] [CrossRef]
- Cai, X.; Lin, M.S.; Tan, S.Z.; Mai, W.J.; Zhang, Y.M.; Liang, Z.W.; Lin, Z.D.; Zhang, X.J. The use of polyethyleneimine-modified reduced graphene oxide as a substrate for silver nanoparticles to produce a material with lower cytotoxicity and long-term antibacterial activity. Carbon 2012, 50, 3407–3415. [Google Scholar] [CrossRef]
- Kim, S.T.; Saha, K.; Kim, C.; Rotello, V.M. The role of surface functionality in determining nanoparticle cytotoxicity. Acc. Chem. Res. 2013, 46, 681–691. [Google Scholar] [CrossRef]
- Kirschling, T.L.; Golas, P.L.; Unrine, J.M.; Matyjaszewski, K.; Gregory, K.B.; Lowry, G.V.; Tilton, R.D. Microbial bioavailability of covalently bound polymer coatings on model engineered nanomaterials. Environ. Sci. Technol. 2011, 45, 5253–5259. [Google Scholar] [CrossRef]
- Banerjee, I.; Mondal, D.; Martin, J.; Kane, R.S. Photoactivated antimicrobial activity of carbon nanotube-porphyrin conjugates. Langmuir 2010, 26, 17369–17374. [Google Scholar] [CrossRef]
- Jia, G.; Wang, H.F.; Yan, L.; Wang, X.; Pei, R.J.; Yan, T.; Zhao, Y.L.; Guo, X.B. Cytotoxicity of carbon nanomaterials: Single-wall nanotube, multi-wall nanotube, and fulleren. Environ. Sci. Technol. 2005, 39, 1378–1383. [Google Scholar] [CrossRef]
- Chen, L.Q.; Hu, P.P.; Zhang, L.; Huang, S.Z.; Luo, L.F.; Huang, C.Z. Toxicity of graphene oxide and multi-walled carbon nanotubes against human cells and zebrafish. Sci. China Chem. 2012, 55, 2209–2216. [Google Scholar] [CrossRef]
- Schaeublin, N.M.; Braydich-Stolle, L.K.; Maurer, E.I.; Park, K.; MacCuspie, R.I.; Afrooz, A.R.M.N.; Vaia, R.A.; Saleh, N.B.; Hussain, S.M. Does shape matter? Bioeffects of gold nanomaterials in a human skin cell model. Langmuir 2012, 28, 3248–3258. [Google Scholar] [CrossRef]
- Smith, B.W.; Luzzi, D.E. Formation mechanism of fullerene peapods and coaxial tubes: A path to large scale synthesis. Chem. Phys. Lett. 2000, 321, 169–174. [Google Scholar] [CrossRef]
- Wu, X.; Zeng, X.C. Periodic graphene nanobuds. Nano Lett. 2009, 9, 250–256. [Google Scholar] [CrossRef]
- Poland, C.A.; Duffin, R.; Kinloch, I.; Maynard, A.; Wallace, W.A.H.; Seaton, A.; Stone, V.; Brown, S.; MacNee, W.; Donaldson, K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat. Nanotechnol. 2008, 3, 423–428. [Google Scholar] [CrossRef]
- Lyon, D.Y.; Alvarez, P.J.J. Fullerene water suspension (nC60) exerts antibacterial effects via ROS-independent protein oxidation. Environ. Sci. Technol. 2008, 42, 8127–8132. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, H.; von dem Bussche, A.; Creighton, M.; Hurt, R.H.; Kane, A.B.; Gao, H. Graphene microsheets enter cells through spontaneous membrane penetration at edge asperities and corner sites. Proc. Natl. Acad. Sci. USA 2013, 110, 12295–12300. [Google Scholar]
- Salvetat, J.-P.; Bonard, J.-M.; Thomson, N.; Kulik, A.; Forro, L.; Benoit, W.; Zuppiroli, L. Mechanical properties of carbon nanotubes. Appl. Phys. A 1999, 69, 255–260. [Google Scholar]
- Zhu, J.; Pan, Z.Y.; Wang, Y.X.; Zhou, L.; Jiang, Q. The effects of encapsulating C60 fullerenes on the bending flexibility of carbon nanotubes. Nanotechnology 2007, 18. [Google Scholar] [CrossRef]
- Zheng, Y.P.; Xu, L.Q.; Fan, Z.Y.; Wei, N.; Lu, Y.; Huang, Z.G. Mechanical properties of graphene nanobuds: A molecular dynamics study. Curr. Nanosci. 2012, 8, 89–96. [Google Scholar]
- Gao, R.; Hu, N.; Yang, Z.; Zhu, Q.; Chai, J.; Su, Y.; Zhang, L.; Zhang, Y. Paper-like graphene-Ag composite films with enhanced mechanical and electrical properties. Nanoscale Res. Lett. 2013, 8, 1–8. [Google Scholar]
- Bu, Y.; Chen, Z.; Li, W. Dramatically enhanced photocatalytic properties of Ag-modified graphene-ZnO quasi-shell-core heterojunction composite material. RSC Adv. 2013, 3, 24118–24125. [Google Scholar]
- Tseng, W.J.; Cheng, C.C.; Hsieh, J.H. Rattle-structured Ag/TiO2 nanocomposite capsules with bactericide and photocatalysis activities. J. Am. Ceram. Soc. 2014, 97, 407–412. [Google Scholar] [CrossRef]
- Li, M.; Noriega-Trevino, M.E.; Nino-Martinez, N.; Marambio-Jones, C.; Wang, J.; Damoiseaux, R.; Ruiz, F.; Hoek, E.M.V. Synergistic bactericidal activity of Ag-TiO2 nanoparticles in both light and dark conditions. Environ. Sci. Technol. 2011, 45, 8989–8995. [Google Scholar] [CrossRef]
- Maurer-Jones, M.A.; Gunsolus, I.L.; Murphy, C.J.; Haynes, C.L. Toxicity of engineered nanoparticles in the environment. Anal. Chem. 2013, 85, 3036–3049. [Google Scholar]
- Hall, S.; Bradley, T.; Moore, J.T.; Kuykindall, T.; Minella, L. Acute and chronic toxicity of nano-scale TiO2 particles to freshwater fish, cladocerans, and green algae, and effects of organic and inorganic substrate on TiO2 toxicity. Nanotoxicology 2009, 3, 91–97. [Google Scholar] [CrossRef]
- Jo, H.J.; Choi, J.W.; Lee, S.H.; Hong, S.W. Acute toxicity of Ag and CuO nanoparticle suspensions against Daphnia magna: The importance of their dissolved fraction varying with preparation methods. J. Hazard. Mater. 2012, 227, 301–308. [Google Scholar]
- Griffitt, R.J.; Weil, R.; Hyndman, K.A.; Denslow, N.D.; Powers, K.; Taylor, D.; Barber, D.S. Exposure to copper nanoparticles causes gill injury and acute lethality in zebrafish (Danio rerio). Environ. Sci. Technol. 2007, 41, 8178–8186. [Google Scholar] [CrossRef]
- Bouldin, J.L.; Ingle, T.M.; Sengupta, A.; Alexander, R.; Hannigan, R.E.; Buchanan, R.A. Aqueous toxicity and food chain transfer of quantum dotsTM in freshwater algae and Ceriodaphnia dubia. Environ. Toxicol. Chem. 2008, 27, 1958–1963. [Google Scholar] [CrossRef]
- Strigul, N.; Vaccari, L.; Galdun, C.; Wazne, M.; Liu, X.; Christodoulatos, C.; Jasinkiewicz, K. Acute toxicity of boron, titanium dioxide, and aluminum nanoparticles to Daphnia magna and Vibrio fischeri. Desalination 2009, 248, 771–782. [Google Scholar] [CrossRef]
- Gong, N.; Shao, K.; Feng, W.; Lin, Z.; Liang, C.; Sun, Y. Biotoxicity of nickel oxide nanoparticles and bio-remediation by microalgae Chlorella vulgaris. Chemosphere 2011, 83, 510–516. [Google Scholar] [CrossRef]
- Gaiser, B.K.; Biswas, A.; Rosenkranz, P.; Jepson, M.A.; Lead, J.R.; Stone, V.; Tyler, C.R.; Fernandes, T.F. Effects of silver and cerium dioxide micro- and nano-sized particles on Daphnia magna. J. Environm. Monit. 2011, 13, 1227–1235. [Google Scholar] [CrossRef]
- Zhu, X.; Tian, S.; Cai, Z. Toxicity assessment of iron oxide nanoparticles in zebrafish (Danio rerio) early life stages. PLoS One 2012, 7. [Google Scholar] [CrossRef]
- Pablo Garcia-Cambero, J.; Nunez Garcia, M.; Diaz Lopez, G.; Lopez Herranz, A.; Cuevas, L.; Perez-Pastrana, E.; Sendra Cuadal, J.; Ramis Castelltort, M.; Castano Calvo, A. Converging hazard assessment of gold nanoparticles to aquatic organisms. Chemosphere 2013, 93, 1194–1200. [Google Scholar]
- Petersen, E.J.; Zhang, L.; Mattison, N.T.; O’Carroll, D.M.; Whelton, A.J.; Uddin, N.; Nguyen, T.; Huang, Q.; Henry, T.B.; Holbrook, R.D.; et al. Potential release pathways, environmental fate, and ecological risks of carbon nanotubes. Environ. Sci. Technol. 2011, 45, 9837–9856. [Google Scholar] [CrossRef]
- Pretti, C.; Oliva, M.; Pietro, R.D.; Monni, G.; Cevasco, G.; Chiellini, F.; Pomelli, C.; Chiappe, C. Ecotoxicity of pristine graphene to marine organisms. Ecotoxicol. Environ. Saf. 2014, 101, 138–145. [Google Scholar] [CrossRef]
- Santos, S.M.; Dinis, A.M.; Rodrigues, D.M.; Peixoto, F.; Videira, R.A.; Jurado, A.S. Studies on the toxicity of an aqueous suspension of C60 nanoparticles using a bacterium (gen. Bacillus) and an aquatic plant (Lemna gibba) as in vitro model systems. Aquat. Toxicol. 2013, 142–143, 347–354. [Google Scholar] [CrossRef]
- Handy, R.D.; Cornelis, G.; Fernandes, T.; Tsyusko, O.; Decho, A.; Sabo-Attwood, T.; Metcalfe, C.; Steevens, J.A.; Klaine, S.J.; Koelmans, A.A.; et al. Ecotoxicity test methods for engineered nanomaterials: Practical experiences and recommendations from the bench. Environ. Toxicol. Chem. 2012, 31, 15–31. [Google Scholar] [CrossRef]
- Massarsky, A.; Dupuis, L.; Taylor, J.; Eisa-Beygi, S.; Strek, L.; Trudeau, V.L.; Moon, T.W. Assessment of nanosilver toxicity during zebrafish (Danio rerio) development. Chemosphere 2013, 92, 59–66. [Google Scholar] [CrossRef]
- Buffet, P.-E.; Tankoua, O.F.; Pan, J.-F.; Berhanu, D.; Herrenknecht, C.; Poirier, L.; Amiard-Triquet, C.; Amiard, J.-C.; Berard, J.-B.; Risso, C.; et al. Behavioural and biochemical responses of two marine invertebrates scrobicularia plana and hediste diversicolor to copper oxide nanoparticles. Chemosphere 2011, 84, 166–174. [Google Scholar] [CrossRef]
- Glenn, J.B.; Klaine, S.J. Abiotic and biotic factors that influence the bioavailability of gold nanoparticles to aquatic macrophytes. Environ. Sci. Technol. 2013, 47, 10223–10230. [Google Scholar]
- Lee, S.-W.; Kim, S.-M.; Choi, J. Genotoxicity and ecotoxicity assays using the freshwater crustacean Daphnia magna and the larva of the aquatic midge Chironomus riparius to screen the ecological risks of nanoparticle exposure. Environ. Toxicol. Pharmacol. 2009, 28, 86–91. [Google Scholar] [CrossRef]
- Gagne, F.; Turcotte, P.; Auclair, J.; Gagnon, C. The effects of zinc oxide nanoparticles on the metallome in freshwater mussels. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2013, 158, 22–28. [Google Scholar] [CrossRef]
- Bernot, R.J.; Brandenburg, M. Freshwater snail vital rates affected by non-lethal concentrations of silver nanoparticles. Hydrobiologia 2013, 714, 25–34. [Google Scholar] [CrossRef]
- Battin, T.J.; Kammer, F.V.D.; Weilhartner, A.; Ottofuelling, S.; Hofmann, T. Nanostructured TiO2: Transport behavior and effects on aquatic microbial communities under environmental conditions. Environ. Sci. Technol. 2009, 43, 8098–8104. [Google Scholar] [CrossRef]
- Dasari, T.P.; Hwang, H.-M. Effect of humic acids and sunlight on the cytotoxicity of engineered zinc oxide and titanium dioxide nanoparticles to a river bacterial assemblage. J. Environ. Sci. China 2013, 25, 1925–1935. [Google Scholar] [CrossRef]
- Pakrashi, S.; Dalai, S.; Humayun, A.; Chakravarty, S.; Chandrasekaran, N.; Mukherjee, A. Ceriodaphnia dubia as a potential bio-indicator for assessing acute aluminum oxide nanoparticle toxicity in fresh water environment. PLoS One 2013, 8, e74003. [Google Scholar]
- Diniz, M.S.; Alves de Matos, A.P.; Lourenco, J.; Castro, L.; Peres, I.; Mendonca, E.; Picado, A. Liver alterations in two freshwater fish species (Carassius auratus and Danio rerio) following exposure to different TiO2 nanoparticle concentrations. Microsc. Microanal. 2013, 19, 1131–1140. [Google Scholar]
- Federici, G.; Shaw, B.J.; Handy, R.D. Toxicity of titanium dioxide nanoparticles to rainbow trout (Oncorhynchus mykiss): Gill injury, oxidative stress, and other physiological effects. Aquat. Toxicol. 2007, 84, 415–430. [Google Scholar] [CrossRef]
- Hao, L.; Chen, L. Oxidative stress responses in different organs of carp (Cyprinus carpio) with exposure to ZnO nanoparticles. Ecotoxicol. Environ. Saf. 2012, 80, 103–110. [Google Scholar] [CrossRef]
- Govindasamy, R.; Rahuman, A.A. Histopathological studies and oxidative stress of synthesized silver nanoparticles in mozambique tilapia (Oreochromis mossambicus). J. Environ. Sci. China 2012, 24, 1091–1098. [Google Scholar] [CrossRef]
- Fan, W.; Shi, Z.; Yang, X.; Cui, M.; Wang, X.; Zhang, D.; Liu, H.; Guo, L. Bioaccumulation and biomarker responses of cubic and octahedral Cu2O micro/nanocrystals in Daphnia magna. Water Res. 2012, 46, 5981–5988. [Google Scholar] [CrossRef]
- Buffet, P.-E.; Richard, M.; Caupos, F.; Vergnoux, A.; Perrein-Ettajani, H.; Luna-Acosta, A.; Akcha, F.; Amiard, J.-C.; Amiard-Triquet, C.; Guibbolini, M.; et al. A mesocosm study of fate and effects of CuO nanoparticles on endobenthic species (Scrobicularia plana, Hediste diversicolor). Environ. Sci. Technol. 2013, 47, 1620–1628. [Google Scholar]
- Chen, D.; Zhang, D.; Yu, J.C.; Chan, K.M. Effects of Cu2O nanoparticle and CuCl2 on zebrafish larvae and a liver cell-line. Aquatic Toxicol. 2011, 105, 344–354. [Google Scholar] [CrossRef]
- Fan, W.; Wang, X.; Cui, M.; Zhang, D.; Zhang, Y.; Yu, T.; Guo, L. Differential oxidative stress of octahedral and cubic Cu2O micro/nanocrystals to Daphnia magna. Environ. Sci. Technol. 2012, 46, 10255–10262. [Google Scholar]
- Farkas, J.; Christian, P.; Urrea, J.A.G.; Roos, N.; Hassellov, M.; Tollefsen, K.E.; Thomas, K.V. Effects of silver and gold nanoparticles on rainbow trout (Oncorhynchus mykiss) hepatocytes. Aquat. Toxicol. 2010, 96, 44–52. [Google Scholar] [CrossRef]
- Fan, W.H.; Cui, M.M.; Shi, Z.W.; Tan, C.; Yang, X.P. Enhanced oxidative stress and physiological damage in Daphnia magna by copper in the presence of nano-TiO2. J. Nanomater. 2012, 2012. [Google Scholar] [CrossRef]
- Wang, D.; Hu, J.; Forthaus, B.E.; Wang, J. Synergistic toxic effect of nano-Al2O3 and As(V) on Ceriodaphnia dubia. Environ. Pollut. 2011, 159, 3003–3008. [Google Scholar] [CrossRef]
- Griffitt, R.J.; Hyndman, K.; Denslow, N.D.; Barber, D.S. Comparison of molecular and histological changes in zebrafish gills exposed to metallic nanoparticles. Toxicol. Sci. 2009, 107, 404–415. [Google Scholar]
- Dash, A.; Singh, A.P.; Chaudhary, B.R.; Singh, S.K.; Dash, D. Effect of silver nanoparticles on growth of eukaryotic green algae. Nano Micro Lett. 2012, 4, 158–165. [Google Scholar]
- Hu, C.; Liu, X.; Li, X.; Zhao, Y. Evaluation of growth and biochemical indicators of Salvinia natans exposed to zinc oxide nanoparticles and zinc accumulation in plants. Environ. Sci. Pollut. Res. 2014, 21, 732–739. [Google Scholar] [CrossRef]
- Perreault, F.; Samadani, M.; Dewez, D. Effect of soluble copper released from copper oxide nanoparticles solubilisation on growth and photosynthetic processes of Lemna gibba L. Nanotoxicology 2014, 8, 374–382. [Google Scholar] [CrossRef]
- Marambio-Jones, C.; Hoek, E.M.V. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopart. Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Neale, P.A.; Jamting, A.K.; Escher, B.I.; Herrmann, J. A review of the detection, fate and effects of engineered nanomaterials in wastewater treatment plants. Water Sci. Technol. 2013, 68, 1440–1453. [Google Scholar] [CrossRef]
- Von Moos, N.; Slaveykova, V.I. Oxidative stress induced by inorganic nanoparticles in bacteria and aquatic microalgae—State of the art and knowledge gaps. Nanotoxicology 2014, 8, 605–630. [Google Scholar] [CrossRef]
- Bai, W.; Zhang, Z.; Tian, W.; He, X.; Ma, Y.; Zhao, Y.; Chai, Z. Toxicity of zinc oxide nanoparticles to zebrafish embryo: A physicochemical study of toxicity mechanism. J. Nanopart. Res. 2010, 12, 1645–1654. [Google Scholar] [CrossRef]
- Kennedy, A.J.; Hull, M.S.; Bednar, A.J.; Goss, J.D.; Gunter, J.C.; Bouldin, J.L.; Vikesland, P.J.; Steevens, J.A. Fractionating nanosilver: Importance for determining toxicity to aquatic test organisms. Environ. Sci. Technol. 2010, 44, 9571–9577. [Google Scholar]
- Wang, D.; Hu, J.; Irons, D.R.; Wang, J. Synergistic toxic effect of nano-TiO2 and As(V) on Ceriodaphnia dubia. Sci. Total Environ. 2011, 409, 1351–1356. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Von der Kammer, F.; Hofmann, T.; Baalousha, M.; Ottofuelling, S.; Baun, A. Algal testing of titanium dioxide nanoparticles-testing considerations, inhibitory effects and modification of cadmium bioavailability. Toxicology 2010, 269, 190–197. [Google Scholar] [CrossRef]
- Campos, B.; Rivetti, C.; Rosenkranz, P.; Maria Navas, J.; Barata, C. Effects of nanoparticles of TiO2 on food depletion and life-history responses of Daphnia magna. Aquat. Toxicol. 2013, 130, 174–183. [Google Scholar]
- Fouqueray, M.; Dufils, B.; Vollat, B.; Chaurand, P.; Botta, C.; Abacci, K.; Labille, J.; Rose, J.; Garric, J. Effects of aged TiO2 nanomaterial from sunscreen on Daphnia magna exposed by dietary route. Environ. Pollut. 2012, 163, 55–61. [Google Scholar] [CrossRef]
- Croteau, M.-N.; Dybowska, A.D.; Luoma, S.N.; Valsami-Jones, E. A novel approach reveals that zinc oxide nanoparticles are bioavailable and toxic after dietary exposures. Nanotoxicology 2011, 5, 79–90. [Google Scholar] [CrossRef]
- Dabrunz, A.; Duester, L.; Prasse, C.; Seitz, F.; Rosenfeldt, R.; Schilde, C.; Schaumann, G.E.; Schulz, R. Biological surface coating and molting inhibition as mechanisms of TiO2 nanoparticle toxicity in Daphnia magna. PLoS One 2011, 6, e20112. [Google Scholar]
- Zhu, X.; Wang, J.; Zhang, X.; Chang, Y.; Chen, Y. The impact of ZnO nanoparticle aggregates on the embryonic development of zebrafish (Danio rerio). Nanotechnology 2009, 20. [Google Scholar] [CrossRef]
- Lin, S.; Zhao, Y.; Ji, Z.; Ear, J.; Chang, C.H.; Zhang, H.; Low-Kam, C.; Yamada, K.; Meng, H.; Wang, X.; et al. Zebrafish high-throughput screening to study the impact of dissolvable metal oxide nanoparticles on the hatching enzyme, ZHE1. Small 2013, 9, 1776–1785. [Google Scholar] [CrossRef]
- Lin, S.; Zhao, Y.; Xia, T.; Meng, H.; Ji, Z.; Liu, R.; George, S.; Xiong, S.; Wang, X.; Zhang, H.; et al. High content screening in zebrafish speeds up hazard ranking of transition metal oxide nanoparticles. ACS nano 2011, 5, 7284–7295. [Google Scholar] [CrossRef]
- Poda, A.R.; Kennedy, A.J.; Cuddy, M.F.; Bednar, A.J. Investigations of UV photolysis of PVP-capped silver nanoparticles in the presence and absence of dissolved organic carbon. J. Nanopart. Res. 2013, 15, 1673. [Google Scholar] [CrossRef]
- Tong, T.; Chu Thi Thanh, B.; Kelly, J.J.; Gaillard, J.-F.; Gray, K.A. Cytotoxicity of commercial nano-TiO2 to Escherichia coli assessed by high-throughput screening: Effects of environmental factors. Water Res. 2013, 47, 2352–2362. [Google Scholar] [CrossRef]
- Yang, S.P.; Bar-Ilan, O.; Peterson, R.E.; Heideman, W.; Hamers, R.J.; Pedersen, J.A. Influence of humic acid on titanium dioxide nanoparticle toxicity to developing zebrafish. Environ. Sci. Technol. 2013, 47, 4718–4725. [Google Scholar] [CrossRef]
- Kennedy, A.J.; Chappell, M.A.; Bednar, A.J.; Ryan, A.C.; Laird, J.G.; Stanley, J.K.; Steevens, J.A. Impact of organic carbon on the stability and toxicity of fresh and stored silver nanoparticles. Environ. Sci. Technol. 2012, 46, 10772–10780. [Google Scholar]
- Lin, D.; Ji, J.; Long, Z.; Yang, K.; Wu, F. The influence of dissolved and surface-bound humic acid on the toxicity of TiO2 nanoparticles to Chlorella sp. Water Res. 2012, 46, 4477–4487. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Ma, H.; Brennan, A.; Diamond, S.A. Phototoxicity of TiO2 nanoparticles under solar radiation to two aquatic species: Daphnia magna and Japanese medaka. Environ. Toxicol. Chem. 2012, 31, 1621–1629. [Google Scholar] [CrossRef]
- Shi, J.-P.; Ma, C.-Y.; Xu, B.; Zhang, H.-W.; Yu, C.-P. Effect of light on toxicity of nanosilver to Tetrahymena pyriformis. Environ. Toxicol. Chem. 2012, 31, 1630–1638. [Google Scholar] [CrossRef]
- Boczkowski, J.; Lanone, S. Respiratory toxicities of nanomaterials—A focus on carbon nanotubes. Adv. Drug Deliv. Rev. 2012, 64, 1694–1699. [Google Scholar] [CrossRef]
- Boyle, D.; Fox, J.E.; Akerman, J.M.; Sloman, K.A.; Henry, T.B.; Handy, R.D. Minimal effects of waterborne exposure to single-walled carbon nanotubes on behaviour and physiology of juvenile rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol. 2014, 146, 154–164. [Google Scholar] [CrossRef]
- Klaper, R.; Arndt, D.; Setyowati, K.; Chen, J.; Goetz, F. Functionalization impacts the effects of carbon nanotubes on the immune system of rainbow trout, Oncorhynchus mykiss. Aquat. Toxicol. 2010, 100, 211–217. [Google Scholar] [CrossRef]
- Edgington, A.J.; Petersen, E.J.; Herzing, A.A.; Podila, R.; Rao, A.; Klaine, S.J. Microscopic investigation of single-wall carbon nanotube uptake by Daphnia magna. Nanotoxicology 2013, in press. [Google Scholar]
- Petersen, E.J.; Akkanen, J.; Kukkonen, J.V.; Weber, W.J., Jr. Biological uptake and depuration of carbon nanotubes by Daphnia magna. Environ. Sci. Technol. 2009, 43, 2969–2975. [Google Scholar]
- Parks, A.N.; Portis, L.M.; Schierz, P.A.; Washburn, K.M.; Perron, M.M.; Burgess, R.M.; Ho, K.T.; Chandler, G.T.; Ferguson, P.L. Bioaccumulation and toxicity of single-walled carbon nanotubes to benthic organisms at the base of the marine food chain. Environ. Toxicol. Chem. SETAC 2013, 32, 1270–1277. [Google Scholar] [CrossRef]
- Bisesi, J.H., Jr.; Merten, J.; Liu, K.; Parks, A.N.; Afrooz, A.R.; Glenn, J.B.; Klaine, S.J.; Kane, A.S.; Saleh, N.B.; Ferguson, P.L.; et al. Tracking and quantification of single-walled carbon nanotubes in fish using near infrared fluorescence. Environ. Sci. Technol. 2014, 48, 1973–1983. [Google Scholar] [CrossRef]
- Edgington, A.J.; Roberts, A.P.; Taylor, L.M.; Alloy, M.M.; Reppert, J.; Rao, A.M.; Mao, J.; Klaine, S.J. The influence of natural organic matter on the toxicity of multiwalled carbon nanotubes. Environ. Toxicol. Chem. SETAC 2010, 29, 2511–2518. [Google Scholar] [CrossRef]
- Kang, S.; Mauter, M.S.; Elimelech, M. Microbial cytotoxicity of carbon-based nanomaterials: Implications for river water and wastewater effluent. Environ. Sci. Technol. 2009, 43, 2648–2653. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef]
- Arndt, D.A.; Moua, M.; Chen, J.; Klaper, R.D. Core structure and surface functionalization of carbon nanomaterials alter impacts to daphnid mortality, reproduction, and growth: Acute assays do not predict chronic exposure impacts. Environ. Sci. Technol. 2013, 47, 9444–9452. [Google Scholar] [CrossRef]
- Arndt, D.A.; Chen, J.; Moua, M.; Klaper, R.D. Multigeneration impacts on Daphnia magna of carbon nanomaterials with differing core structures and functionalizations. Environ. Toxicol. Chem. SETAC 2014, 33, 541–547. [Google Scholar] [CrossRef]
- Gupta, V.K.; Saleh, T.A. Sorption of pollutants by porous carbon, carbon nanotubes and fullerene—An overview. Environ. Sci. Pollut. Res. Int. 2013, 20, 2828–2843. [Google Scholar] [CrossRef]
- Parks, A.N.; Chandler, G.T.; Portis, L.M.; Sullivan, J.C.; Perron, M.M.; Cantwell, M.G.; Burgess, R.M.; Ho, K.T.; Ferguson, P.L. Effects of single-walled carbon nanotubes on the bioavailability of PCBs in field-contaminated sediments. Nanotoxicology 2013, in press. [Google Scholar]
- Azevedo Costa, C.L.; Chaves, I.S.; Ventura-Lima, J.; Ferreira, J.L.; Ferraz, L.; de Carvalho, L.M.; Monserrat, J.M. In vitro evaluation of co-exposure of arsenium and an organic nanomaterial (fullerene, C(60)) in zebrafish hepatocytes. Comp. Biochem. Physiol. Toxicol. Pharmacol. 2012, 155, 206–212. [Google Scholar] [CrossRef]
- Petersen, E.J.; Henry, T.B.; Zhao, J.; Maccuspie, R.I.; Kirschling, T.L.; Dobrovolskaia, M.A.; Hackley, V.; Xing, B.; White, J.C. Identification and avoidance of potential artifacts and misinterpretations in nanomaterial ecotoxicity measurements. Environ. Sci. Technol. 2014, 48, 4226–4246. [Google Scholar] [CrossRef]
- Guo, L.; Von Dem Bussche, A.; Buechner, M.; Yan, A.; Kane, A.B.; Hurt, R.H. Adsorption of essential micronutrients by carbon nanotubes and the implications for nanotoxicity testing. Small 2008, 4, 721–727. [Google Scholar] [CrossRef]
- Creighton, M.A.; Rangel-Mendez, J.R.; Huang, J.; Kane, A.B.; Hurt, R.H. Graphene-induced adsorptive and optical artifacts during in vitro toxicology assays. Small 2013, 9, 1921–1927. [Google Scholar] [CrossRef]
- Alloy, M.M.; Roberts, A.P. Effects of suspended multi-walled carbon nanotubes on daphnid growth and reproduction. Ecotoxicol. Environ. Saf. 2011, 74, 1839–1843. [Google Scholar] [CrossRef]
- Zhang, W.; Rattanaudompol, U.S.; Li, H.; Bouchard, D. Effects of humic and fulvic acids on aggregation of aqu/nC60 nanoparticles. Water Res. 2013, 47, 1793–1802. [Google Scholar] [CrossRef]
- Kim, K.T.; Jang, M.H.; Kim, J.Y.; Xing, B.; Tanguay, R.L.; Lee, B.G.; Kim, S.D. Embryonic toxicity changes of organic nanomaterials in the presence of natural organic matter. Sci. Total Environ. 2012, 426, 423–429. [Google Scholar] [CrossRef]
- Li, D.; Lyon, D.Y.; Li, Q.; Alvarez, P.J. Effect of soil sorption and aquatic natural organic matter on the antibacterial activity of a fullerene water suspension. Environ. Toxicol. Chem. SETAC 2008, 27, 1888–1894. [Google Scholar] [CrossRef]
- Kim, E.-J.; Le Thanh, T.; Chang, Y.-S. Comparative toxicity of bimetallic Fe nanoparticles toward Escherichia coli: Mechanism and environmental implications. Environ. Sci. Nano 2014, 1, 233–237. [Google Scholar] [CrossRef]
- Gratton, S.E.; Ropp, P.A.; Pohlhaus, P.D.; Luft, J.C.; Madden, V.J.; Napier, M.E.; DeSimone, J.M. The effect of particle design on cellular internalization pathways. Proc. Nat. Acad. Sci. USA 2008, 105, 11613–11618. [Google Scholar]
- Fu, P.P.; Xia, Q.; Hwang, H.-M.; Ray, P.C.; Yu, H. Mechanisms of nanotoxicity: Generation of reactive oxygen species. J. Food Drug Anal. 2014, 22, 64–75. [Google Scholar] [CrossRef]
- Siegrist, K.J.; Reynolds, S.H.; Kashon, M.L.; Lowry, D.T.; Dong, C.; Hubbs, A.F.; Young, S.-H.; Salisbury, J.L.; Porter, D.W.; Benkovic, S.A. Genotoxicity of multi-walled carbon nanotubes at occupationally relevant doses. Part. Fibre Toxicol. 2014, 11. [Google Scholar] [CrossRef]
- Murray, A.R.; Kisin, E.R.; Tkach, A.V.; Yanamala, N.; Mercer, R.; Young, S.-H.; Fadeel, B.; Kagan, V.E.; Shvedova, A.A. Factoring-in agglomeration of carbon nanotubes and nanofibers for better prediction of their toxicity versus asbestos. Part. Fibre Toxicol. 2012, 9, 1–19. [Google Scholar] [CrossRef]
- Nagai, H.; Okazaki, Y.; Chew, S.H.; Misawa, N.; Yamashita, Y.; Akatsuka, S.; Ishihara, T.; Yamashita, K.; Yoshikawa, Y.; Yasui, H. Diameter and rigidity of multiwalled carbon nanotubes are critical factors in mesothelial injury and carcinogenesis. Proc. Nat. Acad. Sci. USA 2011, 108, E1330–E1338. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Saleh, N.B.; Afrooz, A.R.M.N.; Bisesi,, J.H., Jr.; Aich, N.; Plazas-Tuttle, J.; Sabo-Attwood, T. Emergent Properties and Toxicological Considerations for Nanohybrid Materials in Aquatic Systems. Nanomaterials 2014, 4, 372-407. https://doi.org/10.3390/nano4020372
Saleh NB, Afrooz ARMN, Bisesi, JH Jr., Aich N, Plazas-Tuttle J, Sabo-Attwood T. Emergent Properties and Toxicological Considerations for Nanohybrid Materials in Aquatic Systems. Nanomaterials. 2014; 4(2):372-407. https://doi.org/10.3390/nano4020372
Chicago/Turabian StyleSaleh, Navid B., A. R. M. Nabiul Afrooz, Joseph H. Bisesi,, Jr., Nirupam Aich, Jaime Plazas-Tuttle, and Tara Sabo-Attwood. 2014. "Emergent Properties and Toxicological Considerations for Nanohybrid Materials in Aquatic Systems" Nanomaterials 4, no. 2: 372-407. https://doi.org/10.3390/nano4020372
APA StyleSaleh, N. B., Afrooz, A. R. M. N., Bisesi,, J. H., Jr., Aich, N., Plazas-Tuttle, J., & Sabo-Attwood, T. (2014). Emergent Properties and Toxicological Considerations for Nanohybrid Materials in Aquatic Systems. Nanomaterials, 4(2), 372-407. https://doi.org/10.3390/nano4020372