2.1. Physicochemical Characterization
Table 1 gives the elemental composition of SAZ materials, Al-SBA-15 and H-ZSM-5 analyzed by inductively coupled plasma atomic emission spectroscopy (ICP-AES) and atomic absorption spectroscopy (AAS). Most of aluminum in the initial gel of ZSM-5 nanoseeds has been successfully incorporated in the final SAZ solids. For example, the final Si/Al ratio of SAZ-20 is 23, which is close to 20 of the initial Si/Al ratio in the gel. This further confirms the beneficial role of pH adjusting method in introducing aluminum in SBA-15 type materials [
9,
10,
11]. Compared to SAZ samples, Al-SBA-15 prepared from conventional silica based sources (TEOS) has a relatively higher Si/Al ratio than that of the initial gel mixture, showing the advantages of zeolite nanoseed precursors over conventional ones, being consistent with the earlier reports [
6,
7,
8].
Table 1.
Physicochemical properties of SAZ materials, Al-SBA-15 and H-ZSM-5.
Table 1.
Physicochemical properties of SAZ materials, Al-SBA-15 and H-ZSM-5.
| Sample a | Initial Si/Al d | Final Si/Al e | d100 (nm) | a0 (nm) | Dp (nm) | SBET (m2/g) | Vt (cm3/g) | Total acidity f (mmol NH3/g) |
|---|
| SAZ-50 | 50 | 56 | 11.2 | 12.9 | 9.1 | 411 | 1.19 | 0.19 |
| SAZ-30 | 30 | 35 | 11.2 | 12.9 | 9.1 | 367 | 1.03 | 0.24 |
| SAZ-20 | 20 | 23 | 11.1 | 12.8 | 9.2 | 342 | 0.99 | 0.32 |
| SAZ-10 | 10 | 16 | 11.6 | 13.3 | - | 262 | 1.02 | 0.21 |
| Al-SBA-15 b | 30 | 45 | 10.2 | 11.7 | 7.2 | 466 | 0.93 | 0.18 |
| H-ZSM-5 c | 30 | 26 | - | - | - | 361 | 0.17 | 1.15 |
Figure 1a illustrates the small angle X-ray Scattering (SAXS) patterns of SAZ materials assembled from ZSM-5 nanoseeds with different initial Si/Al ratios and those of Al-SBA-15 and H-ZSM-5. It can be seen that samples SAZ-50, SAZ-30 and SAZ-20, prepared from ZSM-5 precursors having the initial Si/Al of 50, 30, and 20, respectively, exhibit three well-resolved peaks indexed as (100), (110) and (200) reflections of an ordered 2D hexagonal structure with a p6mm symmetry, which is typical of SBA-15 type materials. However, when reducing the initial Si/Al ratio to 10 (SAZ-10), only one reflection (100) can be observed, indicating the formation of a disordered mesostructure. For Al-SBA-15, the same three reflections with higher intensity are visible, suggesting a better ordering of its hexagonal mesostructure than that of SAZ materials. The unit cell parameter a
0 (calculated from the d
100 spacing) of SAZ samples is almost unchanged with the lowering of the initial Si/Al ratio from 50 to 20 (a
0 = 12.8–12.9 nm), but is considerably higher at the initial Si/Al ratio = 10 (a
0 = 13.3 nm) (
Table 1). Al-SBA-15 exhibits the smallest unit cell of 10.2 nm among others, possibly due to the different nature of zeolite nanoseeds and conventional silica based precursors. According to Han
et al. [
6], ZSM-5 nanoseeds containing zeolite building units have stronger rigidity and larger volume than non-structured silica based species. Thus, the self-assembly of ZSM-5 nanoseed precursors with the template appears more difficult and requires more space to connect each other compared to the self-assembly of conventional silica species, which leads to the lesser ordering and larger unit cell of SBA-15 analogs.
Figure 1.
(a) Small angle X-ray Scattering (SAXS); (b) X-ray diffraction (XRD) patterns; and (c) Fourier transform infrared (FTIR) spectra of SAZ materials, Al-SBA-15 and H-ZSM-5.
Figure 1.
(a) Small angle X-ray Scattering (SAXS); (b) X-ray diffraction (XRD) patterns; and (c) Fourier transform infrared (FTIR) spectra of SAZ materials, Al-SBA-15 and H-ZSM-5.
The nature of the framework of SAZ materials and reference samples Al-SBA-15 and H-ZSM-5 was investigated by X-ray diffraction (XRD) and Fourier transform infrared (FTIR) spectroscopy. As can be seen from
Figure 1b, there are no diffraction reflections in the wide angle XRD pattern of Al-SBA-15, confirming its X-ray amorphous nature. In contrast, H-ZSM-5 displays well-resolved diffraction reflections, characteristic of a highly crystalline ZSM-5. For SAZ samples, the absence of any resolved characteristic diffraction peaks in the wide angle XRD patterns suggests that either their mesostructures are still amorphous at the atomic level or the preserved zeolite building units in their mesopore walls have not connected together to form the long range atomic order of a zeolite framework that can be detected by XRD. To give more insights, FTIR was employed since it is a well-known technique for the identification of zeolite building units in aluminosilicates (
Figure 1c) [
6,
13]. Generally, all SAZ samples exhibit a small band at
ca. 548 cm
−1, indicative of five-membered ring subunits of ZSM-5 present [
13]. Notably, this band looks more obvious for SAZ-30 and SAZ-50, suggesting a better atomic order for the SAZ samples prepared from lower aluminum containing ZSM-5 nanoclusters. In fact, SAZ-10 and Al-SBA-15 show a quite similar FTIR spectrum, signifying that the ZSM-5 subunits are hardly present in the mesopore walls of SAZ-10. On the other hand, H-ZSM-5 shows a sharp absorption peak at
ca. 542 cm
−1, indicative of a much higher degree of zeolite crystallinity, in line with the result of XRD. These observations can be rationalized by considering the fact that the formation of zeolite precursors and their evolution to zeolite crystals decline with increasing aluminum content in the initial gel mixture [
14]. Hence, at a high aluminum concentration, it is likely that only few ZSM-5 nanoseed precursors containing zeolite building units are formed and thereby the nearly complete absence of ZSM-5 subunits in sample SAZ-10 is explained.
The textural properties of SAZ solids, Al-SBA-15 and H-ZSM-5 were studied by N
2 adsorption/desorption. The N
2 sorption isotherms and corresponding pore size distribution curves of SAZ and Al-SBA-15 samples are presented in
Figure 2a,b, respectively. Detailed textural parameters of all samples are summarized in
Table 1.
Figure 2.
(a) N2 sorption isotherms; and (b) corresponding pore size distributions of SAZ and Al-SBA-15 materials.
Figure 2.
(a) N2 sorption isotherms; and (b) corresponding pore size distributions of SAZ and Al-SBA-15 materials.
In line with SAXS results, all SAZ samples exhibit a type IV isotherm with H1-type hysteresis loop, confirming their mesoporous nature. The samples with higher Si/Al ratios (SAZ-50, SAZ-30 and SAZ-20) show a steep capillary condensation step occurring at the relative pressure (
p/p0) range of 0.7–0.9, characteristic of an ordered mesoporous solid with large and uniform pore size. Lowering the initial Si/Al ratio to 10, the hysteresis loop of the isotherm of SAZ-10 becomes irregular and shifts to higher relative pressures, suggesting that its pore uniformity is much degraded. The uniformity and dimension of mesopores can be directly evaluated by their pore size distributions (
Figure 2b). Sample SAZ-10 shows a broader pore size distribution ranging from 8 to 24 nm while SAZ-50, SAZ-30 and SAZ-20 samples yield a narrow pore size distribution centered at
ca. 9.1 nm. The disordered mesostructure of SAZ-10 can be also evidenced by the lowest BET surface area (262 m
2/g) in relation to that of the other SAZ samples (342–411 m
2/g) (
Table 1). Compared to SAZ samples, the higher ordering of the mesostructure of Al-SBA-15 is further proved by its better defined hysteresis loop and narrower pore size distribution (
Figure 2a,b, respectively). On the other hand, the microporous nature of H-ZSM-5 is proven by the type I isotherm with sharply increased nitrogen update at
p/
p0 < 0.01 (not shown). More details of the textural properties of the reference samples are given in
Table 1.
Thus, it is evidenced that the mesostructural ordering of SAZ materials decreases with reducing the initial Si/Al ratio of ZSM-5 nanoseeds. In other words, a high aluminum concentration in the initial gel is not favorable for the formation of an ordered mesostructure. This behavior can be explained based on the synthesis conditions applied in this study. It is generally accepted that the ordered mesostructure of SAZ materials (SBA-15 analogs) is formed by the self-assembly of ZSM-5 precursors with the P123 polymer as a structure directing agent in strongly acidic media at low temperature (1.6 M HCl, 313 K). Under such strongly acidic conditions, it is likely that only part of aluminum, which is presumed to be fixed in the zeolite building units, can be introduced into the mesoporous framework of SBA-15 analogs [
6]. The remaining fraction of aluminum still exists in the cationic form that is unlikely to be incorporated in the mesopore walls. Indeed, MAS-9 prepared from ZSM-5 precursors in strongly acidic media gave a relatively low aluminum content, e.g., the Si/Al ratio in the initial gel of 60 resulted in a product with Si/Al = 256 [
7]. Therefore, in order to introduce more aluminum in SBA-15 analogs, in this work, the pH of the synthesis mixture was adjusted to 3.5 before hydrothermal treatment at high temperature (473 K) for further silica condensation. At this pH value (mildly acidic media), most of aluminum atoms change into their corresponding oxo species and undergo further condensation with surface silanol groups of the preformed mesostructure, forming Si–O–Al linkages [
15,
16]. The incorporated aluminum on the surface of mesopores tends to form a layer that can play a protecting role by repelling the attack of water, thus hindering the hydrolysis of Si–O–Si surface bonds, finally preventing the mesostructure from degradation in boiling water. However, Li
et al. [
17] found out that when a large amount of aluminum was introduced into the mesoporous framework, a large fraction of octahedrally-coordinated aluminum species was formed. Under severe hydrothermal treatment, these aluminum species are easily aggregated; thus, the protecting layer is damaged. As a result, the mesostructure of SAZ-10 has partially collapsed during hydrothermal treatment at 473 K.
The number and strength of acid sites of SAZ solids and reference samples Al-SBA-15 and H-ZSM-5 were studied by temperature-programmed desorption of ammonia (NH
3-TPD). The results are depicted in
Figure 3 and
Table 1.
It can be seen from
Figure 3 that all SAZ materials yield a similar TPD profile with two desorption peaks. The dominant peak at
ca. 473 K corresponds to weak acid sites and the second broader peak like a tail in the range of 573–773 K indicates the presence of medium and strong acid sites. For the reference samples, the second peak of H-ZSM-5 looks well-developed while that of Al-SBA-15 is hardly visible. This implies that the acidity of SAZ solids is weaker than H-ZSM-5, but stronger than conventional Al-SBA-15.
The number of acid sites estimated from the peak area in the TPD profiles is listed in
Table 1. It is apparent that the total acidity of SAZ samples increases with increasing the incorporated aluminum content (SAZ-20 > SAZ-30 > SAZ-50). However, the acid site density of SAZ-10 (0.21 mmol NH
3/g) is noticeably lower than that of SAZ-20 (0.32 mmol NH
3/g) although the former solid contains a higher incorporated aluminum content. The reason might be the fact that the high incorporated aluminum content causes the formation and aggregation of octahedrally coordinated aluminum species, which decrease the density of acid sites [
17]. On the other hand, it should be noted that at the same initial Si/Al ratio of 30, the acid site density of SAZ-30 is higher than that of Al-SBA-15. This result further supports the work of Han
et al. [
6] and others [
7,
8,
9,
18] who found that the introduction of zeolite building units in the mesopore walls induced an improved acidity of SBA-15 analogs.
Figure 3.
Temperature-programmed desorption of ammonia (NH3-TPD) profiles of SAZ solids compared to reference samples Al-SBA-15 and H-ZSM-5.
Figure 3.
Temperature-programmed desorption of ammonia (NH3-TPD) profiles of SAZ solids compared to reference samples Al-SBA-15 and H-ZSM-5.
2.2. Hydrothermal Stability Test
Hydrothermal stability is of crucial importance as potential catalysts are targeted for the fluid catalytic cracking (FCC) process, wherein the catalyst has to withstand severe conditions,
i.e., 973–1073 K in the presence of steam during regeneration step [
2]. Therefore, the hydrothermal stability was evaluated by treatment of the representative sample SAZ-30 (1073 K, 30% of steam in 30 mL/min He flow) and subsequent SAXS, N
2 sorption, and TEM analyses. The results for the fresh and steamed samples are shown in
Figure 4,
Figure 5 and
Table 2.
Figure 4.
Effect of steaming on the structural and textural properties of SAZ-30: (a) SAXS patterns, (b) N2 sorption isotherms.
Figure 4.
Effect of steaming on the structural and textural properties of SAZ-30: (a) SAXS patterns, (b) N2 sorption isotherms.
Figure 5.
TEM images of (a) fresh and (b) steamed SAZ-30 viewed along [110] direction.
Figure 5.
TEM images of (a) fresh and (b) steamed SAZ-30 viewed along [110] direction.
Table 2.
Effect of steaming on structural and textural properties of SAZ-30.
Table 2.
Effect of steaming on structural and textural properties of SAZ-30.
| SAZ-30 | Dp (nm) | Vt (cm3/g) | Vt decrease, % | SBET (m2/g) | SBET decrease (%) |
|---|
| Fresh | 9.1 | 1.03 | - | 367 | - |
| Steamed | 9.1 | 0.85 | 19.4 | 263 | 28.3 |
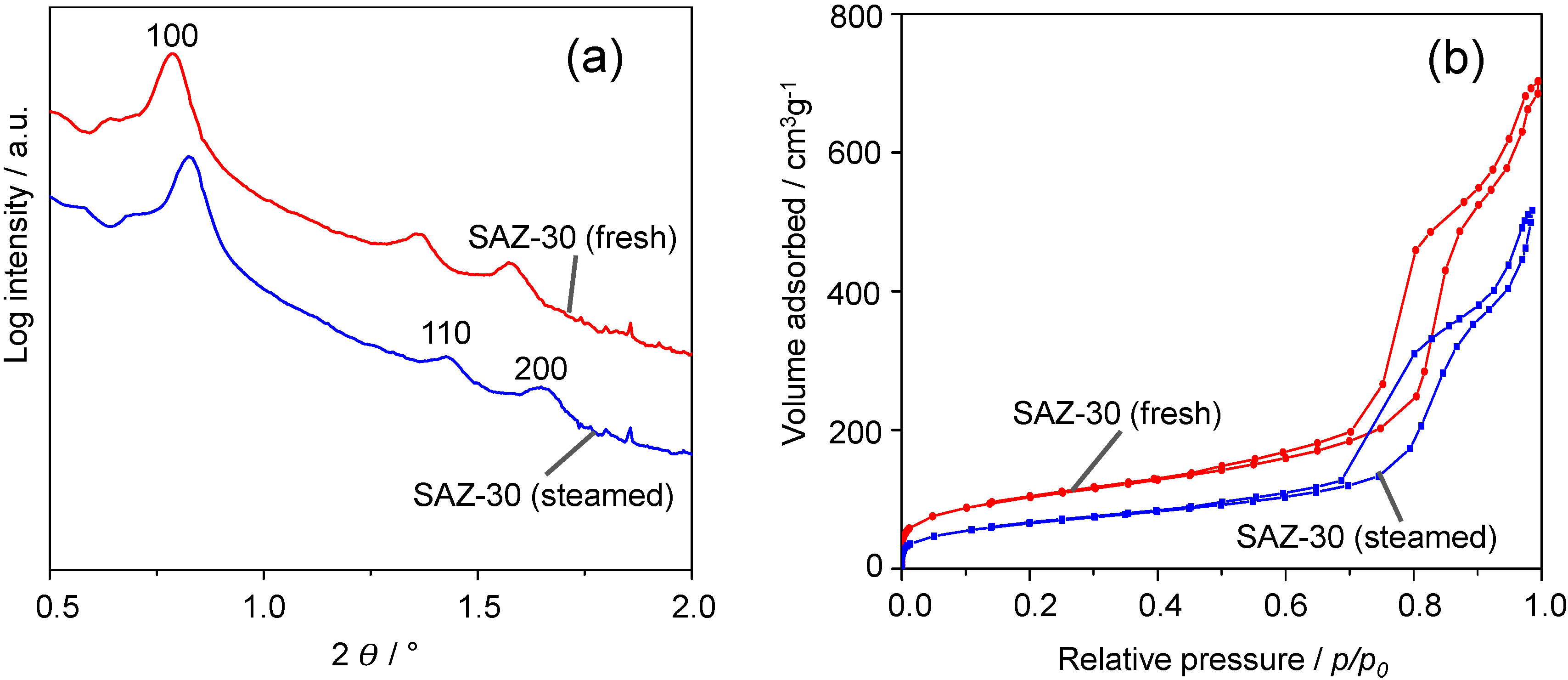
Figure 4a shows the SAXS patterns of fresh and steamed SAZ-30. Upon steaming at 1073 K for 24 h, SAZ-30 still displays three main reflections indexed as (100), (110) and (200) planes of the ordered hexagonal symmetry. The remarkable stability of SAZ-30 against steaming is further supported by N
2 adsorption/desorption studies (
Figure 4b). They reveal a similar shape of the adsorption and desorption isotherms of fresh and steamed SAZ-30. Thus, SAXS and BET results suggest the preservation of the SAZ-30 ordered mesostructure. The pore volume and BET surface area of SAZ-30 decrease by about 19% and 28%, respectively, whereas during steaming under the same conditions, the ordered mesostructure of SBA-15 partially collapsed already after 4 h, and the pore volume and BET surface area dropped by 60% and 65% respectively [
9]. The mesostructure preservation upon steam treatment is of practical importance because the future FCC process is likely to operate with very short contact time (less than 2 s) [
19] where large pores ease molecular transport, and consequently improve catalytic performance.
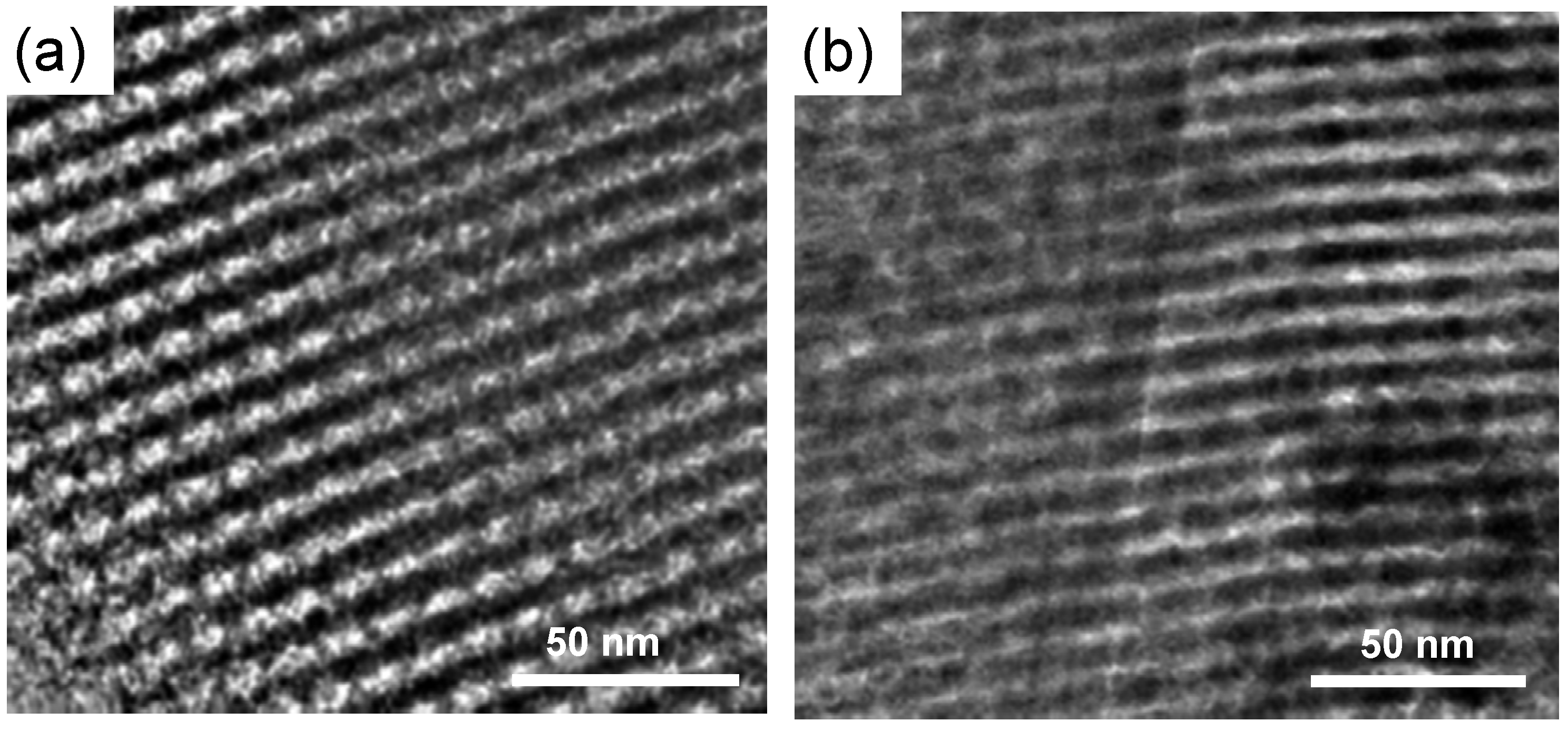
The high steaming stability is finally confirmed by transmission electron microscopy (TEM) analysis. The TEM image of fresh SAZ-30 (
Figure 5a) taken along a direction perpendicular to the pore axis reveals well-ordered hexagonal arrays with large uniform pores. Upon the steam treatment, the mesostructure of SAZ-30 apparently looks degraded, but the regularity of its mesopores is clearly evidenced (
Figure 5b). It was reported that there are many factors such as “zeolite-like” connectivity, high temperature synthesis or the salt effect which are favorable for the hydrothermal stability of mesoporous materials [
5,
8,
9,
20,
21]. Taking these factors into account, the remarkable steaming stability of SAZ-30 can be attributed to the retention of zeolite building units and highly condensed mesopore walls caused by high temperature synthesis.
2.3. Catalytic Cracking of Cumene
The gas phase cracking of cumene was carried out as probe reaction to evaluate Brønsted acidity of SAZ solids compared to that of conventional Al-SBA-15 and H-ZSM-5. To reach this goal, cumene cracking over SAZ solids, Al-SBA-15 and H-ZSM-5 was run at low temperature (573 K) and a weight hourly space velocity (WHSV) of 1.5 h
−1 under ambient pressure. The effect of thermal cracking was checked with inert material (glass beads) and found to be negligible. Under the chosen conditions in the presence of a catalyst in this study, cumene was mainly dealkylated to form benzene as a main product, indicating that Brønsted acidity is at play in this reaction [
22]. The cumene conversion over various SAZ catalysts, Al-SBA-15 and H-ZSM-5 with time-on-stream is depicted in
Figure 6a and the cumene conversion upon 1 h on-stream in relation with their acid site density and initial Si/Al ratios is shown in
Figure 6b.
Figure 6.
(a) Cumene conversion over SAZ materials compared to Al-SBA-15 and H-ZSM-5 as a function of time-on-stream; (b) cumene conversion over SAZ materials after 1 h on-stream and their total acidity plotted against the initial Si/Al ratio.
Figure 6.
(a) Cumene conversion over SAZ materials compared to Al-SBA-15 and H-ZSM-5 as a function of time-on-stream; (b) cumene conversion over SAZ materials after 1 h on-stream and their total acidity plotted against the initial Si/Al ratio.
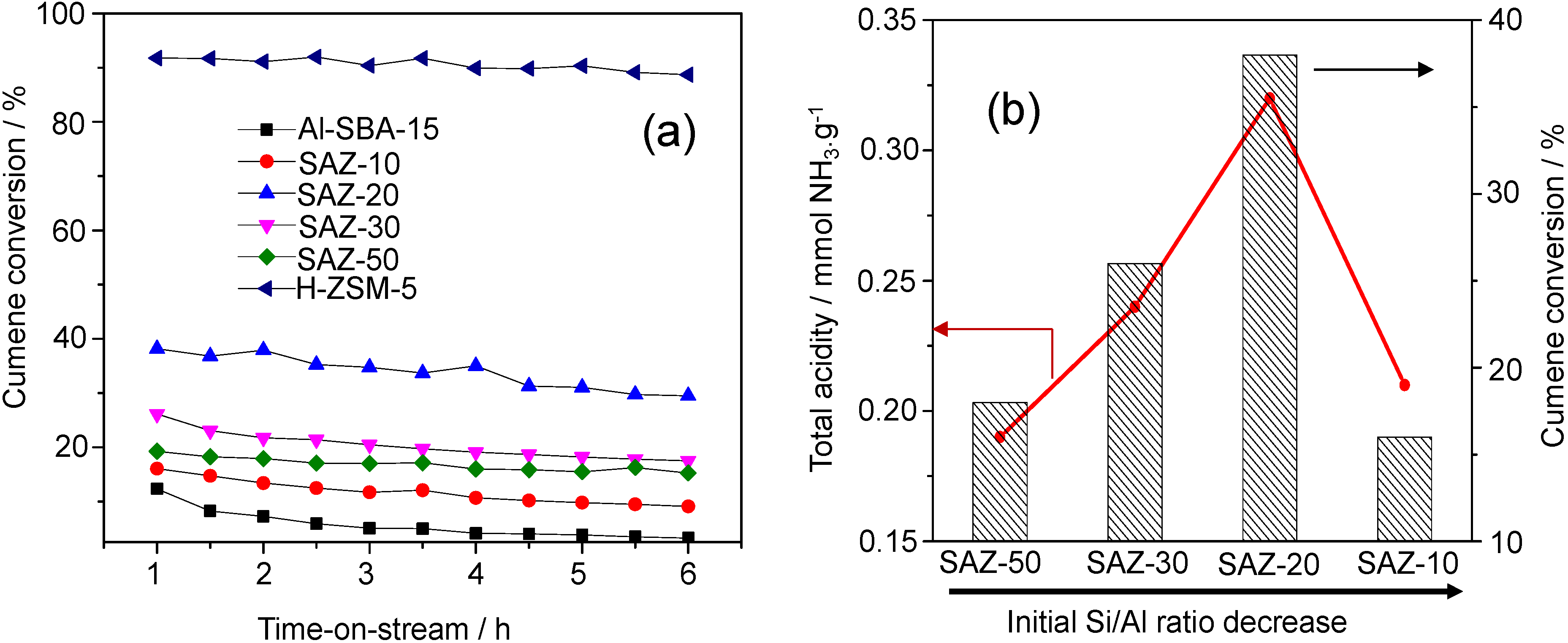
From
Figure 6a, it is obvious that H-ZSM-5 and Al-SBA-15 show the highest and lowest cumene conversions, respectively, and SAZ catalysts display cumene cracking activities in between. The highest conversion of cumene over H-ZSM-5 can be attributed to the highest density and strength of acid sites, in particular Brønsted acid sites [
1,
2]. In contrast, most of incorporated aluminum in amorphous Al-SBA-15 appears to contribute to Lewis acidity due to a lack of a long-range atomic order [
16]. Since Lewis acidity is apparently little active under the mild cracking conditions in this study, the lowest cumene conversion over Al-SBA-15 among others has been obtained. The SAZ catalysts, for instance, SAZ-50, have a similar density of acid sites compared to that Al-SBA-15 but exhibit a higher cumene conversion, which further supports the conclusion that the incorporation of zeolite building units in the mesopore walls results in an improved number of acid sites, particularly of active Brønsted acid sites [
6,
7,
8,
9].
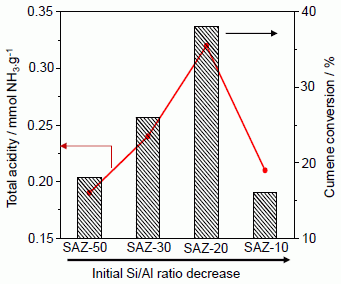
The effect of the initial Si/Al ratio of ZSM-5 nanoseeds on the catalytic activity of SAZ catalysts is illustrated in
Figure 6b. It can be seen that the cumene conversion increases from 18.2% to 38.1% with reducing the initial Si/Al ratio from 50 to 20. Within this initial Si/Al ratio range, a good correlation between the number of acid sites and cumene conversion has been observed. However, adding more aluminum to the initial gel (Si/Al = 10), the acidity and cumene cracking activity of SAZ-10 drop sharply, due possibly to the partial collapse of its mesostructure and the aggregation of octahedral aluminum as above discussed. Thus, in order to obtain an ordered mesostructure of SBA-15 analogs with the enhanced density of acid sites, particularly Brønsted acid sites and high hydrothermal stability, the initial Si/Al ratio of ZSM-5 precursors should not be lower than 20.