Optical and Structural Properties of Si Nanocrystals in SiO2 Films
Abstract
:1. Introduction
2. Results and Discussion
2.1. Furnace-Annealed Films
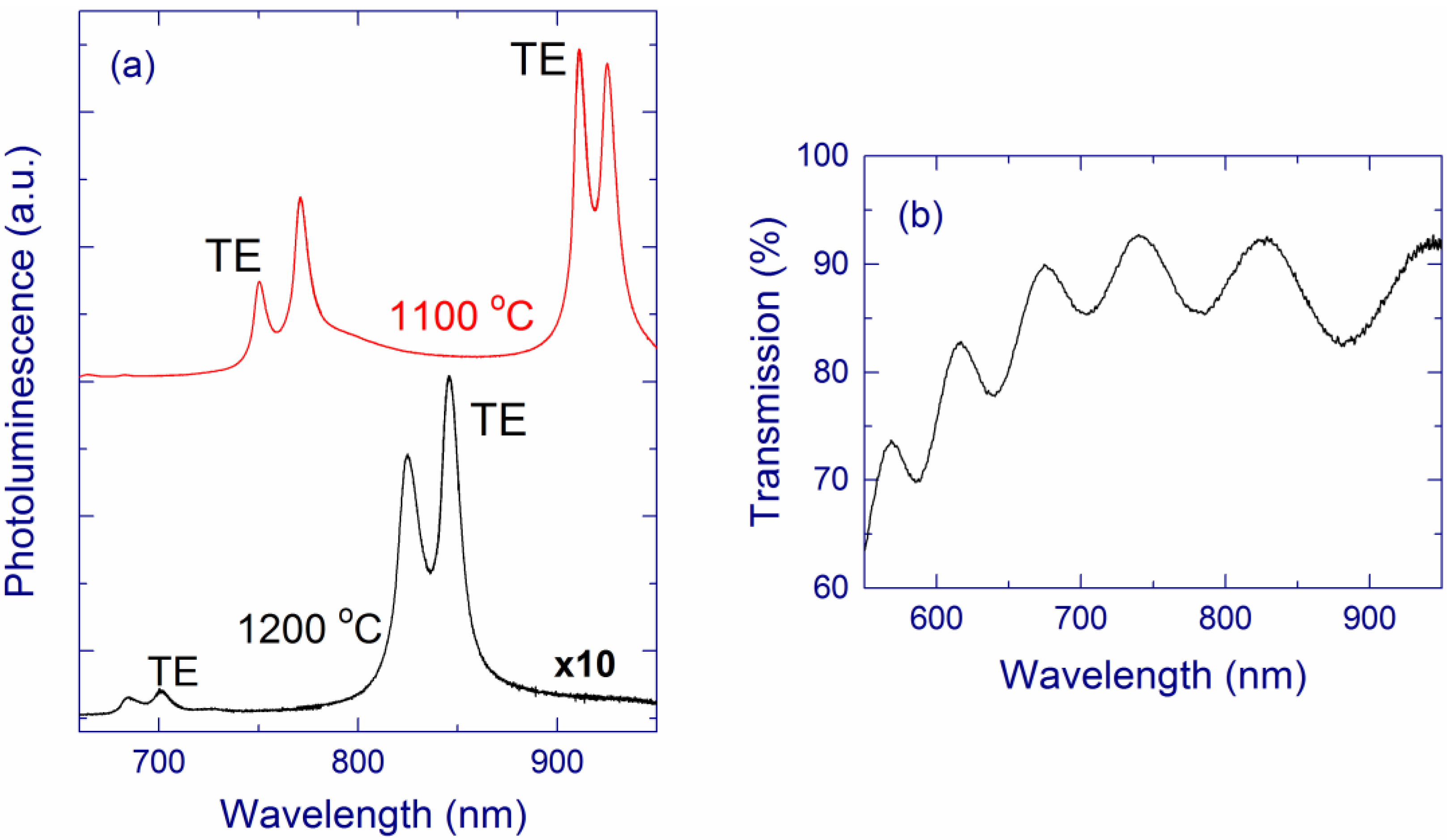
2.1.1. Correlation between Optical and Structural Properties




| Tann (°C) | nexp | αexp (104 cm–1) | nest | kest(SiO) | kest(Si) |
|---|---|---|---|---|---|
| 400 | - | 1.57 | 1.575 | 0.45 | 1.25 |
| 900 | - | 0.56 | 1.57 | 0.07 | 1.25 |
| 1000 | 1.64 * | 0.134 * | 1.56 * | 0 | 0.77 |
| 1100 | 1.61 | 0.126 | 1.56 | 0 | 0.53 |
| 1150 | 1.64 | 0.120 | 1.565 | 0 | 0.535 |
| 1200 | 1.67 | 0.128 | 1.57 | 0 | 0.48 |

2.1.2. Photoluminescence of the MBD Samples


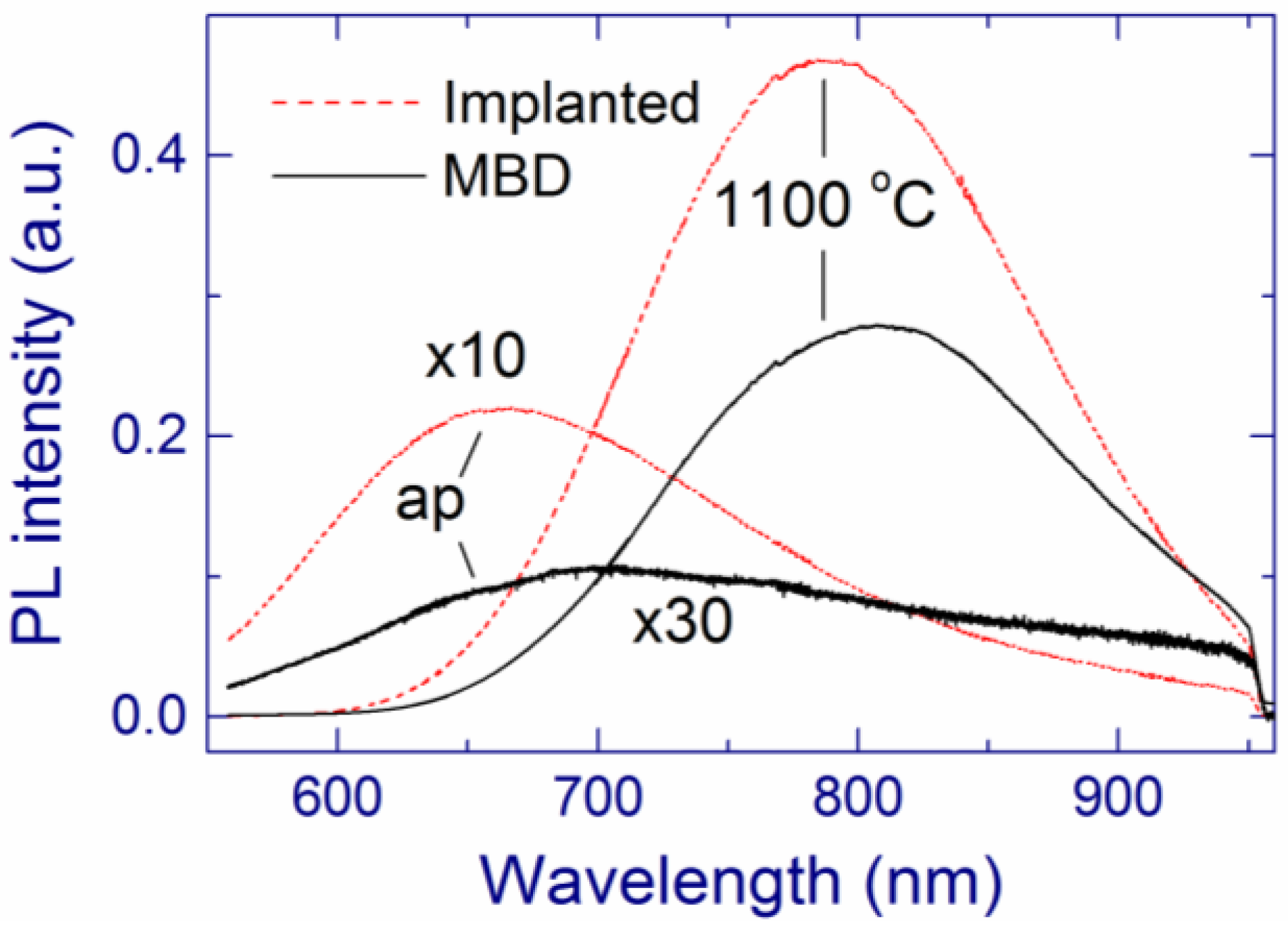
2.1.3. Comparison of the Implanted and MBD SiOx Samples (x ~ 1.8)

2.1.4. Laser Heating of Films on Substrates

2.2. Laser Annealing of Free-Standing Films
2.2.1. Laser-Annealed Areas

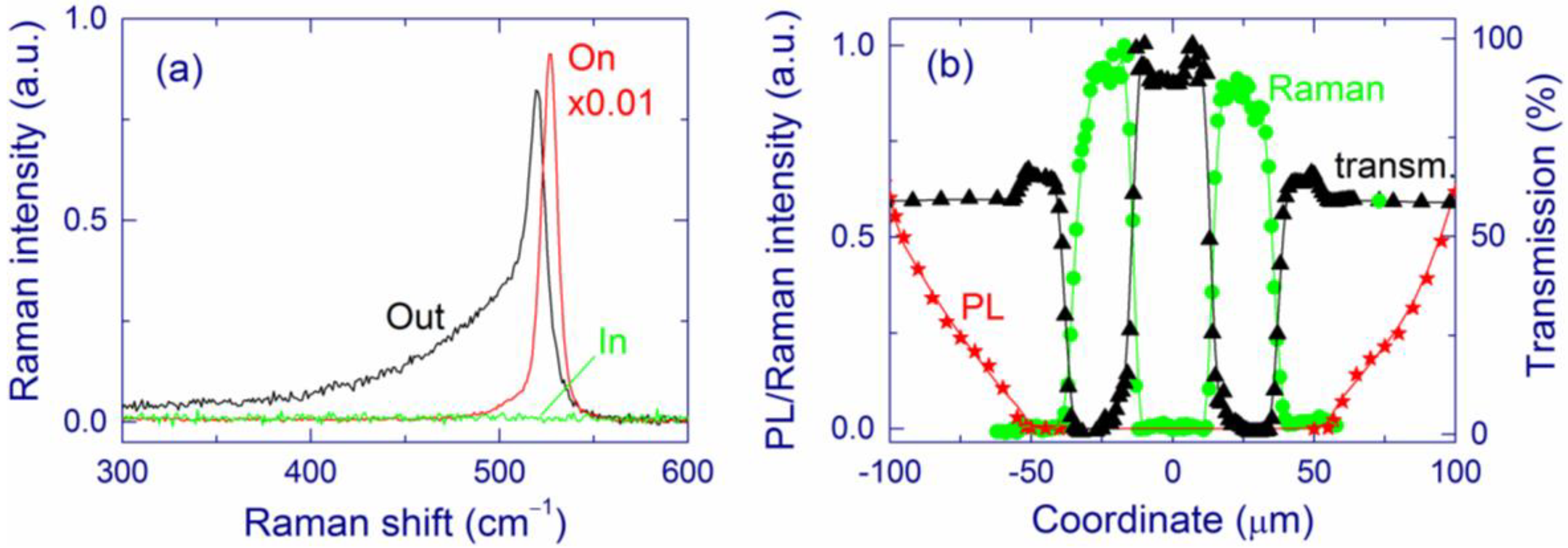
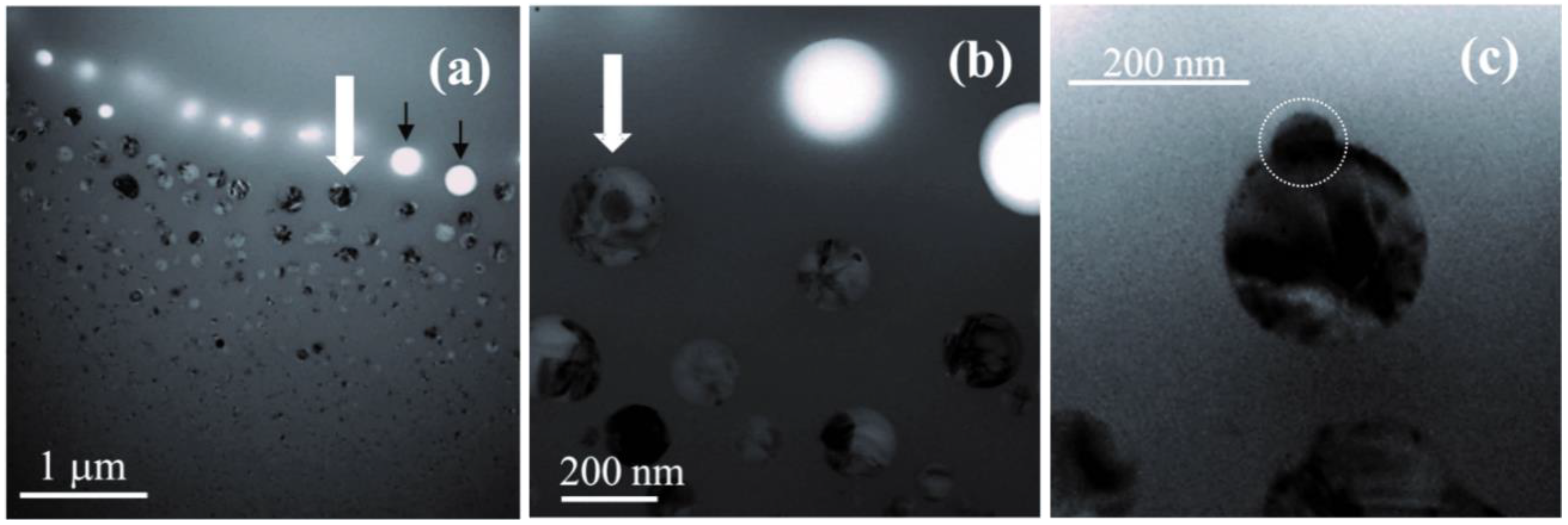
2.2.2. Surface Structure

2.2.3. Effect of the Annealing Atmosphere


2.2.4. Effect of the Exposure Period

2.2.5. Optical Memory

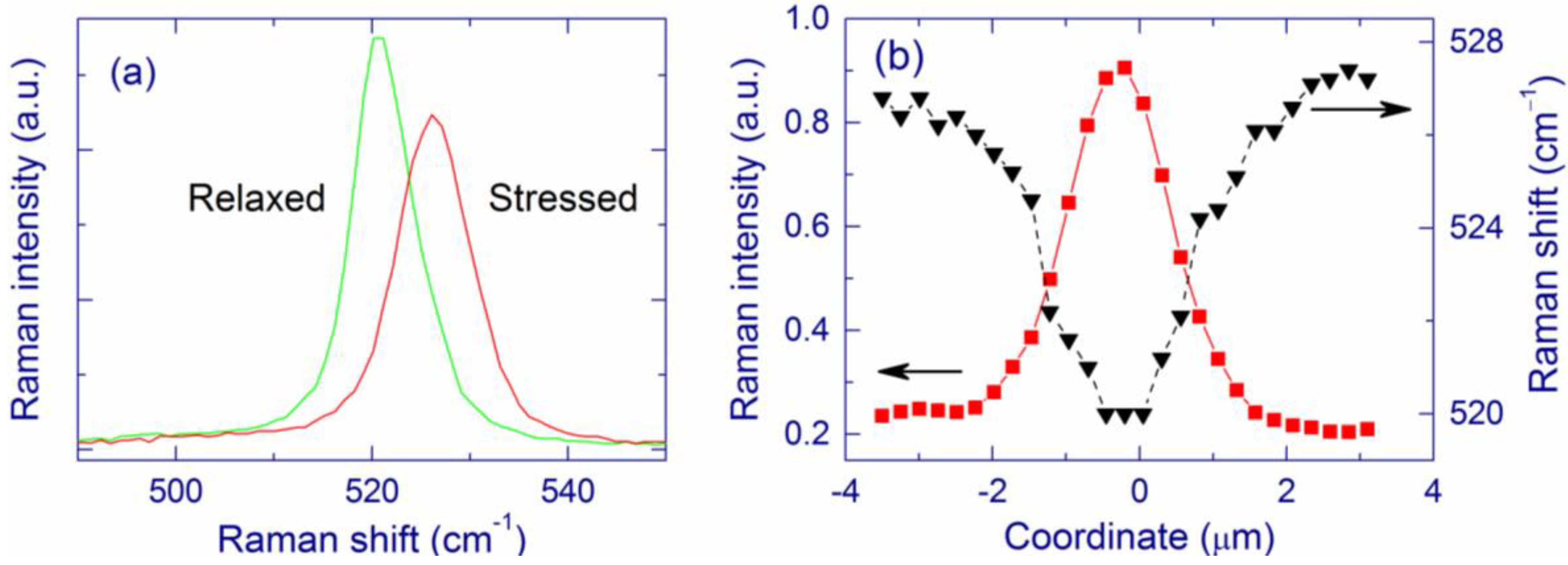
3. Experimental Section
3.1. Samples
3.2. Equipment
3.3. PL-Filtering Effect
3.4. X-Ray Photoelectron Spectroscopy

3.5. Effective Medium Approximation
3.6. Temperature Measurements

3.7. Optical Absorption
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Silicon Nanophotonics: Basic Principles, Present Status and Perspectives; Khriachtchev, L. (Ed.) Pan Stanford: Singapore, Singapore, 2008.
- Silicon Photonics II: Components and Integration; Lockwood, D.J.; Pavesi, L. (Eds.) Springer-Verlag: Berlin, Germany, 2011.
- Nanostructures in Electronics and Photonics; Rahman, F. (Ed.) Pan Stanford: Singapore, Singapore, 2008.
- Yariv, A.; Yeh, P. Photonics: Optical Electronics in Modern Communications; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Jalali, B.; Fathpour, S. Silicon photonics. J. Lightwave Technol. 2006, 24, 4600–4615. [Google Scholar]
- Soref, R. The past, present, and future of silicon photonics. IEEE J. Sel. Top. Quant. Electron. 2006, 12, 1678–1687. [Google Scholar] [CrossRef]
- Daldosso, N.; Pavesi, L. Nanosilicon photonics. Laser Photonics Rev. 2009, 3, 508–534. [Google Scholar] [CrossRef]
- Silicon Photonics: The State of the Art; Reed, G.T. (Ed.) John Wiley & Sons, Ltd: Chichester, UK, 2008.
- Pavesi, L. Will silicon be the photonic material of the third millennium? J. Phys. Cond. Matter 2003, 15, R1169–R1196. [Google Scholar] [CrossRef]
- Lockwood, D.J. Light emission in silicon nanostructures. NATO Asi Ser. Ser. E 1998, 348, 185–209. [Google Scholar]
- Lockwood, D.J.; Pavesi, L. Silicon fundamentals for photonics applications. Top. Appl. Phys. 2004, 94, 1–50. [Google Scholar]
- Delley, B.; Steigmeier, E.F. Quantum confinement in Si nanocrystals. Phys. Rev. B 1993, 47, 1397–1400. [Google Scholar] [CrossRef]
- Ogut, S.; Chelikowsky, J.R.; Louie, S.G. Quantum confinement and optical gaps in Si nanocrystals. Phys. Rev. Lett. 1997, 79, 1770–1773. [Google Scholar] [CrossRef]
- Balberg, I. Electrical transport mechanisms in three dimensional ensembles of silicon quantum dots. J. App. Phys. 2011, 110, 061301. [Google Scholar] [CrossRef]
- Canham, L.T. Silicon quantum wire array fabrication by electrochemical and chemical dissolution of wafers. Appl. Phys. Lett. 1990, 57, 1046–1048. [Google Scholar] [CrossRef]
- Cullis, A.G.; Canham, L.T. Visible-light emission due to quantum size effects in highly porous crystalline silicon. Nature 1991, 353, 335–338. [Google Scholar] [CrossRef]
- Gardelis, S.; Rimmer, J.S.; Dawson, P.; Hamilton, B.; Kubiak, R.A.; Whall, T.E.; Parker, E.H.C. Evidence for quantum confinement in the photoluminescence of porous Si and SiGe. Appl. Phys. Lett. 1991, 59, 2118–2120. [Google Scholar] [CrossRef]
- Bsiesy, A.; Vial, J.C.; Gaspard, F.; Herino, R.; Ligeon, M.; Muller, F.; Romestain, R.; Wasiela, A.; Halimaoui, A.; Bomchil, G. Photoluminescence of high porosity and of electrochemically oxidized porous silicon layers. Surf. Sci. 1991, 254, 195–200. [Google Scholar] [CrossRef]
- Delerue, C.; Allan, G.; Lannoo, M. Theoretical aspects of the luminescence of porous silicon. Phys. Rev. B 1993, 48, 11024–11036. [Google Scholar] [CrossRef]
- Prokes, S.M. Light emission in thermally oxidized porous silicon: Evidence for oxide-related luminescence. Appl. Phys. Lett. 1993, 62, 3244–3246. [Google Scholar] [CrossRef]
- Gole, J.L.; Dudel, F.P.; Grantier, D.; Dixon, D.A. Origin of porous silicon photoluminescence: Evidence for a surface bound oxyhydride-like emitter. Phys. Rev. B 1997, 56, 2137–2153. [Google Scholar] [CrossRef]
- Wolkin, M.V.; Jorne, J.; Fauchet, P.M.; Allan, G.; Delerue, C. Electronic states and luminescence in porous silicon quantum dots: The role of oxygen. Phys. Rev. Lett. 1999, 82, 197–200. [Google Scholar] [CrossRef]
- Ledoux, G.; Gong, J.; Huisken, F.; Guillois, O.; Reynaud, C. Photoluminescence of size-separated silicon nanocrystals: Confirmation of quantum confinement. Appl. Phys. Lett. 2002, 80, 4834–4836. [Google Scholar] [CrossRef]
- Hannah, D.C.; Yang, J.H.; Podsiadlo, P.; Chan, M.K.Y.; Demortiere, A.; Gosztola, D.J.; Prakapenka, V.B.; Schatz, G.C.; Kortshagen, U.; Schaller, R.D. On the origin of photoluminescence in silicon nanocrystals: Pressure-dependent structural and optical studies. Nano Lett. 2012, 12, 4200–4205. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.Y.; Park, N.M.; Kim, K.H.; Sung, G.Y.; Ok, Y.W.; Seong, T.Y.; Choi, C.J. Quantum confinement effect of silicon nanocrystals in situ grown in silicon nitride films. Appl. Phys. Lett. 2004, 85, 5355–5357. [Google Scholar] [CrossRef]
- Rezgui, B.; Sibai, A.; Nychyporuk, T.; Lemiti, M.; Bremond, G. Photoluminescence and optical absorption properties of silicon quantum dots embedded in Si-rich silicon nitride matrices. J. Lumin. 2009, 129, 1744–1746. [Google Scholar] [CrossRef]
- Khriachtchev, L.; Novikov, S.; Lahtinen, J. Thermal annealing of Si/SiO2 materials: Modification of structural and photoluminescence emission properties. J. Appl. Phys. 2002, 92, 5856–5862. [Google Scholar] [CrossRef]
- Lu, Z.H.; Lockwood, D.J.; Baribeau, J.M. Quantum confinement and light emission in SiO2/Si superlattices. Nature 1995, 378, 258–260. [Google Scholar] [CrossRef]
- Zhuravlev, K.S.; Gilinsky, A.M.; Kobitsky, A.Y. Mechanism of photoluminescence of Si nanocrystals fabricated in a SiO2 matrix. Appl. Phys. Lett. 1998, 73, 2962–2964. [Google Scholar] [CrossRef]
- Dhara, S.; Lu, C.Y.; Nair, K.G.M.; Chen, K.H.; Chen, C.P.; Huang, Y.F.; David, C.; Chen, L.C.; Raj, B. Mechanism of bright red emission in Si nanoclusters. Nanotechnology 2008, 19, 395401. [Google Scholar] [CrossRef] [PubMed]
- Garrido Fernandez, B.; Lopez, M.; Garcia, C.; Perez-Rodriguez, A.; Morante, J.R.; Bonafos, C.; Carrada, M.; Claverie, A. Influence of average size and interface passivation on the spectral emission of Si nanocrystals embedded in SiO2. J. Appl. Phys. 2002, 91, 798–807. [Google Scholar]
- Guha, S. Characterization of Si+ ion-implanted SiO2 films and silica glasses. J. Appl. Phys. 1998, 84, 5210–5217. [Google Scholar] [CrossRef]
- Mirabella, S.; Agosta, R.; Franzo, G.; Crupi, I.; Miritello, M.; Lo Savio, R.; Di Stefano, M.A.; Di Marco, S.; Simone, F.; Terrasi, A. Light absorption in silicon quantum dots embedded in silica. J. Appl. Phys. 2009, 106, 103505. [Google Scholar] [CrossRef]
- Franzo, G.; Miritello, M.; Boninelli, S.; Lo Savio, R.; Grimaldi, M.G.; Priolo, F.; Iacona, F.; Nicotra, G.; Spinella, C.; Coffa, S. Microstructural evolution of SiOx films and its effect on the luminescence of Si nanoclusters. J. Appl. Phys. 2008, 104, 094306. [Google Scholar] [CrossRef]
- Ternon, C.; Dufour, C.; Gourbilleau, F.; Rizk, R. Roles of interfaces in nanostructured silicon luminescence. Eur. Phys. J. B 2004, 41, 325–332. [Google Scholar] [CrossRef]
- Alonso, M.I.; Marcus, I.C.; Garriga, M.; Goñi, A.R.; Jedrzejewski, J.; Balberg, I. Evidence of quantum confinement effects on interband optical transitions in Si nanocrystals. Phys. Rev. B 2010, 82, 045302. [Google Scholar] [CrossRef]
- Roussel, M.; Talbot, E.; Pareige, P.; Gourbilleau, F. Influence of the supersaturation on Si diffusion and growth of Si nanoparticles in silicon-rich silica. J. Appl. Phys. 2013, 113, 063519. [Google Scholar] [CrossRef]
- Antonova, I.V.; Gulyaev, M.; Savir, E.; Jedrzejewski, J.; Balberg, I. Charge storage, photoluminescence, and cluster statistics in ensembles of Si quantum dots. Phys. Rev. B 2008, 77, 125318. [Google Scholar] [CrossRef]
- Balberg, I.; Savir, E.; Jedrzejewski, J.; Nassiopoulou, A.G.; Gardelis, S. Fundamental transport processes in ensembles of silicon quantum dots. Phys. Rev. B 2007, 75, 235329. [Google Scholar] [CrossRef]
- Iacona, F.; Franzo, G.; Spinella, C. Correlation between luminescence and structural properties of Si nanocrystals. J. Appl. Phys. 2000, 87, 1295–1303. [Google Scholar] [CrossRef]
- Trwoga, P.F.; Kenyon, A.J.; Pitt, C.W. Modeling the contribution of quantum confinement to luminescence from silicon nanoclusters. J. Appl. Phys. 1998, 83, 3789–3794. [Google Scholar] [CrossRef]
- Boninelli, S.; Iacona, F.; Franzo, G.; Bongiorno, C.; Spinella, C.; Priolo, F. Formation, evolution and photoluminescence properties of Si nanoclusters. J. Phys.: Condens. Matter 2007, 19, 225003. [Google Scholar] [CrossRef]
- Nassiopoulou, A.G. Encyclopedia of Nanoscience and Nanotechnology; Nalwa, H.S., Ed.; American Scientific: Valencia, Spain, 2004; Volume 9, pp. 793–813. [Google Scholar]
- Gardelis, S.; Nassiopoulou, A.G.; Manousiadis, P.; Milita, S.; Gkanatsiou, A.; Frangis, N.; Lioutas, C.B. Structural and optical characterization of two-dimensional arrays of Si nanocrystals embedded in SiO2 for photovoltaic applications. J. Appl. Phys. 2012, 111, 083536. [Google Scholar] [CrossRef]
- Gardelis, S.; Nassiopoulou, A.G.; Vouroutzis, N.; Frangis, N. Effect of exciton migration on the light emission properties in silicon nanocrystal ensembles. J. Appl. Phys. 2009, 105, 113509. [Google Scholar] [CrossRef]
- Hiller, D.; Goetze, S.; Munnik, F.; Jivanescu, M.; Gerlach, J.W.; Vogt, J.; Pippel, E.; Zakharov, N.; Stesmans, A.; Zacharias, M. Nitrogen at the Si-nanocrystal/SiO2 interface and its influence on luminescence and interface defects. Phys. Rev. B 2010, 82, 195401. [Google Scholar] [CrossRef]
- Seguini, G.; Castro, C.; Schamm-Chardon, S.; BenAssayag, G.; Pellegrino, P.; Perego, M. Scaling size of the interplay between quantum confinement and surface related effects in nanostructured silicon. Appl. Phys. Lett. 2013, 103, 023103. [Google Scholar] [CrossRef]
- Khriachtchev, L.; Räsänen, M.; Novikov, S.; Pavesi, L. Systematic correlation between Raman spectra, photoluminescence intensity, and absorption coefficient of silica layers containing Si nanocrystals. Appl. Phys. Lett. 2004, 85, 1511–1513. [Google Scholar] [CrossRef]
- Khriachtchev, L.; Räsänen, M.; Novikov, S.; Lahtinen, J. Tunable wavelength-selective waveguiding of photoluminescence in Si-rich silica optical wedges. J. Appl. Phys. 2004, 95, 7592–7601. [Google Scholar] [CrossRef]
- Zacharias, M.; Blasing, J.; Veit, P.; Tsybeskov, L.; Hirschman, K.; Fauchet, P.M. Thermal crystallization of amorphous Si/SiO2 superlattices. Appl. Phys. Lett. 1999, 74, 2614–2616. [Google Scholar] [CrossRef]
- Zacharias, M.; Heitmann, J.; Scholz, R.; Kahler, U.; Schmidt, M.; Blasing, J. Size-controlled highly luminescent silicon nanocrystals: A SiO/SiO2 superlattice approach. Appl. Phys. Lett. 2002, 80, 661–663. [Google Scholar] [CrossRef]
- Godefroo, S.; Hayne, M.; Jivanescu, M.; Stesmans, A.; Zacharias, M.; Lebedev, O.I.; van Tendeloo, G.; Moshchalkov, V.V. Classification and control of the origin of photoluminescence from Si nanocrystals. Nat. Nanotechnol. 2008, 3, 174–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiller, D.; Jivanescu, M.; Stesmans, A.; Zacharias, M. P-b(0) centers at the Si-nanocrystal/SiO2 interface as the dominant photoluminescence quenching defect. J. Appl. Phys. 2010, 107, 084309. [Google Scholar] [CrossRef]
- Novikov, S.V.; Sinkkonen, J.; Kilpela, O.; Gastev, S.V. Visible light emission from MBD-grown Si/SiO2 superlattices. J. Cryst. Growth 1997, 175, 514–518. [Google Scholar] [CrossRef]
- Khriachtchev, L.; Räsänen, M.; Novikov, S.; Kilpela, O.; Sinkkonen, J. Raman scattering from very thin Si layers of Si/SiO2 superlattices: Experimental evidence of structural modification in the 0.8-3.5 nm thickness region. J. Appl. Phys. 1999, 86, 5601–5608. [Google Scholar] [CrossRef]
- Khriachtchev, L.; Räsänen, M.; Novikov, S. Laser-controlled stress of Si nanocrystals in a free-standing Si/SiO2 superlattice. Appl. Phys. Lett. 2006, 88, 013102. [Google Scholar] [CrossRef]
- Khriachtchev, L.; Novikov, S.; Lahtinen, J.; Räsänen, M. Wavelength-selective optical waveguiding of photoluminescence in a thermally annealed Si/SiO2 superlattice. J. Phys.: Condens. Matter 2004, 16, 3219–3228. [Google Scholar] [CrossRef]
- Pavesi, L.; Dal Negro, L.; Mazzoleni, C.; Franzo, G.; Priolo, F. Optical gain in silicon nanocrystals. Nature 2000, 408, 440–444. [Google Scholar] [CrossRef]
- Khriachtchev, L.; Räsänen, M.; Novikov, S.; Sinkkonen, J. Optical gain in Si/SiO2 lattice: Experimental evidence with nanosecond pulses. Appl. Phys. Lett. 2001, 79, 1249–1251. [Google Scholar] [CrossRef]
- Koshida, N.; Koyama, H. Visible electroluminescence from porous silicon. Appl. Phys. Lett. 1992, 60, 347–349. [Google Scholar] [CrossRef]
- Franzo, G.; Irrera, A.; Moreira, E.C.; Miritello, M.; Iacona, F.; Sanfilippo, D.; Di Stefano, G.; Fallica, P.G.; Priolo, F. Electroluminescence of silicon nanocrystals in MOS structures. Appl. Phys. A 2002, 74, 1–5. [Google Scholar] [CrossRef]
- Zhou, F.L.; Head, J.D. Role of Si=O in the photoluminescence of porous silicon. J. Phys. Chem. B 2000, 104, 9981–9986. [Google Scholar] [CrossRef]
- Takeoka, S.; Fujii, M.; Hayashi, S. Size-dependent photoluminescence from surface-oxidized Si nanocrystals in a weak confinement regime. Phys. Rev. B 2000, 62, 16820–16825. [Google Scholar] [CrossRef]
- Nesheva, D.; Raptis, C.; Perakis, A.; Bineva, I.; Aneva, Z.; Levi, Z.; Alexandrova, S.; Hofmeister, H. Raman scattering and photoluminescence from Si nanoparticles in annealed SiOx thin films. J. Appl. Phys. 2002, 92, 4678–4683. [Google Scholar] [CrossRef]
- Lioudakis, E.; Othonos, A.; Hadjisavvas, G.C.; Kelires, P.C.; Nassiopoulou, A.G. Quantum confinement and interface structure of Si nanocrystals of sizes 3–5 nm embedded in a-SiO2. Phys. E 2007, 38, 128–134. [Google Scholar] [CrossRef]
- Garcia, C.; Garrido, B.; Pellegrino, P.; Ferre, R.; Moreno, J.A.; Morante, J.R.; Pavesi, L.; Cazzanelli, M. Size dependence of lifetime and absorption cross section of Si nanocrystals embedded in SiO2. Appl. Phys. Lett. 2003, 82, 1595–1597. [Google Scholar] [CrossRef]
- Kanemitsu, Y.; Ogawa, T.; Shiraishi, K.; Takeda, K. Visible photoluminescence from oxidized Si nanometer-sized spheres: Exciton confinement on a spherical shell. Phys. Rev. B 1993, 48, 4883–4886. [Google Scholar] [CrossRef]
- Kanemitsu, Y.; Uto, H.; Masumoto, Y.; Matsumoto, T.; Futagi, T.; Mimura, H. Microstructure and optical properties of free-standing porous silicon films: Size dependence of absorption spectra in Si nanometer-sized srystallites. Phys. Rev. B 1993, 48, 2827–2830. [Google Scholar] [CrossRef]
- Garoufalis, C.S.; Zdetsis, A.D. High accuracy calculations of the optical gap and absorption spectrum of oxygen contaminated Si nanocrystals. Phys. Chem. Chem. Phys. 2006, 8, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Dohnalova, K.; Kusova, K.; Pelant, I. Time-resolved photoluminescence spectroscopy of the initial oxidation stage of small silicon nanocrystals. Appl. Phys. Lett. 2009, 94, 211903. [Google Scholar] [CrossRef]
- Averboukh, B.; Huber, R.; Cheah, K.W.; Shen, Y.R.; Qin, G.G.; Ma, Z.C.; Zong, W.H. Luminescence studies of a Si/SiO2 superlattice. J. Appl. Phys. 2002, 92, 3564–3568. [Google Scholar] [CrossRef]
- Iacona, F.; Bongiorno, C.; Spinella, C.; Boninelli, S.; Priolo, F. Formation and evolution of luminescent Si nanoclusters produced by thermal annealing of SiOx films. J. Appl. Phys. 2004, 95, 3723–3732. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Smirani, R.; Ross, G.G. The formation mechanism of Si nanocrystals in SiO2. J. Cryst. Growth 2006, 294, 486–489. [Google Scholar] [CrossRef]
- Naciri, A.E.; Mansour, M.; Johann, L.; Grob, J.J.; Eckert, C. Optical study of Si nanocrystals in Si/SiO2 layers by spectroscopic ellipsometry. Nucl. Instrum. Meth. B 2004, 216, 167–172. [Google Scholar] [CrossRef]
- Falcony, C.; Calleja, W.; Aceves, M.; Siqueiros, J.M.; Machorro, R.; CotaAraiza, L.; Soto, G.; Farias, M.H. Characterization of excess Si in nonstoichiometric SiO2 films by optical and surface analysis techniques. J. Electrochem. Soc. 1997, 144, 379–383. [Google Scholar] [CrossRef]
- Charvet, S.; Madelon, R.; Gourbilleau, F.; Rizk, R. Ellipsometric spectroscopy study of photoluminescent Si/SiO2 systems obtained by magnetron co-sputtering. J. Lumin. 1998, 80, 257–261. [Google Scholar] [CrossRef]
- Daldosso, N.; Melchiorri, M.; Pavesi, L.; Pucker, G.; Gourbilleau, F.; Chausserie, S.; Belarouci, A.; Portier, X.; Dufour, C. Optical losses and absorption cross-section of silicon nanocrystals. J. Lumin. 2006, 121, 344–348. [Google Scholar] [CrossRef]
- Navarro-Urrios, D.; Riboli, F.; Cazzanelli, M.; Chiasera, A.; Daldosso, N.; Pavesi, L.; Oton, C.J.; Heitmann, J.; Yi, L.X.; Scholz, R.; et al. Birefringence characterization of mono-dispersed silicon nanocrystals planar waveguides. Opt. Mater. 2005, 27, 763–768. [Google Scholar] [CrossRef]
- Khriachtchev, L.; Navarro-Urrios, D.; Pavesi, L.; Oton, C.J.; Capuj, N.E.; Novikov, S. Spectroscopy of silica layers containing Si nanocrystals: Experimental evidence of optical birefringence. J. Appl. Phys. 2007, 101, 044310. [Google Scholar] [CrossRef]
- Moreno, J.A.; Garrido, B.; Pellegrino, P.; Garcia, C.; Arbiol, J.; Morante, J.R.; Marie, P.; Gourbilleau, F.; Rizk, R. Size dependence of refractive index of Si nanoclusters embedded in SiO2. J. Appl. Phys. 2005, 98, 013523. [Google Scholar] [CrossRef]
- Chen, T.P.; Liu, Y.; Tse, M.S.; Fung, S.; Dong, G. Profile of optical constants of SiO2 thin films containing Si nanocrystals. J. Appl. Phys. 2004, 95, 8481–8483. [Google Scholar] [CrossRef] [Green Version]
- Amans, D.; Callard, S.; Gagnaire, A.; Joseph, J.; Ledoux, G.; Huisken, F. Ellipsometric study of silicon nanocrystal optical constants. J. Appl. Phys. 2003, 93, 4173–4179. [Google Scholar] [CrossRef]
- Mishra, P.; Jain, K.P. Raman, photoluminescence and optical absorption studies on nanocrystalline silicon. Mat. Sci. Eng. B 2002, 95, 202–213. [Google Scholar] [CrossRef]
- von Behren, J.; van Buuren, T.; Zacharias, M.; Chimowitz, E.H.; Fauchet, P.M. Quantum confinement in nanoscale silicon: The correlation of size with bandgap and luminescence. Solid State Commun. 1998, 105, 317–322. [Google Scholar]
- Khriachtchev, L.; Räsänen, M.; Novikov, S. Efficient wavelength-selective optical waveguiding in a silica layer containing Si nanocrystals. Appl. Phys. Lett. 2003, 83, 3018–3020. [Google Scholar] [CrossRef]
- Valenta, J.; Ostatnicky, T.; Pelant, I.; Elliman, R.G.; Linnros, J.; Honerlage, B. Microcavity-like leaky mode emission from a planar optical waveguide made of luminescent silicon nanocrystals. J. Appl. Phys. 2004, 96, 5222–5225. [Google Scholar] [CrossRef]
- Pelant, I.; Ostatnicky, T.; Valenta, J.; Luterova, K.; Skopalova, E.; Mates, T.; Elliman, R.G. Waveguide cores containing silicon nanocrystals as active spectral filters for silicon-based photonics. Appl. Phys. B 2006, 83, 87–91. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorov, R.; Vancu, A. Optical properties and electronic structure of amorphous germanium. Phys. Status Solidi B 1966, 15, 627–637. [Google Scholar] [CrossRef]
- BenChorin, M.; Averboukh, B.; Kovalev, D.; Polisski, G.; Koch, F. Influence of quantum confinement on the critical points of the band structure of Si. Phys. Rev. Lett. 1996, 77, 763–766. [Google Scholar] [CrossRef]
- Podhorodecki, A.; Zatryb, G.; Misiewicz, J.; Wojcik, J.; Mascher, P. Influence of the annealing temperature and silicon concentration on the absorption and emission properties of Si nanocrystals. J. Appl. Phys. 2007, 102, 043104. [Google Scholar] [CrossRef]
- Inokuma, T.; Wakayama, Y.; Muramoto, T.; Aoki, R.; Kurata, Y.; Hasegawa, S. Optical properties of Si clusters and Si nanocrystallites in high-temperature annealed SiOx films. J. Appl. Phys. 1998, 83, 2228–2234. [Google Scholar] [CrossRef]
- Thompson, M.O.; Galvin, G.J.; Mayer, J.W.; Peercy, P.S.; Poate, J.M.; Jacobson, D.C.; Cullis, A.G.; Chew, N.G. Melting temperature and explosive crystallization of amorphous silicon during pulsed laser irradiation. Phys. Rev. Lett. 1984, 52, 2360–2363. [Google Scholar] [CrossRef]
- Koyama, H.; Fauchet, P.M. Laser-induced thermal effects on the optical properties of free-standing porous silicon films. J. Appl. Phys. 2000, 87, 1788–1794. [Google Scholar] [CrossRef]
- Khriachtchev, L.; Räsänen, M.; Novikov, S. Free-standing silica film containing Si nanocrystals: Photoluminescence, Raman scattering, optical waveguiding, and laser-induced thermal effects. Appl. Phys. Lett. 2005, 86, 141911. [Google Scholar] [CrossRef]
- Khriachtchev, L.; Räsänen, M.; Novikov, S. Continuous-wave laser annealing of free-standing Si/SiO2 superlattice: Modification of optical, structural, and light-emitting properties. J. Appl. Phys. 2006, 100, 053502. [Google Scholar] [CrossRef]
- Khriachtchev, L.; Novikov, S. Laser-induced thermal effects on Si/SiO2 free-standing superlattices. Appl. Phys. A 2007, 87, 761–766. [Google Scholar] [CrossRef]
- Koyama, H.; Fauchet, P.M. Very large continuous-wave-laser-induced optical absorption in porous silicon films: Evidence for thermal effects. Appl. Phys. Lett. 1998, 73, 3259–3261. [Google Scholar] [CrossRef]
- Mchedlidze, T.; Arguirov, T.; Kouteva-Arguirova, S.; Kittler, M. Light induced solid-phase crystallization of Si nanolayers in Si/SiO2 multiple quantum wells. J. Appl. Phys. 2010, 107, 124302. [Google Scholar] [CrossRef]
- Khriachtchev, L.; Ossicini, S.; Iacona, F.; Gourbilleau, F. Silicon nanoscale materials: From theoretical simulations to photonic applications. Int. J. Photoenergy 2012, 2012, 872576. [Google Scholar]
- Daldosso, N.; Luppi, M.; Ossicini, S.; Degoli, E.; Magri, R.; Dalba, G.; Fornasini, P.; Grisenti, R.; Rocca, F.; Pavesi, L.; et al. Role of the interface region on the optoelectronic properties of silicon nanocrystals embedded in SiO2. Phys. Rev. B 2003, 68, 085327. [Google Scholar] [CrossRef]
- Djurabekova, F.; Nordlund, K. Atomistic simulation of the interface structure of Si nanocrystals embedded in amorphous silica. Phys. Rev. B 2008, 77, 115325. [Google Scholar] [CrossRef]
- Luppi, M.; Ossicini, S. Multiple SiO bonds at the silicon cluster surface. J. Appl. Phys. 2003, 94, 2130–2132. [Google Scholar] [CrossRef]
- Vasiliev, I.; Chelikowsky, J.R.; Martin, R.M. Surface oxidation effects on the optical properties of silicon nanocrystals. Phys. Rev. B 2002, 65, 121302. [Google Scholar] [CrossRef]
- Puzder, A.; Williamson, A.J.; Grossman, J.C.; Galli, G. Computational studies of the optical emission of silicon nanocrystals. J. Am. Chem. Soc 2003, 125, 2786–2791. [Google Scholar] [CrossRef] [PubMed]
- Pennycook, T.J.; Hadjisavvas, G.; Idrobo, J.C.; Kelires, P.C.; Pantelides, S.T. Optical gaps of free and embedded Si nanoclusters: Density functional theory calculations. Phys. Rev. B 2010, 82, 125310. [Google Scholar] [CrossRef]
- Luppi, E.; Iori, F.; Magri, R.; Pulci, O.; Ossicini, S.; Degoli, E.; Olevano, V. Excitons in silicon nanocrystallites: The nature of luminescence. Phys. Rev. B 2007, 75, 033303. [Google Scholar] [CrossRef]
- Kleovoulou, K.; Kelires, P.C. Stress state of embedded Si nanocrystals. Phys. Rev. B 2013, 88, 085424. [Google Scholar] [CrossRef]
- Hadjisavvas, G.; Kelires, P.C. Structure and energetics of Si nanocrystals embedded in a-SiO2. Phys. Rev. Lett. 2004, 93, 226104. [Google Scholar] [CrossRef] [PubMed]
- Kleovoulou, K.; Kelires, P.C. Local rigidity and physical trends in embedded Si nanocrystals. Phys. Rev. B 2013, 88, 245202. [Google Scholar] [CrossRef]
- Guerra, R.; Degoli, E.; Ossicini, S. Size, oxidation, and strain in small Si/SiO2 nanocrystals. Phys. Rev. B 2009, 80, 155332. [Google Scholar] [CrossRef]
- Watanabe, T.; Tatsumura, K.; Ohdomari, I. SiO2/Si interface structure and its formation studied by large-scale molecular dynamics simulation. Appl. Surf. Sci. 2004, 237, 125–133. [Google Scholar] [CrossRef]
- Guerra, R.; Marri, I.; Magri, R.; Martin-Samos, L.; Pulci, O.; Degoli, E.; Ossicini, S. Silicon nanocrystallites in a SiO2 matrix: Role of disorder and size. Phys. Rev. B 2009, 79, 155320. [Google Scholar] [CrossRef]
- Guerra, R.; Marri, I.; Magri, R.; Martin-Samos, L.; Pulci, O.; Degoli, E.; Ossicini, S. Optical properties of silicon nanocrystallites in SiO2 matrix: Crystalline vs. amorphous case. Superlattices Microstruct. 2009, 46, 246–253. [Google Scholar] [CrossRef]
- Guerra, R.; Ossicini, S. High luminescence in small SiO/SiO2 nanocrystals: A theoretical study. Phys. Rev. B 2010, 81, 245307. [Google Scholar] [CrossRef]
- Khriachtchev, L.; Nikitin, T.; Oton, C.J.; Velagapudi, R.; Sainio, J.; Lahtinen, J.; Novikov, S. Optical properties of silicon nanocrystals in silica: Results from spectral filtering effect, m-line technique, and X-ray photoelectron spectroscopy. J. Appl. Phys. 2008, 104, 104316. [Google Scholar] [CrossRef]
- Nikitin, T.; Velagapudi, R.; Sainio, J.; Lahtinen, J.; Räsänen, M.; Novikov, S.; Khriachtchev, L. Optical and structural properties of SiOx films grown by molecular beam deposition: Effect of the Si concentration and annealing temperature. J. Appl. Phys. 2012, 112, 094316. [Google Scholar] [CrossRef]
- Khriachtchev, L.; Nikitin, T.; Velagapudi, R.; Lahtinen, J.; Novikov, S. Light-emission mechanism of thermally annealed silicon-rich silicon oxide revisited: What is the role of silicon nanocrystals? Appl. Phys. Lett. 2009, 94, 043115. [Google Scholar] [CrossRef]
- Nikitin, T.; Aitola, K.; Novikov, S.; Räsänen, M.; Velagapudi, R.; Sainio, J.; Lahtinen, J.; Mizohata, K.; Ahlgren, T.; Khriachtchev, L. Optical and structural properties of silicon-rich silicon oxide films: Comparison of ion implantation and molecular beam deposition methods. Phys. Status Solidi A 2011, 208, 2176–2181. [Google Scholar] [CrossRef]
- Hartstein, A.; Tsang, J.C.; Dimaria, D.J.; Dong, D.W. Observation of amorphous silicon regions in silicon-rich silicon dioxide films. Appl. Phys. Lett. 1980, 36, 836–837. [Google Scholar] [CrossRef]
- Khriachtchev, L.; Kilpela, O.; Karirinne, S.; Keranen, J.; Lepisto, T. Substrate-dependent crystallization and enhancement of visible photoluminescence in thermal annealing of Si/SiO2 superlattices. Appl. Phys. Lett. 2001, 78, 323–325. [Google Scholar] [CrossRef]
- Faraci, G.; Gibilisco, S.; Russo, P.; Pennisi, A.R.; La Rosa, S. Modified Raman confinement model for Si nanocrystals. Phys. Rev. B 2006, 73, 033307. [Google Scholar] [CrossRef]
- Campbell, I.H.; Fauchet, P.M. The effects of microcrystal size and shape on the one phonon Raman spectra of crystalline semiconductors. Solid State Commun. 1986, 58, 739–741. [Google Scholar] [CrossRef]
- Khriachtchev, L.; Novikov, S.; Kilpela, O. Optics of Si/SiO2 superlattices: Application to Raman scattering and photoluminescence measurements. J. Appl. Phys. 2000, 87, 7805–7813. [Google Scholar] [CrossRef]
- Seino, K.; Bechstedt, F.; Kroll, P. Influence of SiO2 matrix on electronic and optical properties of Si nanocrystals. Nanotechnology 2009, 20, 135702. [Google Scholar] [CrossRef] [PubMed]
- Novikov, S.; Sinkkonen, J.; Nikitin, T.; Khriachtchev, L.; Räsänen, M.; Haimi, E. Free-standing SiO2 films containing Si nanocrystals directly suitable for transmission electron microscopy. Microelectron. J. 2008, 39, 518–522. [Google Scholar] [CrossRef]
- Kogelnik, H.; Ramaswamy, V. Scaling rules for thin-film optical waveguides. Appl. Opt. 1974, 13, 1857–1862. [Google Scholar] [CrossRef] [PubMed]
- Unger, H.G. Planar Optical Waveguides and Fibres; Oxford University Press: Oxford, UK, 1977. [Google Scholar]
- Yurtsever, A.; Weyland, M.; Muller, D.A. Three-dimensional imaging of nonspherical silicon nanoparticles embedded in silicon oxide by plasmon tomography. Appl. Phys. Lett. 2006, 89, 151920. [Google Scholar] [CrossRef]
- Zatryb, G.; Podhorodecki, A.; Misiewicz, J.; Cardin, J.; Gourbilleau, F. Correlation between matrix structural order and compressive stress exerted on silicon nanocrystals embedded in silicon-rich silicon oxide. Nanoscale Res. Lett. 2013, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Munekuni, S.; Yamanaka, T.; Shimogaichi, Y.; Tohmon, R.; Ohki, Y.; Nagasawa, K.; Hama, Y. Various types of nonbridging oxygen hole center in highpurity silica glass. J. Appl. Phys. 1990, 68, 1212–1217. [Google Scholar] [CrossRef]
- Glinka, Y.D.; Lin, S.H.; Hwang, L.P.; Chen, Y.T. Photoluminescence from mesoporous silica: Similarity of properties to porous silicon. Appl. Phys. Lett. 2000, 77, 3968–3970. [Google Scholar] [CrossRef]
- Borsella, E.; D'Amato, R.; Fabbri, F.; Falconieri, M.; Trave, E.; Bello, V.; Mattei, G.; Nie, Y.R.; Wang, D.Y. On the role of non-bridging oxygen centers in the red luminescence emission from silicon nanocrystals. Phys. Status Solidi C 2011, 8, 974–978. [Google Scholar] [CrossRef]
- Koponen, L.; Tunturivuori, L.O.; Puska, M.J.; Nieminen, R.M. Effect of the surrounding oxide on the photoabsorption spectra of Si nanocrystals. Phys. Rev. B 2009, 79, 235332. [Google Scholar] [CrossRef]
- Garcia, C.; Garrido, B.; Pellegrino, P.; Ferre, R.; Moreno, J.A.; Pavesi, L.; Cazzanelli, M.; Morante, J.R. Absorption cross-sections and lifetimes as a function of size in Si nanocrystals embedded in SiO2. Phys. E 2003, 16, 429–433. [Google Scholar] [CrossRef]
- Shimizu-Iwayama, T.; Kurumado, N.; Hole, D.E.; Townsend, P.D. Optical properties of silicon nanoclusters fabricated by ion implantation. J. Appl. Phys. 1998, 83, 6018–6022. [Google Scholar] [CrossRef]
- Lee, B.G.; Hiller, D.; Luo, J.W.; Semonin, O.E.; Beard, M.C.; Zacharias, M.; Stradins, P. Strained interface defects in silicon nanocrystals. Adv. Funct. Mater. 2012, 22, 3223–3232. [Google Scholar] [CrossRef]
- Kusova, K.; Ondic, L.; Klimesova, E.; Herynkova, K.; Pelant, I.; Danis, S.; Valenta, J.; Gallart, M.; Ziegler, M.; Honerlage, B.; et al. Luminescence of free-standing versus matrix-embedded oxide-passivated silicon nanocrystals: The role of matrix-induced strain. Appl. Phys. Lett. 2012, 101, 143101. [Google Scholar] [CrossRef]
- Faraci, G.; Gibilisco, S.; Pennisi, A.R. Quantum confinement and thermal effects on the Raman spectra of Si nanocrystals. Phys. Rev. B 2009, 80, 193410. [Google Scholar] [CrossRef]
- Balkanski, M.; Wallis, R.F.; Haro, E. Anharmonic effects in light scattering due to optical phonons in silicon. Phys. Rev. B 1983, 28, 1928–1934. [Google Scholar] [CrossRef]
- Khriachtchev, L.; Nikitin, T.; Räsänen, M.; Domanskaya, A.; Boninelli, S.; Iacona, F.; Engdahl, A.; Juhanoja, J.; Novikov, S. Continuous-wave laser annealing of Si-rich oxide: A microscopic picture of macroscopic Si-SiO2 phase separation. J. Appl. Phys. 2010, 108, 124301. [Google Scholar] [CrossRef]
- Nikitin, T.; Kemell, M.; Puukilainen, E.; Boninelli, S.; Iacona, F.; Räsänen, M.; Ritala, M.; Novikov, S.; Khriachtchev, L. Surface fingerprints of individual silicon nanocrystals in laser-annealed Si/SiO2 superlattice: Evidence of nanoeruptions of laser-pressurized silicon. J. Appl. Phys. 2012, 111, 124302. [Google Scholar] [CrossRef]
- Nikitin, T.; Kemell, M.; Puukilainen, E.; Räsänen, M.; Ritala, M.; Novikov, S.; Khriachtchev, L. Continuous-wave laser annealing of a Si/SiO2 superlattice: Effect of the ambient atmosphere and exposure period. Sci. Adv. Mater. 2014, 6, 1000–1010. [Google Scholar] [CrossRef]
- Bachels, T.; Schafer, R. Binding energies of neutral silicon clusters. Chem. Phys. Lett. 2000, 324, 365–372. [Google Scholar] [CrossRef]
- Murphy, D.V.; Brueck, S.R.J. Enhanced raman-scattering from silicon microstructures. Opt. Lett. 1983, 8, 494–496. [Google Scholar] [CrossRef] [PubMed]
- Doremus, R.H. Viscosity of silica. J. Appl. Phys. 2002, 92, 7619–7629. [Google Scholar] [CrossRef]
- Wada, K.; Suzuki, A.; Sato, H.; Kikuchi, R. Soret effect in solids. J. Phys. Chem. Solids 1985, 46, 1195–1205. [Google Scholar] [CrossRef]
- Zhang, K.J.; Briggs, M.E.; Gammon, R.W.; Sengers, J.V. Optical measurement of the Soret coefficient and the diffusion coefficient of liquid mixtures. J. Chem. Phys. 1996, 104, 6881–6892. [Google Scholar] [CrossRef]
- Susa, M.; Nagata, K. Thermal oxidation of silicon substrates through oxygen diffusion. Mat. Sci. Eng. A 1991, 146, 51–62. [Google Scholar] [CrossRef]
- Deal, B.E.; Grove, A.S. General relationship for thermal oxidation of silicon. J. App. Phys. 1965, 36, 3770–3778. [Google Scholar] [CrossRef]
- Lukes, F.; Schmidt, E. Oxidation of silicon in dry oxygen. J. Phys. Chem. Solids 1965, 26, 1353–1357. [Google Scholar] [CrossRef]
- Nikitin, T.; Khriachtchev, L.; Räsänen, M.; Novikov, S. Optical memory of silicon nanocrystals with submicron spatial resolution and very high thermal stability. Appl. Phys. Lett. 2009, 94, 173116. [Google Scholar] [CrossRef]
- Khriachtchev, L. Comment on Optical absorption measurements of silica containing Si nanocrystals produced by ion implantation and thermal annealing. Appl. Phys. Lett. 2002, 81, 1357–1358. [Google Scholar] [CrossRef]
- Oton, C.J.; Ghulinyan, M.; Gaburro, Z.; Bettotti, P.; Pavesi, L.; Pancheri, L.; Gialanella, S.; Capuj, N.E. Scattering rings as a tool for birefringence measurements in porous silicon. J. Appl. Phys. 2003, 94, 6334–6340. [Google Scholar] [CrossRef]
- Renault, O.; Marlier, R.; Gely, M.; De Salvo, B.; Baron, T.; Hansson, M.; Barrett, N.T. Synchrotron radiation X-ray photoelectron spectroscopy of Si nanocrystals grown onto Al2O3/Si surfaces. Appl. Phys. Lett. 2005, 87, 163119. [Google Scholar] [CrossRef]
- Kim, S.; Kim, M.C.; Choi, S.H.; Kim, K.J.; Hwang, H.N.; Hwang, C.C. Size dependence of Si 2p core-level shift at Si nanocrystal/SiO2 interfaces. Appl. Phys. Lett. 2007, 91, 103113. [Google Scholar] [CrossRef]
- Barbagiovanni, E.G.; Goncharova, L.V.; Simpson, P.J. Electronic structure study of ion-implanted Si quantum dots in a SiO2 matrix: Analysis of quantum confinement theories. Phys. Rev. B 2011, 83, 035112. [Google Scholar] [CrossRef]
- Bruggeman, D.A.G. Berechnung verschiedener physikalischer Konstanten von heterogenen Substanzen. I. Dielektrizitätskonstanten und Leitfähigkeiten der Mischkörper aus isotropen Substanzen. Ann. Phys. 1935, 416, 636–664. [Google Scholar] [CrossRef]
- Knief, S.; von Niessen, W. Disorder, defects, and optical absorption in a-Si and a-Si:H. Phys. Rev. B 1999, 59, 12940–12946. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikitin, T.; Khriachtchev, L. Optical and Structural Properties of Si Nanocrystals in SiO2 Films. Nanomaterials 2015, 5, 614-655. https://doi.org/10.3390/nano5020614
Nikitin T, Khriachtchev L. Optical and Structural Properties of Si Nanocrystals in SiO2 Films. Nanomaterials. 2015; 5(2):614-655. https://doi.org/10.3390/nano5020614
Chicago/Turabian StyleNikitin, Timur, and Leonid Khriachtchev. 2015. "Optical and Structural Properties of Si Nanocrystals in SiO2 Films" Nanomaterials 5, no. 2: 614-655. https://doi.org/10.3390/nano5020614
APA StyleNikitin, T., & Khriachtchev, L. (2015). Optical and Structural Properties of Si Nanocrystals in SiO2 Films. Nanomaterials, 5(2), 614-655. https://doi.org/10.3390/nano5020614







