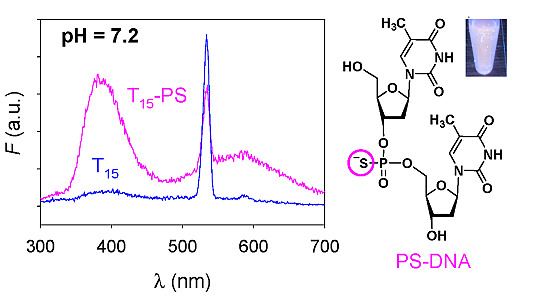
Phosphorothioate DNA Stabilized Fluorescent Gold and Silver Nanoclusters
Abstract
:1. Introduction
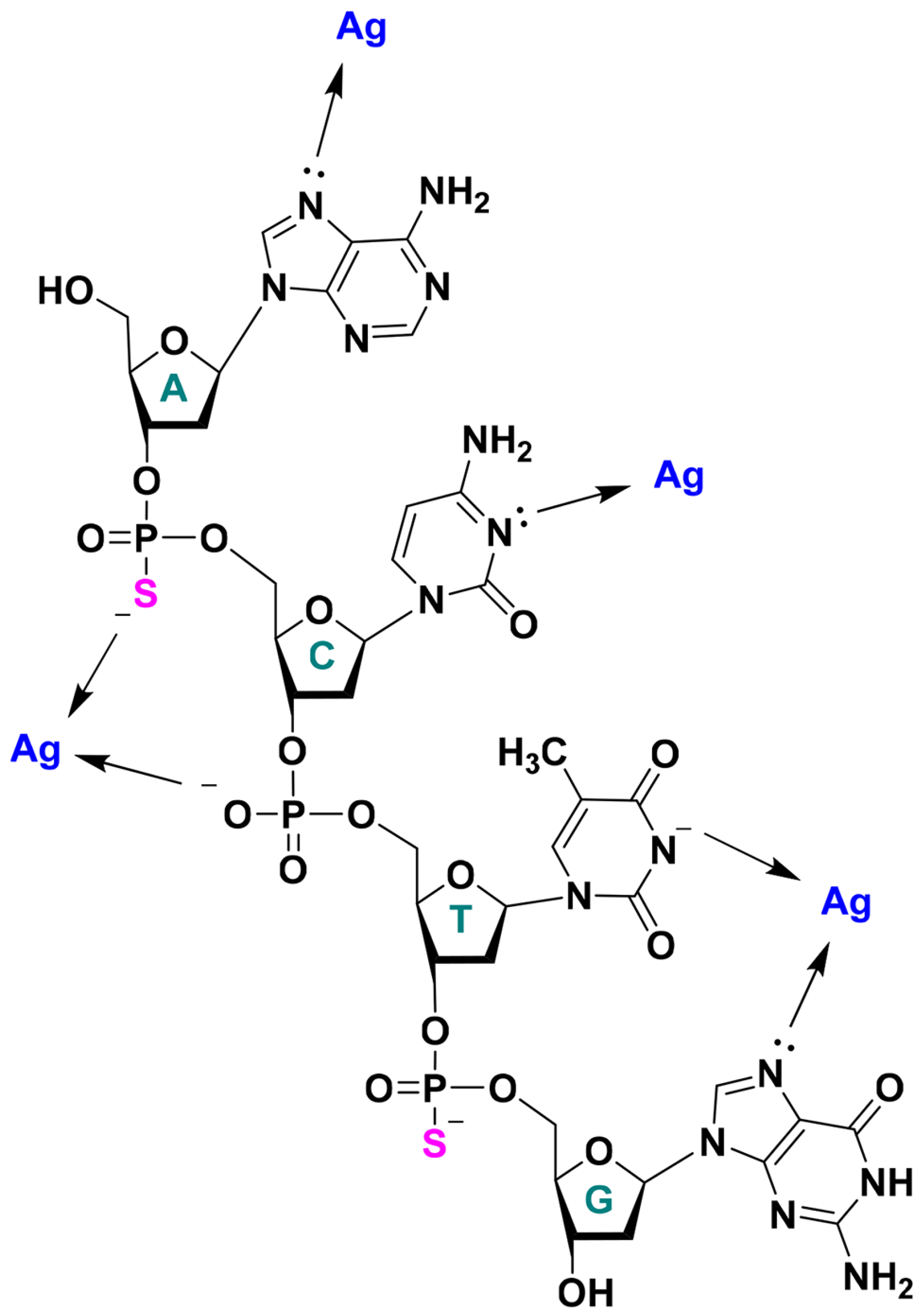
2. Results and Discussion
2.1. DNA Sequence Screening for AgNCs
| DNA Name | Sequence and Modifications |
|---|---|
| A15-PO | AAAAAAAAAAAAAAA |
| A15-PS | A*A*A*A*A*A*A*A*A*A*A*A*A*A*A |
| C15-PO | CCCCCCCCCCCCCCC |
| C15-PS | C*C*C*C*C*C*C*C*C*C*C*C*C*C*C |
| T15-PO | TTTTTTTTTTTTTTT |
| T15-PS | T*T*T*T*T*T*T*T*T*T*T*T*T*T*T |
| T15-PS1 | TTTTTTTT*T*T*T*T*T*T*T |
| T15-PS2 | T*TT*TT*TT*TT*TT*TT*TT |
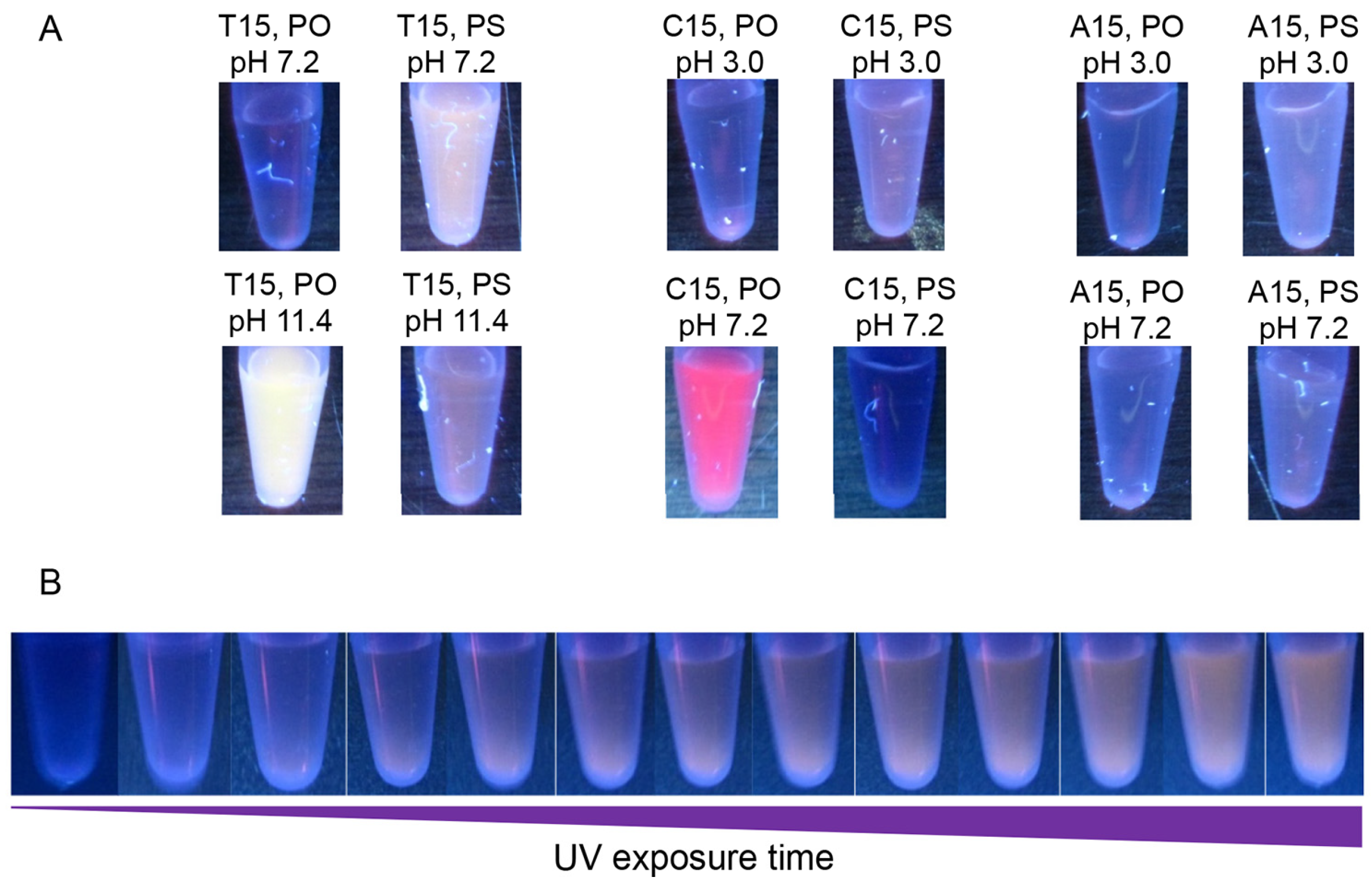
2.2. Different PS Compositions
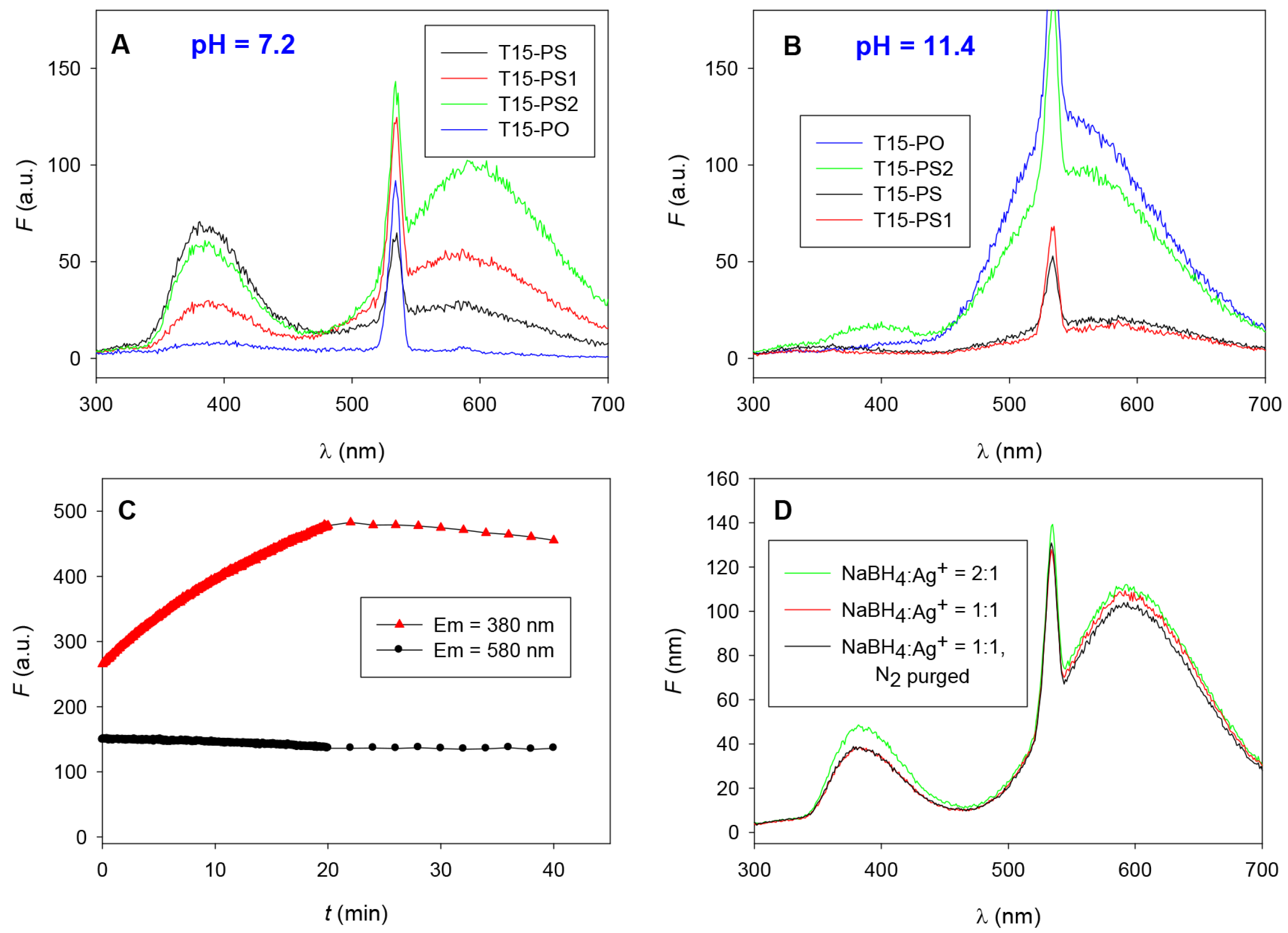
2.3. UV-Light Activated Fluorescence
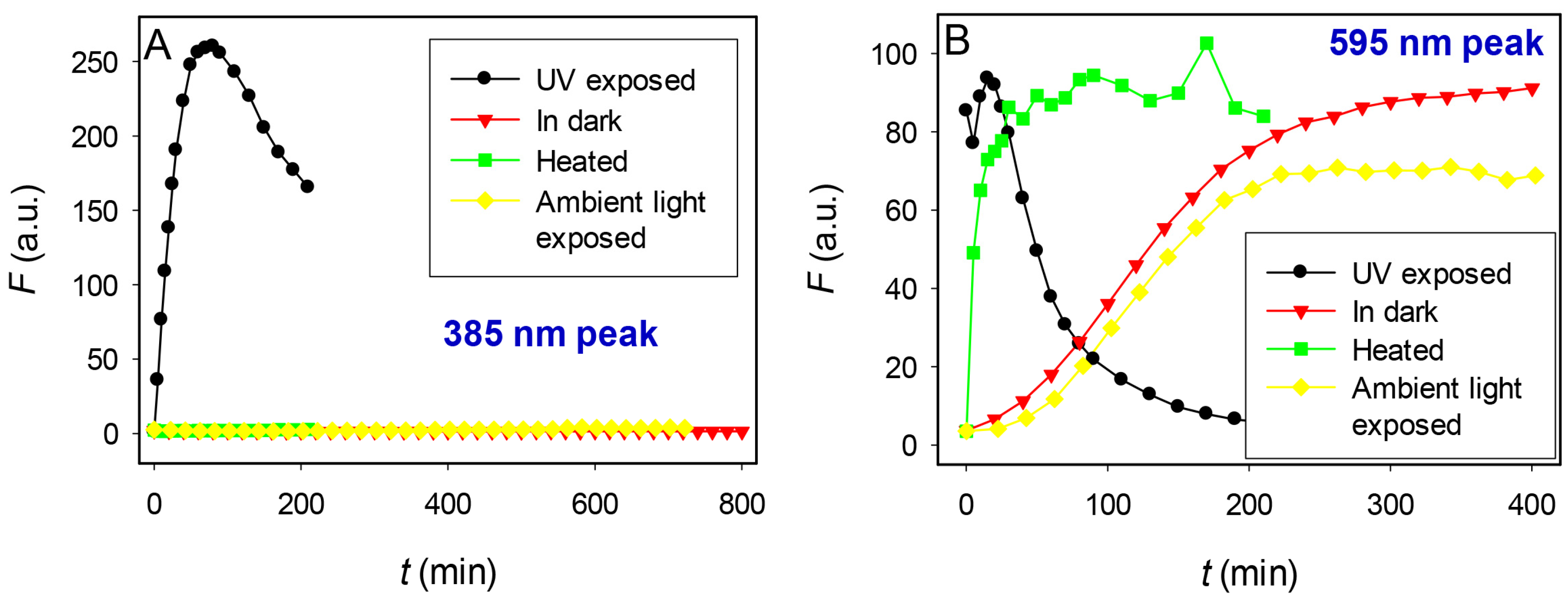
2.4. Effect of Heat and Light on Kinetics of Cluster Formation
3. Experimental Section
3.1. Reagents
3.2. AgNC Synthesis
3.3. Fluorescence Spectroscopy
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Díez, I.; Ras, R.H.A. Fluorescent silver nanoclusters. Nanoscale 2011, 3, 1963–1970. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. DNA-stabilized, fluorescent, metal nanoclusters for biosensor development. Trends Anal. Chem. 2014, 58, 99–111. [Google Scholar] [CrossRef]
- Shang, L.; Dong, S.; Nienhaus, G.U. Ultra-small fluorescent metal nanoclusters: Synthesis and biological applications. Nano Today 2011, 6, 401–418. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, E. Metal nanoclusters: New fluorescent probes for sensors and bioimaging. Nano Today 2014, 9, 132–157. [Google Scholar] [CrossRef]
- Wei, H.; Wang, Z.; Yang, L.; Tian, S.; Hou, C.; Lu, Y. Lysozyme-stabilized gold fluorescent cluster: Synthesis and application as Hg2+ sensor. Analyst 2010, 135, 1406–1410. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Peng, M.; Shi, L.; Du, Y.; Cai, N.; He, Y.; Chang, H.T.; Yeung, E.S. Disassembly mediated fluorescence recovery of gold nanodots for selective sulfide sensing. Nanoscale 2013, 5, 4683–4686. [Google Scholar] [CrossRef] [PubMed]
- Deleavey, G.F.; Damha, M.J. Designing chemically modified oligonucleotides for targeted gene silencing. Chem. Biol. 2012, 19, 937–954. [Google Scholar] [CrossRef] [PubMed]
- Sekhon, G.S.; Sen, D. A stereochemical glimpse of the active site of the 8–17 deoxyribozyme from iodine-mediated cross-links formed with the substrate’s scissile site. Biochemistry 2010, 49, 9072–9077. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Karbstein, K.; Peracchi, A.; Beigelman, L.; Herschlag, D. Identification of the hammerhead ribozyme metal ion binding site responsible for rescue of the deleterious effect of a cleavage site phosphorothioate. Biochemistry 1999, 38, 14363–14378. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.H.; Xing, H.; Lu, Y. DNA as a powerful tool for morphology control, spatial positioning, and dynamic assembly of nanoparticles. Acc. Chem. Res. 2014, 47, 1881–1890. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Sargent, E.H.; Kelley, S.O. One-step DNA-programmed growth of luminescent and biofunctionalized nanocrystals. Nat. Nanotechnol. 2009, 4, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.J.J.; Liu, J. Sensing parts-per-trillion Cd2+, Hg2+, and Pb2+ collectively and individually using phosphorothioate DNAzymes. Anal. Chem. 2014, 86, 5999–6005. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Deng, M.; Xu, L.; Zhou, Y.; Yuwen, J.; Zhou, X. The sensitive and selective optical detection of mercury(II) ions by using a phosphorothioate DNAzyme strategy. Chem. Eur. J. 2009, 15, 8117–8120. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Wernette, D.P.; Yigit, M.V.; Liu, J.; Wang, Z.; Lu, Y. Site-specific control of distances between gold nanoparticles using phosphorothioate anchors on DNA and a short bifunctional molecular fastener. Angew. Chem. Int. Ed. Engl. 2007, 46, 9006–9010. [Google Scholar] [CrossRef] [PubMed]
- Link, S.; Beeby, A.; FitzGerald, S.; el-Sayed, M.A.; Schaaff, T.G.; Whetten, R.L. Visible to infrared luminescence from a 28-atom gold cluster. J. Phys. Chem. B 2002, 106, 3410–3415. [Google Scholar] [CrossRef]
- Han, B.; Wang, E. DNA-templated fluorescent silver nanoclusters. Anal. Bioanal. Chem. 2012, 402, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Liang, J.; Pu, T.; Xu, F.; Yao, F.; Yang, Y.; Zhao, Y.L.; You, D.; Zhou, X.; Deng, Z.; et al. Phosphorothioate DNA as an antioxidant in bacteria. Nucleic Acids Res. 2012, 40, 9115–9124. [Google Scholar] [CrossRef] [PubMed]
- Guga, P.; Koziołkiewicz, M. Phosphorothioate nucleotides and oligonucleotides—Recent progress in synthesis and application. Chem. Biodivers. 2011, 8, 1642–1681. [Google Scholar] [CrossRef] [PubMed]
- Cruse, W.B.; Salisbury, S.A.; Brown, T.; Cosstick, R.; Eckstein, F.; Kennard, O. Chiral phosphorothioate analogues of B-DNA. The crystal structure of Rp-d[Gp(S)CpGp(S)CpGp(S)C]. J. Mol. Biol. 1986, 192, 891–905. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Nikonowicz, E.P. Phosphorothioate substitution can substantially alter RNA conformation. Biochemistry 2000, 39, 5642–5652. [Google Scholar] [CrossRef] [PubMed]
- Moody, E.M.; Brown, T.S.; Bevilacqua, P.C. Simple method for determining nucleobase pKa values by indirect labeling and demonstration of a pKa of neutrality in dsDNA. J. Am. Chem. Soc. 2004, 126, 10200–10201. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. Adsorption of DNA onto gold nanoparticles and graphene oxide: Surface science and applications. Phys. Chem. Chem. Phys. 2012, 14, 10485–10496. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, C.M.; Johnsen, K.R.; Kiser, J.R.; Antoku, Y.; Dickson, R.M.; Petty, J.T. Ag nanocluster formation using a cytosine oligonucleotide template. J. Phys. Chem. C 2007, 111, 175–181. [Google Scholar] [CrossRef]
- Sengupta, B.; Ritchie, C.M.; Buckman, J.G.; Johnsen, K.R.; Goodwin, P.M.; Petty, J.T. Base-directed formation of fluorescent silver clusters. J. Phys. Chem. C 2008, 112, 18776–18782. [Google Scholar] [CrossRef]
- Han, B.; Wang, E. Oligonucleotide-stabilized fluorescent silver nanoclusters for sensitive detection of biothiols in biological fluids. Biosens. Bioelectron. 2011, 26, 2585–2589. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Pu, F.; Lin, Y.; Ren, J.; Qu, X. Modulating DNA-templated silver nanoclusters for fluorescence turn-on detection of thiol compounds. Chem. Commun. 2011, 47, 3487–3489. [Google Scholar] [CrossRef]
- Lopez, A.; Liu, J. Light-activated metal-coordinated supramolecular complexes with charge-directed self-assembly. J. Phys. Chem. C 2013, 117, 3653–3661. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weadick, D.S.; Liu, J. Phosphorothioate DNA Stabilized Fluorescent Gold and Silver Nanoclusters. Nanomaterials 2015, 5, 804-813. https://doi.org/10.3390/nano5020804
Weadick DS, Liu J. Phosphorothioate DNA Stabilized Fluorescent Gold and Silver Nanoclusters. Nanomaterials. 2015; 5(2):804-813. https://doi.org/10.3390/nano5020804
Chicago/Turabian StyleWeadick, Daniel S., and Juewen Liu. 2015. "Phosphorothioate DNA Stabilized Fluorescent Gold and Silver Nanoclusters" Nanomaterials 5, no. 2: 804-813. https://doi.org/10.3390/nano5020804
APA StyleWeadick, D. S., & Liu, J. (2015). Phosphorothioate DNA Stabilized Fluorescent Gold and Silver Nanoclusters. Nanomaterials, 5(2), 804-813. https://doi.org/10.3390/nano5020804