Comparison of the in Vitro Uptake and Toxicity of Collagen- and Synthetic Polymer-Coated Gold Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Collagen-Coated Au NPs
2.2. Fluorescence-Labelling of Collagen-Coated Au NPs
2.3. Synthesis of PMA-Coated Au NPs
2.4. Nanoparticle Purification and Characterization
2.5. Cell Culture
2.6. Particle Internalization and Sample Preparation for ICP-MS Measurements
2.7. Cell Viability
3. Results and Discussion
3.1. Synthesis and Characterization of Au NPs

| Type of Nanoparticle | dh (nm) | |||
|---|---|---|---|---|
| t0 | t2h | t4h | ||
| Collagen-Coated Au NPs | + serum | 18.1 | 18.1 | 18.1 |
| Collagen-Coated Au NPs | − serum | 13.5 | 141.8 | 220 |
| PMA-Coated Au NPs | + serum | 8.7 | 8.7 | 8.7 |
| PMA-Coated Au NPs | − serum | 15.6 | 15.6 | 15.6 |
3.2. Cellular Internalization of NPs


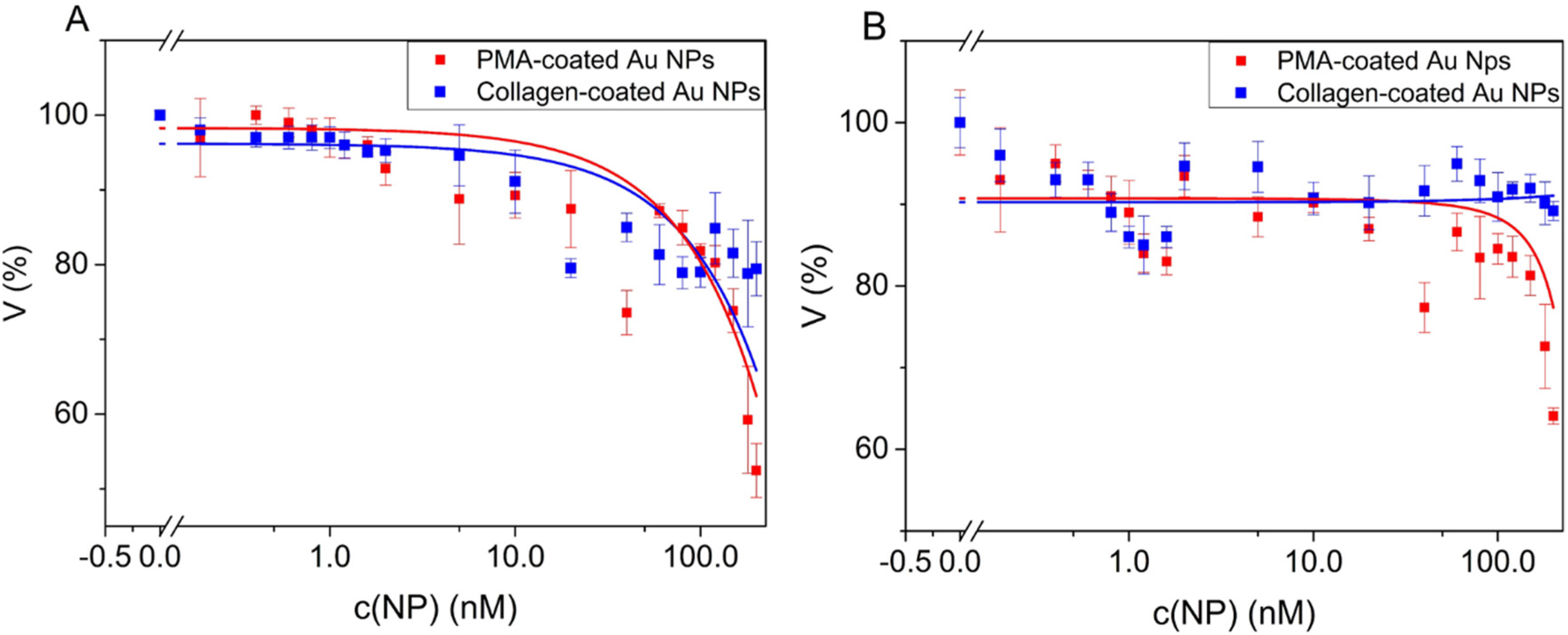
3.3. Cell Viability upon Incubation with Au NPs
4. Conclusions
Supplementary Materials
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Sperling, R.A.; Rivera Gil, P.; Zhang, F.; Zanella, M.; Parak, W.J. Biological applications of gold nanoparticles. Chem. Soc. Rev. 2008, 37, 1896–1908. [Google Scholar] [CrossRef] [PubMed]
- Aroca, R.; Alvarez-Puebla, R.; Pieczonka, N.; Sanchez-Cortez, S.; Garcia-Ramos, J. Surface-enhanced raman scattering on colloidal nanostructures. Adv. Colloid Interf. Sci. 2005, 116, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Leopold, N.; Chiş, V.; Mircescu, N.E.; Marişca, O.T.; Buja, O.M.; Leopold, L.F.; Socaciu, C.; Braicu, C.; Irimie, A.; Berindan-Neagoe, I. One step synthesis of sers active colloidal gold nanoparticles by reduction with polyethylene glycol. Colloid Surf. A 2013, 436, 133–138. [Google Scholar] [CrossRef]
- Rivera-Gil, P.; Jimenez De Aberasturi, D.; Wulf, V.; Pelaz, B.; Del Pino, P.; Zhao, Y.; de la Fuente, J.M.; Ruiz de Larramendi, I.; Rojo, T.; Liang, X.-J.; et al. The challenge to relate the physicochemical properties of colloidal nanoparticles to their cytotoxicity. Accounts Chem. Res. 2013, 46, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Yao, P. Gold nanoparticles with different amino acid surfaces: Serum albumin adsorption, intracellular uptake and cytotoxicity. Colloid Surf. B 2014, 123, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Klingberg, H.; Oddershede, L.B.; Loeschner, K.; Larsen, E.H.; Loft, S.; Møller, P. Uptake of gold nanoparticles in primary human endothelial cells. Toxicol. Res. 2015, 4, 655–666. [Google Scholar] [CrossRef]
- Pyshnaya, I.A.; Razum, K.V.; Poletaeva, J.E.; Pyshnyi, D.V.; Zenkova, M.A.; Ryabchikova, E.I. Comparison of behaviour in different liquids and in cells of gold nanorods and spherical nanoparticles modified by linear polyethyleneimine and bovine serum albumin. BioMed Res. Int. 2014, 2014, 908175. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-A.J.; Sperling, R.A.; Li, J.K.; Yang, T.-Y.; Li, P.-Y.; Zanella, M.; Chang, W.H.; Parak, W.J. Design of an amphiphilic polymer for nanoparticle coating and functionalization. Small 2008, 4, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Geidel, C.; Schmachtel, S.; Riedinger, A.; Pfeiffer, C.; Müllen, K.; Klapper, M.; Parak, W.J. A general synthetic approach for obtaining cationic and anionic inorganic nanoparticles via encapsulation in amphiphilic copolymers. Small 2011, 7, 2929–2934. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.V.; Herst, P.M.; Tan, A.S. Tetrazolium dyes as tools in cell biology: New insights into their cellular reduction. Biotechnol. Ann. Rev. 2005, 11, 127–152. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of size and concentration of gold nanoparticles from UV-VIS spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef] [PubMed]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase liquid-liquid system. J. Chem. Soc. Chem. Commun. 1994, 1994, 801–802. [Google Scholar] [CrossRef]
- von Holt, B.; Kudera, S.; Weiss, A.; Schrader, T.E.; Manna, L.; Parak, W.J.; Braun, M. Ligand exchange of cdse nanocrystals probed by optical spectroscopy in the visible and mid-ir. J. Mater. Chem. 2008, 18, 2728–2732. [Google Scholar] [CrossRef]
- Soliman, M.G.; Pelaz, B.; Parak, W.J.; del Pino, P. Phase transfer and polymer coating methods toward improving the stability of metallic nanoparticles for biological applications. Chem. Mater. 2015, 27, 990–997. [Google Scholar] [CrossRef]
- Tan, G.; Kantner, K.; Zhang, Q.; Soliman, M.G.; Pino, P.d.; Parak, W.J.; Onur, M.A.; Valdepérez, D.; Rejman, J.; Pelaz, B. Conjugation of polymer-coated gold nanoparticles with antibodies—Synthesis and characterization. Nanomaterials 2015, 5, 1297–1316. [Google Scholar] [CrossRef]
- Kreyling, W.G.; Abdelmonem, A.M.; Ali, Z.; Alves, F.; Geiser, M.; Haberl, N.; Hartmann, R.; Hirn, S.; de Aberasturi, D.J.; Kantner, K.; et al. In vivo integrity of polymer-coated gold nanoparticles. Nat. Nano 2015, 10, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, T.; Sperling, R.A.; Alivisatos, A.P.; Parak, W.J. Gel electrophoresis of gold-DNA nanoconjugates. J. Biomed. Biotechnol. 2007, 2007, 26796. [Google Scholar] [CrossRef] [PubMed]
- Rehbock, C.; Merk, V.; Gamrad, L.; Streubel, R.; Barcikowski, S. Size control of laser-fabricated surfactant-free gold nanoparticles with highly diluted electrolytes and their subsequent bioconjugation. Phys. Chem. Chem. Phys. 2013, 15, 3057–3067. [Google Scholar] [CrossRef] [PubMed]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Khlebtsov, N.G.; Dykman, L.A. Optical properties and biomedical applications of plasmonic nanoparticles. J. Quant. Spectrosc. Ra. 2010, 111, 1–35. [Google Scholar] [CrossRef]
- Fu, C.; Yang, H.; Wang, M.; Xiong, H.; Yu, S. Serum albumin adsorbed on au nanoparticles: Structural changes over time induced by s-au interaction. Chem. Commun. 2015, 51, 3634–3636. [Google Scholar] [CrossRef] [PubMed]
- Hühn, D.; Kantner, K.; Geidel, C.; Brandholt, S.; De Cock, I.; Soenen, S.J.H.; Rivera_Gil, P.; Montenegro, J.-M.; Braeckmans, K.; Müllen, K.; et al. Polymer-coated nanoparticles interacting with proteins and cells: Focusing on the sign of the net charge. ACS Nano 2013, 7, 3253–3263. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Díaz, E.; Pfeiffer, C.; Kastl, L.; Rivera-Gil, P.; Simonet, B.; Valcárcel, M.; Jiménez-Lamana, J.; Laborda, F.; Parak, W.J. The toxicity of silver nanoparticles depends on their uptake by cells and thus on their surface chemistry. Part. Part. Syst. Char. 2013, 30, 1079–1085. [Google Scholar] [CrossRef]
- Hauck, T.S.; Ghazani, A.A.; Chan, W.C.W. Assessing the effect of surface chemistry on gold nanorod uptake, toxicity, and gene expression in mammalian cells. Small 2008, 4, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Brewer, S.H.; Glomm, W.R.; Johnson, M.C.; Knag, M.K.; Franzen, S. Probing bsa binding to citrate-coated gold nanoparticles and surfaces. Langmuir 2005, 21, 9303–9307. [Google Scholar] [CrossRef] [PubMed]
- Feliu, N.; Pelaz, B.; Zhang, Q.; Pino, P.d.; Nyström, A.; Parak, W.J. Nanoparticle dosage—A nontrivial task of utmost importance for quantitative nanotoxicology. WIREs Nanomed. Nanobiotechnol. 2015. submitted for publication. [Google Scholar]
- Kumar, A.; Ma, H.; Zhang, X.; Huang, K.; Jin, S.; Liu, J.; Wei, T.; Cao, W.; Zou, G.; Liang, X.J. Gold nanoparticles functionalized with therapeutic and targeted peptides for cancer treatment. Biomaterials 2012, 33, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Khullar, P.; Singh, V.; Mahal, A.; Dave, P.N.; Thakur, S.; Kaur, G.; Singh, J.; Singh Kamboj, S.; Singh Bakshi, M. Bovine serum albumin bioconjugated gold nanoparticles: Synthesis, hemolysis, and cytotoxicity toward cancer cell lines. J. Phys. Chem. C 2012, 116, 8834–8843. [Google Scholar] [CrossRef]
- Choi, S.Y.; Jeong, S.; Jang, S.H.; Park, J.; Park, J.H.; Ock, K.S.; Lee, S.Y.; Joo, S.W. In vitro toxicity of serum protein-adsorbed citrate-reduced gold nanoparticles in human lung adenocarcinoma cells. Toxicol. Vitro 2012, 26, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Soenen, S.J.; Manshian, B.; Montenegro, J.M.; Amin, F.; Meermann, B.; Thiron, T.; Cornelissen, M.; Vanhaecke, F.; Doak, S.; Parak, W.J.; et al. Cytotoxic effects of gold nanoparticles: A multiparametric study. ACS Nano 2012, 6, 5767–5783. [Google Scholar] [CrossRef] [PubMed]
- Chanana, M.; Rivera_Gil, P.; Correa-Duarte, M.A.; Liz-Marzán, L.M.; Parak, W.J. Physicochemical properties of protein-coated gold nanoparticles in biological fluids and cells before and after proteolytic digestion. Angew. Chem. Int. Ed. 2013, 52, 4179–4183. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marisca, O.T.; Kantner, K.; Pfeiffer, C.; Zhang, Q.; Pelaz, B.; Leopold, N.; Parak, W.J.; Rejman, J. Comparison of the in Vitro Uptake and Toxicity of Collagen- and Synthetic Polymer-Coated Gold Nanoparticles. Nanomaterials 2015, 5, 1418-1430. https://doi.org/10.3390/nano5031418
Marisca OT, Kantner K, Pfeiffer C, Zhang Q, Pelaz B, Leopold N, Parak WJ, Rejman J. Comparison of the in Vitro Uptake and Toxicity of Collagen- and Synthetic Polymer-Coated Gold Nanoparticles. Nanomaterials. 2015; 5(3):1418-1430. https://doi.org/10.3390/nano5031418
Chicago/Turabian StyleMarisca, Oana T., Karsten Kantner, Christian Pfeiffer, Qian Zhang, Beatriz Pelaz, Nicolae Leopold, Wolfgang J. Parak, and Joanna Rejman. 2015. "Comparison of the in Vitro Uptake and Toxicity of Collagen- and Synthetic Polymer-Coated Gold Nanoparticles" Nanomaterials 5, no. 3: 1418-1430. https://doi.org/10.3390/nano5031418
APA StyleMarisca, O. T., Kantner, K., Pfeiffer, C., Zhang, Q., Pelaz, B., Leopold, N., Parak, W. J., & Rejman, J. (2015). Comparison of the in Vitro Uptake and Toxicity of Collagen- and Synthetic Polymer-Coated Gold Nanoparticles. Nanomaterials, 5(3), 1418-1430. https://doi.org/10.3390/nano5031418






