Cytotoxicity, Uptake Behaviors, and Oral Absorption of Food Grade Calcium Carbonate Nanomaterials
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization


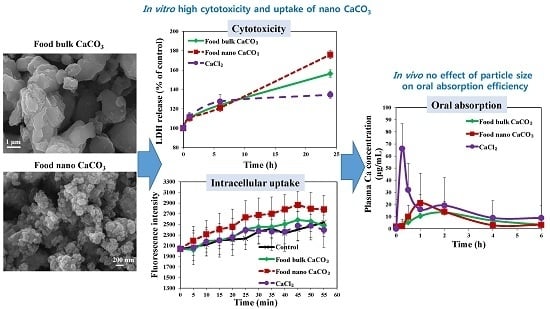
2.2. Cytotoxicity
2.2.1. Cell Proliferation

2.2.2. Reactive oxygen species (ROS) Generation and lactate dehydrogenase (LDH) Release

2.3. Cellular Uptake Behaviors
2.3.1. Cellular Uptake

2.3.2. Intestinal Transport

2.4. Biokinetics

| Biokinetic parameters | Food bulk CaCO3 | Food nano CaCO3 | SS CaCO3 | CaCl2 |
|---|---|---|---|---|
| Cmax (μg/mL) | 13.39 ± 1.63 c | 21.55 ± 6.71 b,c | 31.56 ± 0.99 b | 66.16 ± 12.98 a |
| Tmax (h) | 2.00 c | 1.00 b | 1.00 b | 0.25 a |
| AUC (h × μg/mL) | 63.21 ± 2.04 b | 62.26 ± 2.08 b | 66.40 ± 8.60 b | 120.98 ± 11.14 a |
| T1/2 (h) | 2.50 ± 0.01 c | 2.86 ± 0.22 d | 1.59 ± 0.01 b | 0.97 ± 0.07 a |
| MRT (h) | 4.58 ± 0.18 c | 4.28 ± 0.23 b | 2.94 ± 0.07 a | 2.81 ± 0.20 a |
| Absorption (%) 1 | 4.86 ± 0.16 b | 4.79 ± 0.16 b | 5.11 ± 0.66 b | 8.07 ± 0.74 a |
3. Experimental Section
3.1. Materials and Characterization
3.2. Cell Culture
3.3. Cell Proliferation
3.4. Intracellular ROS Generation
3.5. LDH Leakage
3.6. Cellular Uptake
3.7. Intestinal Transport Mechanism
3.8. Oral Absorption
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Reid, I.R.; Ames, R.W.; Evans, M.C.; Gamble, G.D.; Sharpe, S.J. Effect of calcium supplementation on bone loss in postmenopausal women. N. Engl. J. Med. 1993, 328, 460–464. [Google Scholar] [CrossRef] [PubMed]
- White, E.; Shannon, J.S.; Patterson, R.E. Relationship between vitamin and calcium supplement use and colon cancer. Cancer Epidemiol. Biomarkers Prev. 1997, 6, 769–774. [Google Scholar] [PubMed]
- Straub, D.A. Calcium supplementation in clinical practice: A review of forms, doses, and indications. Nutr. Clin. Pract. 2007, 22, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Hanzlik, R.P.; Fowler, S.C.; Fisher, D.H. Relative bioavailability of calcium from calcium formate, calcium citrate, and calcium carbonate. J. Pharmacol. Exp. Ther. 2005, 313, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Bo-Linn, G.W.; Davis, G.R.; Buddrus, D.J.; Morawski, S.G.; Santa Ana, C.; Fordtran, J.S. An evaluation of the importance of gastric acid secretion in the absorption of dietary calcium. J. Clin. Invest. 1984, 73, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Goss, S.L.; Lemons, K.A.; Kerstetter, J.E.; Bogner, R.H. Determination of calcium salt solubility with changes in pH and PCO2, simulating varying gastrointestinal environments. J. Pharm. Pharmacol. 2007, 59, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-J.; Choy, J.-H. Layered double hydroxide nanoparticles as target-specific delivery carriers: uptake mechanism and toxicity. Nanomedicine-UK 2011, 6, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Canton, I.; Battaglia, G. Endocytosis at the nanoscale. Chem. Soc. Rev. 2012, 41, 2718–2739. [Google Scholar] [CrossRef] [PubMed]
- Bronner, F. Mechanisms of intestinal calcium absorption. J. Cell. Biochem. 2003, 88, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Vilella, A.; Ruozi, B.; Belletti, D.; Pederzoli, F.; Galliani, M.; Semeghini, V.; Forni, F.; Zoli, M.; Vandelli, M.A.; Tosi, G. Endocytosis of Nanomedicines: The Case of Glycopeptide Engineered PLGA Nanoparticles. Pharmaceutics 2015, 7, 74–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, J.M.; Choi, S.J.; Kim, S.T.; Choy, J.H. Cellular uptake mechanism of an inorganic nanovehicle and its drug conjugates: Enhanced efficacy due to clathrin-mediated endocytosis. Bioconjugate Chem. 2006, 17, 1411–1417. [Google Scholar] [CrossRef] [PubMed]
- Oh, N.; Park, J.H. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int. J. Nanomed. 2014, 9, 51–63. [Google Scholar]
- Soni, D.; Naoghare, P.K.; Saravanadevi, S.; Pandey, R.A. Release, transport and toxicity of engineered nanoparticles. Rev. Environ. Contam. Toxicol. 2015, 234, 1–47. [Google Scholar] [PubMed]
- Martirosyan, A.; Schneider, Y.J. Engineered nanomaterials in food: implications for food safety and consumer health. Int. J. Environ. Res. Public Health 2014, 11, 5720–5750. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.S.; Cho, H.S.; Park, S.J.; Song, K.S.; Ahn, K.S.; Cho, M.H.; Kim, J.S. Physico-chemical characterization-based safety evaluation of nanocalcium. Food Chem. Toxicol. 2013, 62, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Lee, J.K.; Jeong, J.; Choy, J.H. Toxicity evaluation of inorganic nanoparticles: considerations and challenges. Mol. Cell. Toxicol. 2013, 9, 205–210. [Google Scholar] [CrossRef]
- Choi, S.J.; Choy, J.H. Biokinetics of zinc oxide nanoparticles: toxicokinetics, biological fates, and protein interaction. Int. J. Nanomed. 2014, 9, 261–269. [Google Scholar]
- Schaumann, G.E.; Philippe, A.; Bundschuh, M.; Metreveli, G.; Klitzke, S.; Rakcheev, D.; Grun, A.; Kumahor, S.K.; Kuhn, M.; Baumann, T.; et al. Understanding the fate and biological effects of Ag- and TiO2-nanoparticles in the environment: The quest for advanced analytics and interdisciplinary concepts. Sci. Total Environ. 2015, 535, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.S.; Chang, J.H.; Wu, J.S. Calcium bioavailability of nanonized pearl powder for adults. J. Food Sci. 2008, 73, H246–H251. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Chen, J.C.; Hsu, C.W.; Chang, W.H. Effects of nano calcium carbonate and nano calcium citrate on toxicity in ICR mice and on bone mineral density in an ovariectomized mice model. Nanotechnology 2009, 20, 375102. [Google Scholar] [CrossRef] [PubMed]
- Shahnazari, M.; Martin, B.R.; Legette, L.L.; Lachcik, P.J.; Welch, J.; Weaver, C.M. Diet calcium level but not calcium supplement particle size affects bone density and mechanical properties in ovariectomized rats. J. Nutr. 2009, 139, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
- Borel, T.; Sabliov, C.M. Nanodelivery of bioactive components for food applications: Types of delivery systems, properties, and their effect on ADME profiles and toxicity of nanoparticles. Annu. Rev. Food Sci. Technol. 2014, 5, 197–213. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Nanoscale Nutrient Delivery Systems for Food Applications: Improving Bioactive Dispersibility, Stability, and Bioavailability. J. Food Sci. 2015, 80, N1602–N1611. [Google Scholar] [CrossRef] [PubMed]
- Rabolli, V.; Thomassen, L.C.; Princen, C.; Napierska, D.; Gonzalez, L.; Kirsch-Volders, M.; Hoet, P.H.; Huaux, F.; Kirschhock, C.E.; Martens, J.A.; et al. Influence of size, surface area and microporosity on the in vitro cytotoxic activity of amorphous silica nanoparticles in different cell types. Nanotoxicology 2010, 4, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.E.; Madler, L.; Velegol, D.; Xia, T.; Hoek, E.M.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Brylev, K.A.; Xu, J.Z.; Mironov, Y.V.; Fedorov, V.E.; Sohn, Y.S.; Kim, S.J.; Choy, J.H. Cellular uptake and cytotoxicity of octahedral rhenium cluster complexes. J. Inorg. Biochem. 2008, 102, 1991–1996. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Paek, H.J.; Yu, J. Oxidative stress by layered double hydroxide nanoparticles via an SFK-JNK and p38-NF-kappaB signaling pathway mediates induction of interleukin-6 and interleukin-8 in human lung epithelial cells. Int. J. Nanomed. 2015, 10, 3217–3229. [Google Scholar] [CrossRef] [PubMed]
- Kerneis, S.; Bogdanova, A.; Kraehenbuhl, J.P.; Pringault, E. Conversion by Peyer’s patch lymphocytes of human enterocytes into M cells that transport bacteria. Science 1997, 277, 949–952. [Google Scholar] [CrossRef] [PubMed]
- Des Rieux, A.; Fievez, V.; Theate, I.; Mast, J.; Preat, V.; Schneider, Y.J. An improved in vitro model of human intestinal follicle-associated epithelium to study nanoparticle transport by M cells. Eur. J. Pharm. Sci. 2007, 30, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Gebert, A. The role of M cells in the protection of mucosal membranes. Histochem. Cell. Biol. 1997, 108, 455–470. [Google Scholar] [CrossRef] [PubMed]
- Gebert, A.; Rothkotter, H.J.; Pabst, R. M cells in Peyer’s patches of the intestine. Int. Rev. Cytol. 1996, 167, 91–159. [Google Scholar] [PubMed]
- Chen, J.F.; Wang, Y.H.; Guo, F.; Wang, X.M.; Zheng, G. Synthesis of nanoparticles with novel technology: high-gravity reactive precipitation. Ind. Eng. Chem. Res. 2000, 39, 948–954. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-K.; Lee, J.-A.; Jo, M.-R.; Kim, M.-K.; Kim, H.-M.; Oh, J.-M.; Song, N.W.; Choi, S.-J. Cytotoxicity, Uptake Behaviors, and Oral Absorption of Food Grade Calcium Carbonate Nanomaterials. Nanomaterials 2015, 5, 1938-1954. https://doi.org/10.3390/nano5041938
Kim M-K, Lee J-A, Jo M-R, Kim M-K, Kim H-M, Oh J-M, Song NW, Choi S-J. Cytotoxicity, Uptake Behaviors, and Oral Absorption of Food Grade Calcium Carbonate Nanomaterials. Nanomaterials. 2015; 5(4):1938-1954. https://doi.org/10.3390/nano5041938
Chicago/Turabian StyleKim, Mi-Kyung, Jeong-A. Lee, Mi-Rae Jo, Min-Kyu Kim, Hyoung-Mi Kim, Jae-Min Oh, Nam Woong Song, and Soo-Jin Choi. 2015. "Cytotoxicity, Uptake Behaviors, and Oral Absorption of Food Grade Calcium Carbonate Nanomaterials" Nanomaterials 5, no. 4: 1938-1954. https://doi.org/10.3390/nano5041938
APA StyleKim, M.-K., Lee, J.-A., Jo, M.-R., Kim, M.-K., Kim, H.-M., Oh, J.-M., Song, N. W., & Choi, S.-J. (2015). Cytotoxicity, Uptake Behaviors, and Oral Absorption of Food Grade Calcium Carbonate Nanomaterials. Nanomaterials, 5(4), 1938-1954. https://doi.org/10.3390/nano5041938