Effect of Saturation Pressure Difference on Metal–Silicide Nanopowder Formation in Thermal Plasma Fabrication
Abstract
:1. Introduction
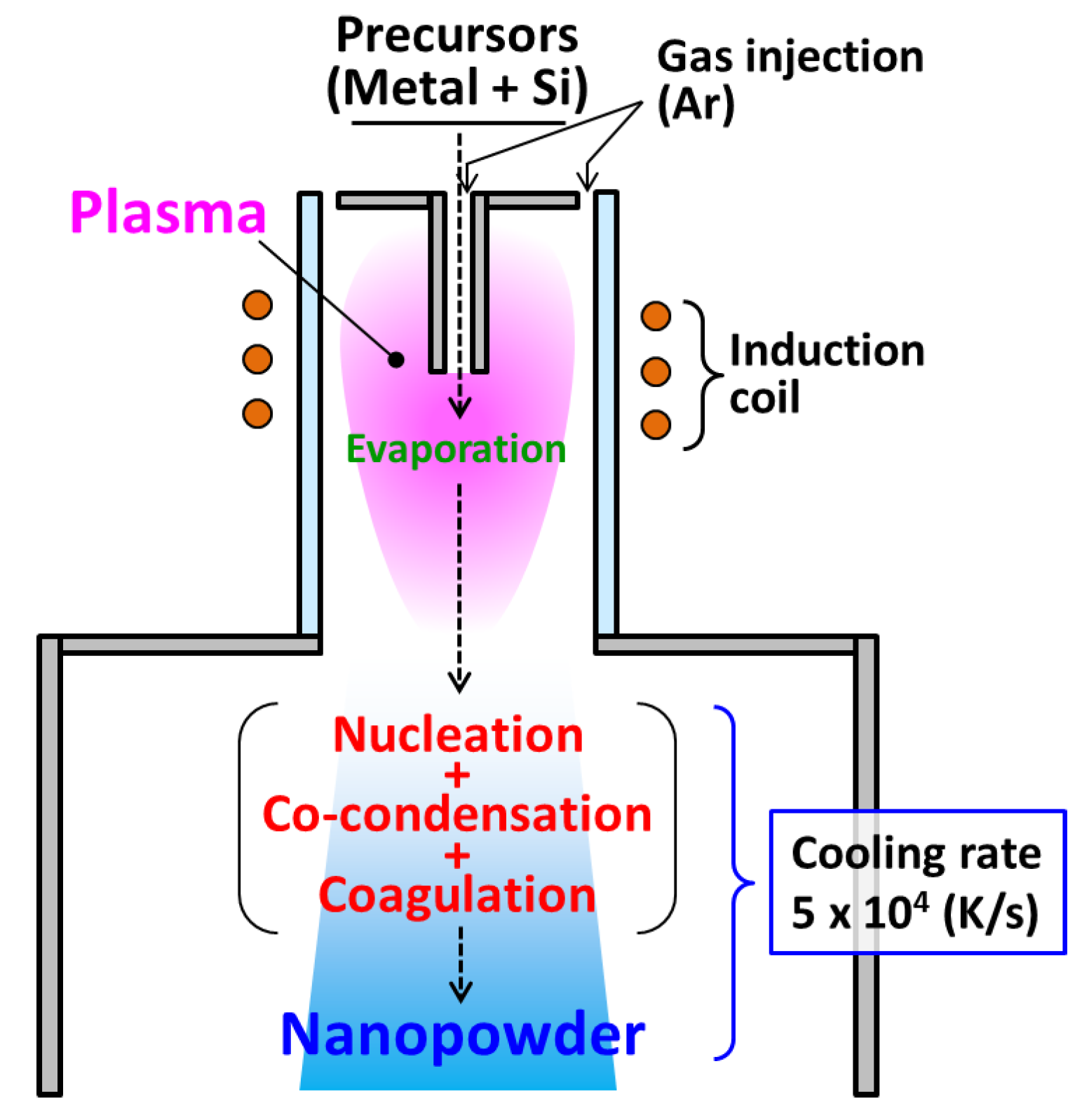
2. Computational Conditions and Strategy
3. Outline of Metal–Silicide Nanopowder Formation Model
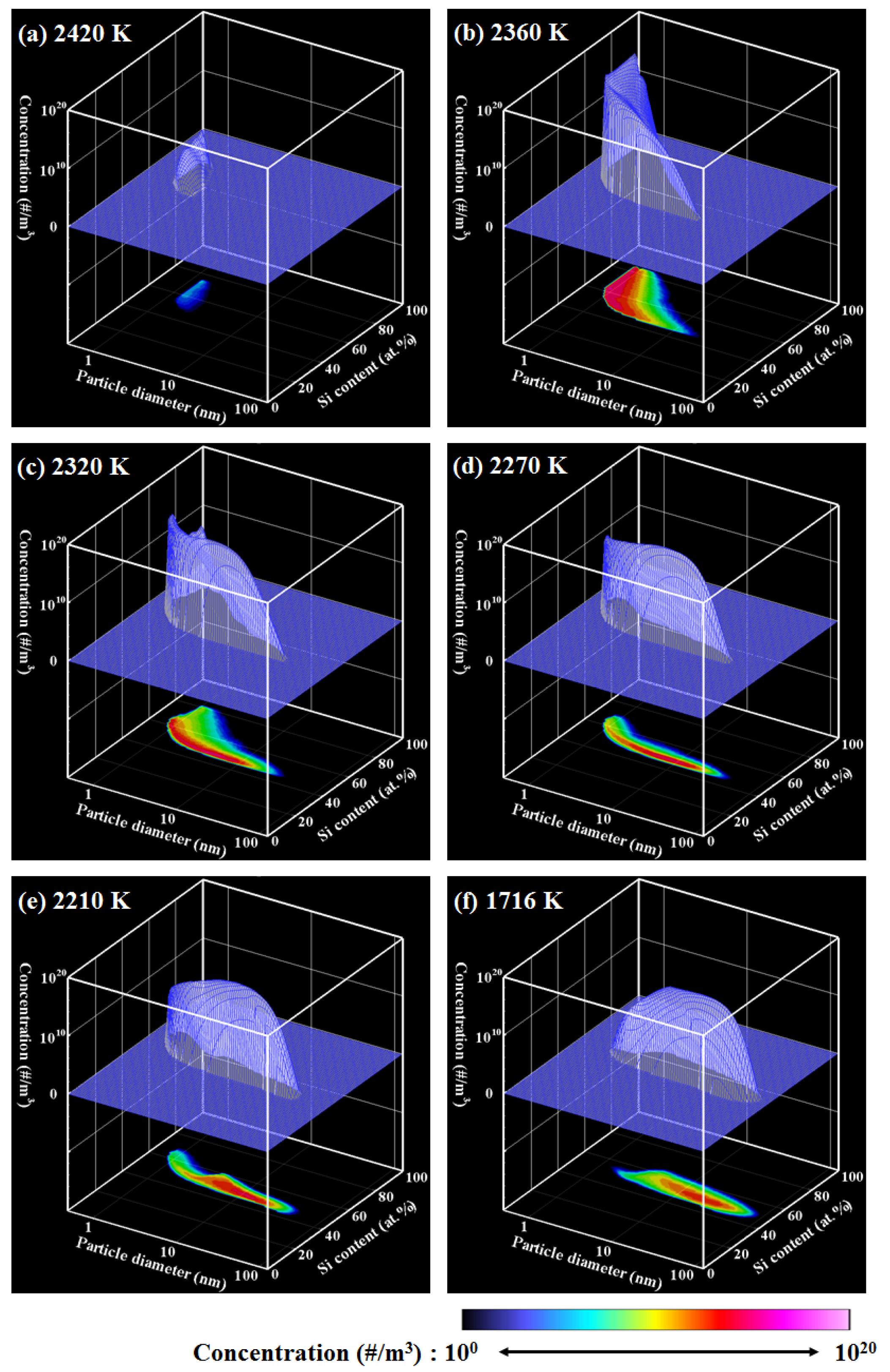
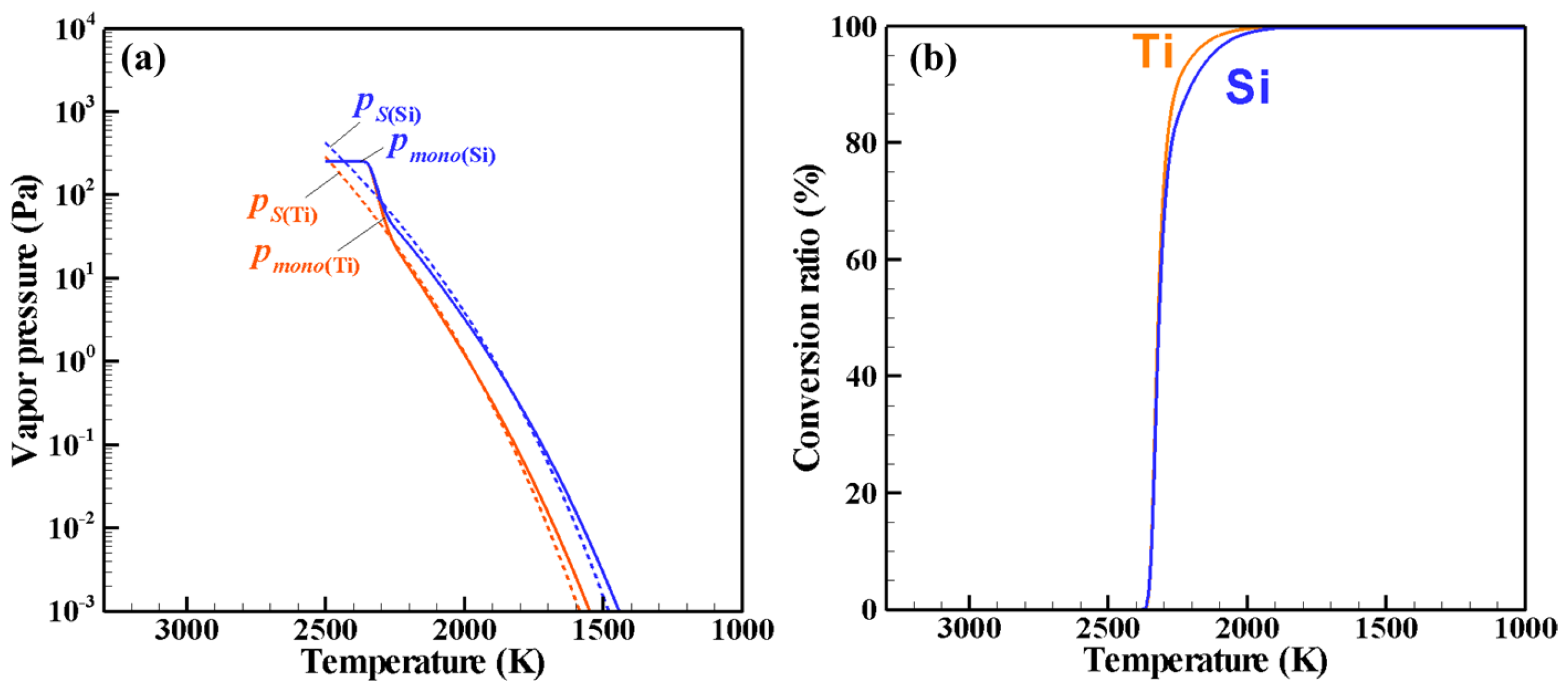
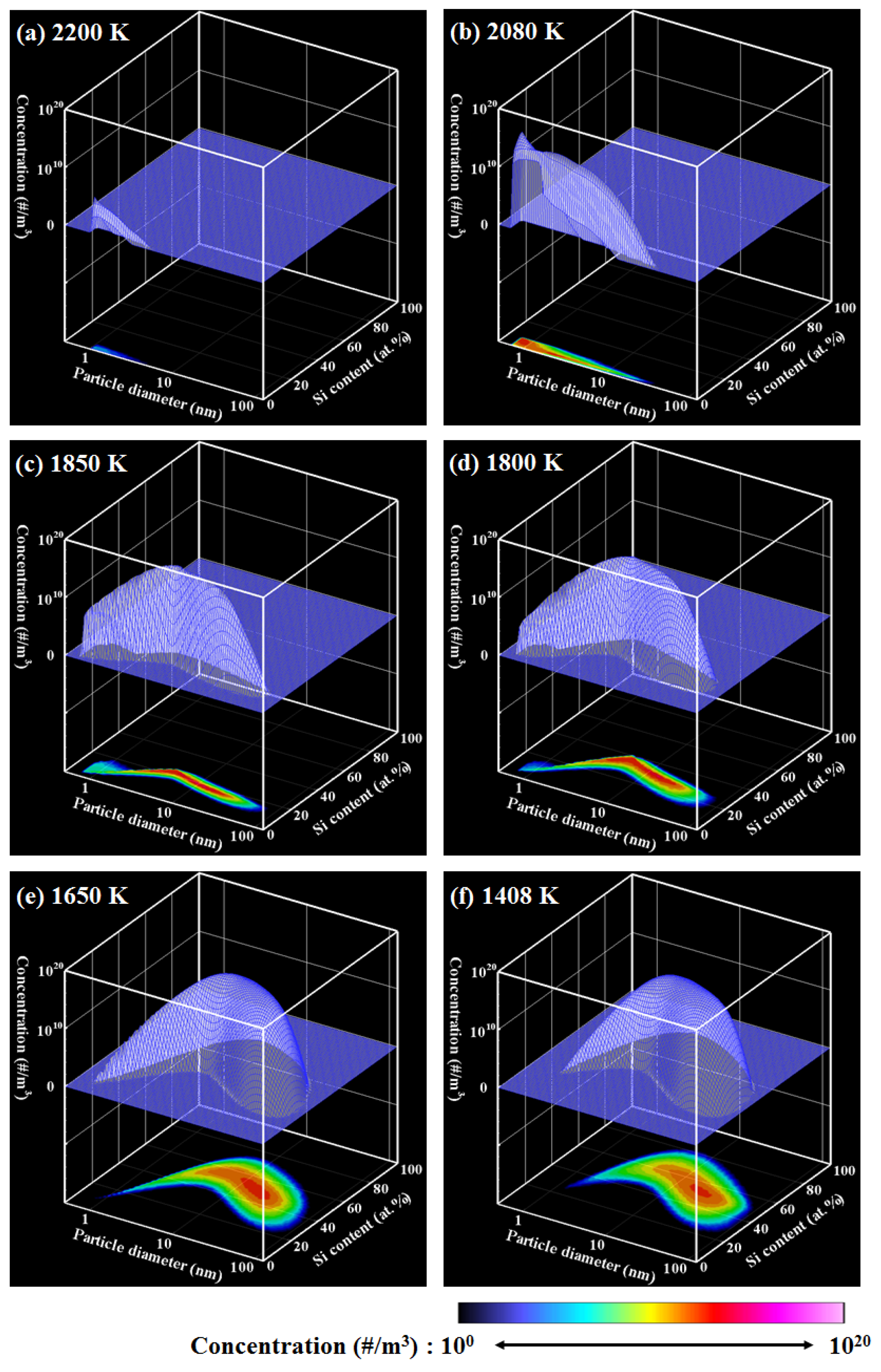
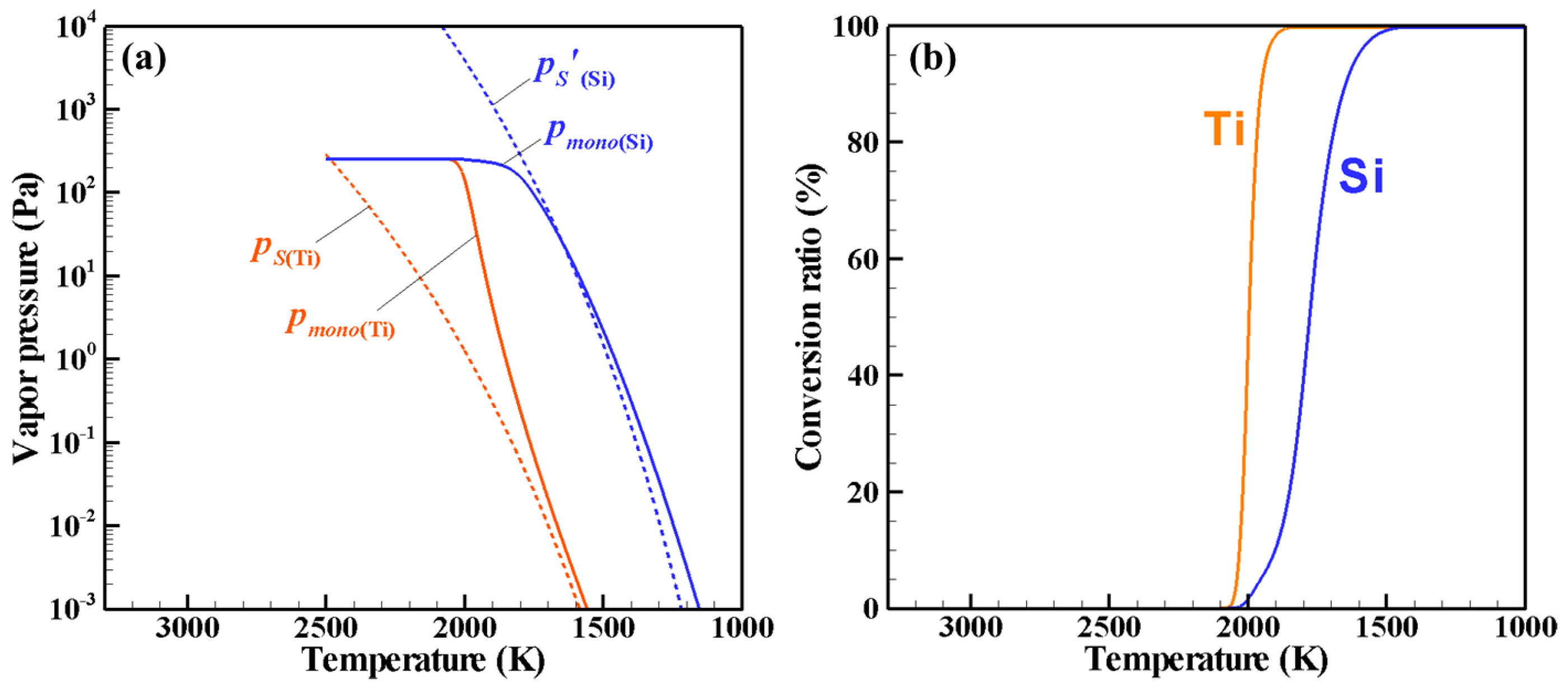
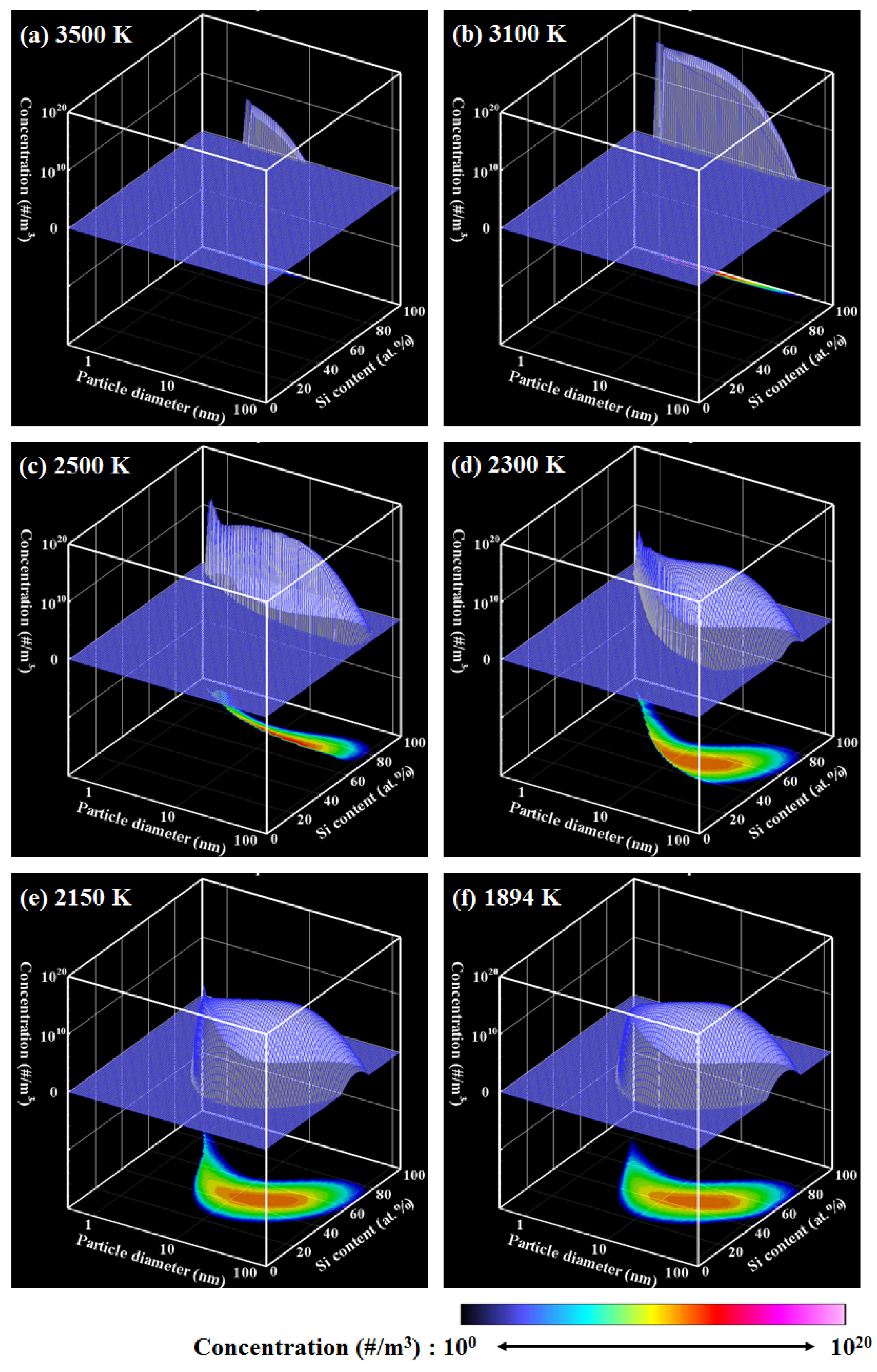
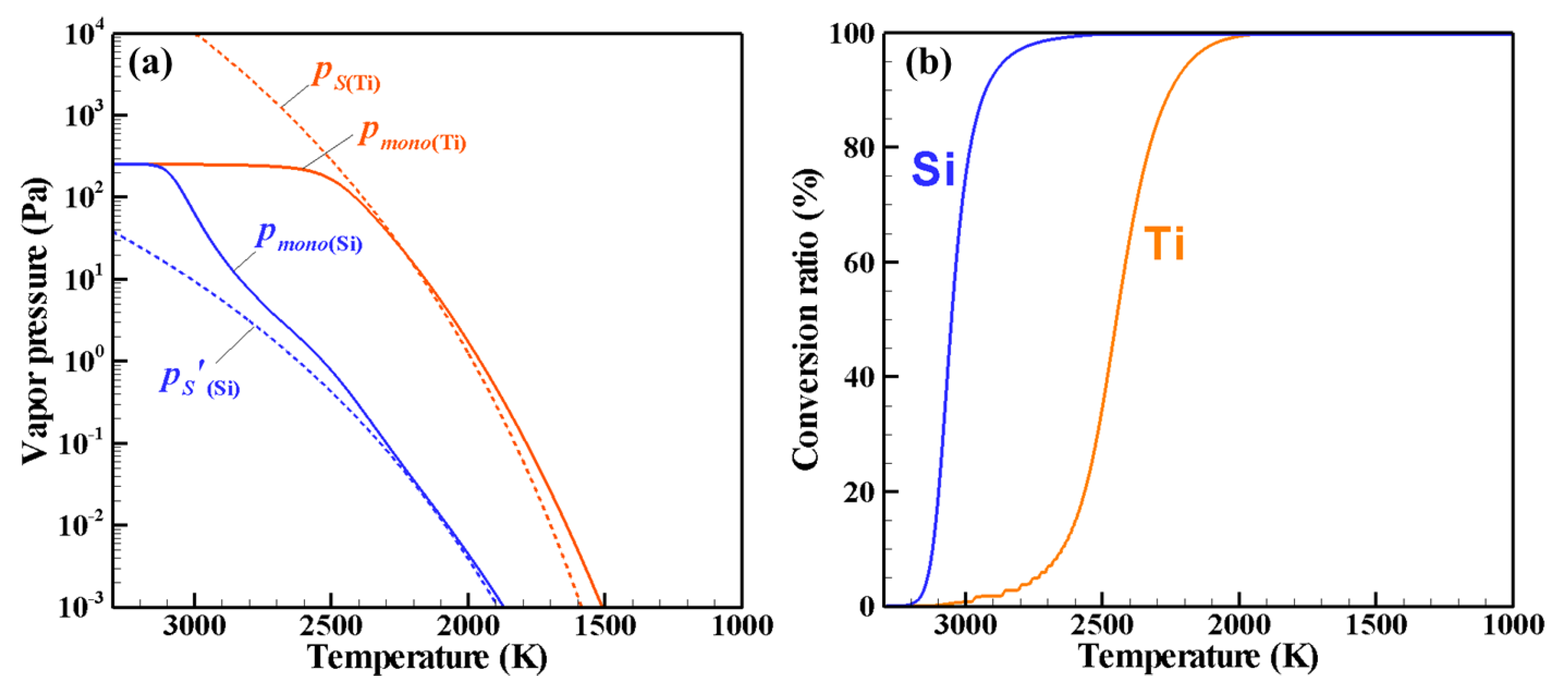
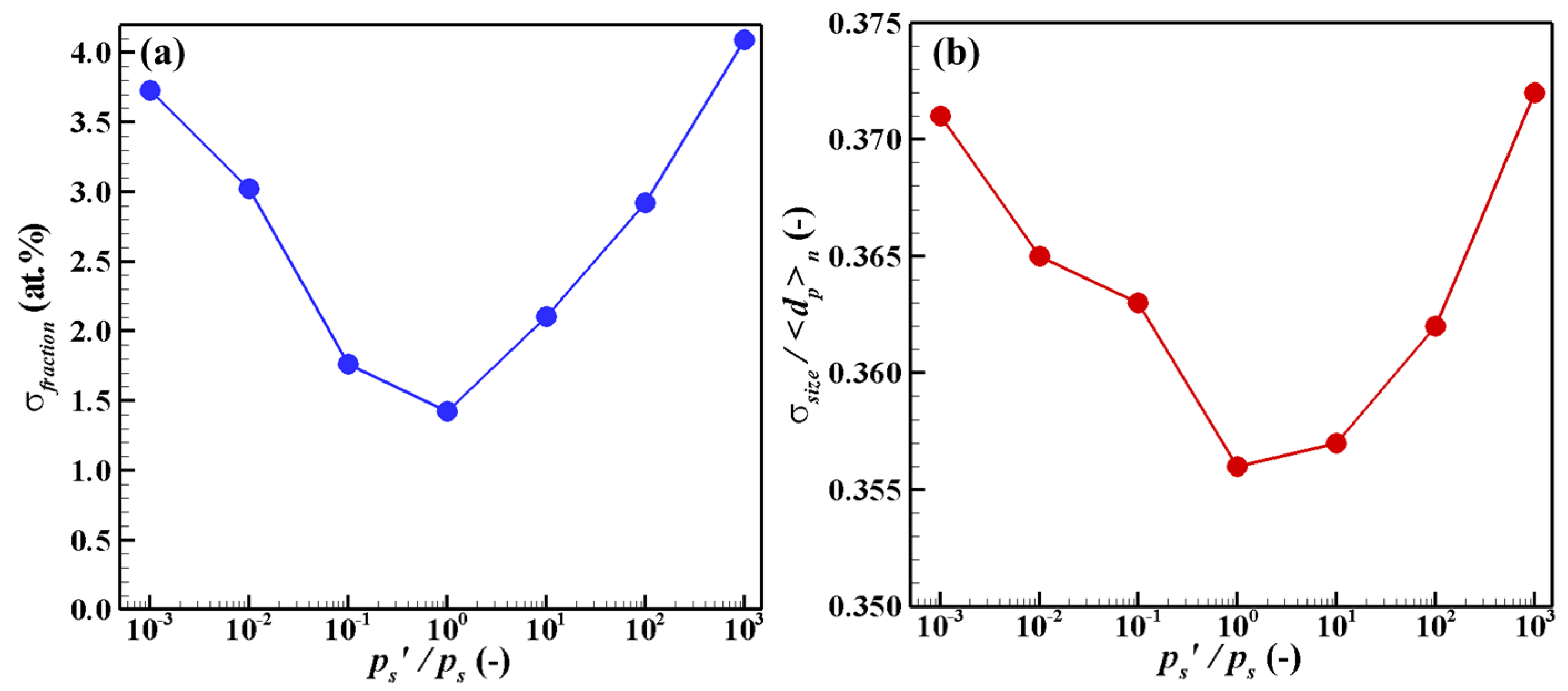
4. Results and Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shigeta, M.; Murphy, A.B. Thermal Plasmas for Nanofabrication. J. Phys. D Appl. Phys. 2011, 44, 174025. [Google Scholar] [CrossRef]
- Siegel, R.W. Synthesis and properties of nanophase materials. Mater. Sci. Eng. 1993, A168, 189–197. [Google Scholar] [CrossRef]
- Matsuura, K.; Hasegawa, T.; Ohmi, T.; Kudoh, M. Synthesis of MoSi2-TiSi2 Pseudobinary Alloys by Reactive Sintering. Metall. Mater. Transact. A 2000, 31, 747–753. [Google Scholar] [CrossRef]
- Kambara, M.; Kitayama, A.; Homma, K.; Hideshima, T.; Kaga, M.; Sheem, K.-Y.; Ishida, S.; Yoshida, T. Nano-composite Si particle formation by plasma spraying for negative electrode of Li ion batteries. J. Appl. Phys. 2014, 115, 143302. [Google Scholar] [CrossRef]
- Sato, T.; Shigeta, M.; Kato, D.; Nishiyama, H. Mixing and magnetic effects on a nonequilibrium argon plasma jet. Int. J. Therm. Sci. 2001, 40, 273–278. [Google Scholar] [CrossRef]
- Shigeta, M.; Sato, T.; Nishiyama, H. Computational simulation of a particle-laden RF inductively coupled plasma with seeded potassium. Int. J. Heat Mass Transf. 2004, 47, 707–716. [Google Scholar] [CrossRef]
- Shigeta, M.; Nishiyama, H. Numerical Analysis of Metallic Nanoparticle Synthesis Using RF Inductively Coupled Plasma Flows. J. Heat Transf. 2005, 127, 1222–1230. [Google Scholar] [CrossRef]
- Fan, X.; Ishigaki, T. Critical free energy for nucleation from the congruent melt of MoSi2. J. Cryst. Growth 1997, 171, 166–173. [Google Scholar] [CrossRef]
- Fan, X.; Ishigaki, T.; Sato, Y. Phase formation in molybdenum disilicide powders during in-flight induction plasma treatment. J. Mater. Res. 1997, 12, 1315–1326. [Google Scholar] [CrossRef]
- Watanabe, T.; Itoh, H.; Ishii, Y. Preparation of ultrafine particles of silicon base intermetallic compound by arc plasma method. Thin Solid Films 2001, 390, 44–50. [Google Scholar] [CrossRef]
- Watanabe, T.; Okumiya, H. Formation mechanism of silicide nanoparticles by induction thermal plasmas. Sci. Technol. Adv. Mater. 2004, 5, 639–646. [Google Scholar] [CrossRef]
- Gerile, N.; Kaga, M.; Kambara, M. Synthesis and Characterization of the Plasma Sprayed Si–Ni Composite Powders as Negative Electrode of lithium-ion Batteries. In Proceedings of the 12th Asia Pacific Physics Conference (APPC12), Makuhari, Japan, 14–19 July 2013.
- Lümmen, N.; Kraska, T. Homogeneous nucleation and growth in iron-platinum vapor investigated by molecular dynamics simulation. Eur. Phys. J. D 2007, 40, 247–260. [Google Scholar] [CrossRef]
- Girshick, S.L.; Chiu, C.-P.; Muno, R.; Wu, C.Y.; Yang, L.; Singh, S.K.; McMurry, P.H. Thermal plasma synthesis of ultrafine iron particles. J. Aerosol Sci. 1993, 24, 367–382. [Google Scholar] [CrossRef]
- Bilodeau, J.F.; Proulx, P. A mathematical model for ultrafine iron powder growth in thermal plasma. Aerosol Sci. Technol. 1996, 24, 175–189. [Google Scholar] [CrossRef]
- Desilets, M.; Bilodeau, J.F.; Proulx, P. Modelling of the reactive synthesis of ultra-fine powders in a thermal plasma reactor. J. Phys. D Appl. Phys. 1997, 30, 1951–1960. [Google Scholar] [CrossRef]
- Cruz, A.C.D.; Munz, R.J. Vapor Phase Synthesis of Fine Particles. IEEE Transact. Plasma Sci. 1997, 25, 1008–1016. [Google Scholar] [CrossRef]
- Murphy, A.B. Formation of titanium nanoparticles from a titanium tetrachloride plasma. J. Phys. D Appl. Phys. 2004, 37, 2841–2847. [Google Scholar] [CrossRef]
- Shigeta, M.; Watanabe, T. Two-dimensional analysis of nanoparticle formation in induction thermal plasmas with counterflow cooling. Thin Solid Films 2008, 516, 4415–4422. [Google Scholar] [CrossRef]
- Shigeta, M.; Watanabe, T. Numerical investigation of cooling effect on platinum nanoparticle formation in inductively coupled thermal plasmas. J. Appl. Phys. 2008, 103, 074903. [Google Scholar] [CrossRef]
- Colombo, V.; Ghedini, E.; Gherardi, M.; Sanibondi, P.; Shigeta, M. A Two-Dimensional nodal model with turbulent effects for the synthesis of Si nano-particles by inductively coupled thermal plasmas. Plasma Sources Sci. Technol. 2012, 21, 025001. [Google Scholar] [CrossRef]
- Shigeta, M.; Watanabe, T. Growth mechanism of silicon-based functional nanoparticles fabricated by inductively coupled thermal plasmas. J. Phys. D Appl. Phys. 2007, 40, 2407–2419. [Google Scholar] [CrossRef]
- Vorobev, A.; Zikanov, O.; Mohanty, P. Modelling of the in-flight synthesis of TaC nanoparticles from liquid precursor in thermal plasma jet. J. Phys. D Appl. Phys. 2008, 41, 085302. [Google Scholar] [CrossRef]
- Vorobev, A.; Zikanov, O.; Mohanty, P. A Co-Condensation Model for In-Flight Synthesis of Metal-Carbide Nanoparticles in Thermal Plasma Jet. J. Therm. Spray Technol. 2008, 17, 956–965. [Google Scholar] [CrossRef]
- Shigeta, M.; Watanabe, T. Two-Directional Nodal Model for Co-Condensation Growth of Multi-Component Nanoparticles in Thermal Plasma Processing. J. Therm. Spray Technol. 2009, 18, 1022–1037. [Google Scholar] [CrossRef]
- Shigeta, M.; Watanabe, T. Growth model of binary alloy nanopowders for thermal plasma synthesis. J. Appl. Phys. 2010, 108, 043306. [Google Scholar] [CrossRef]
- Cheng, Y.; Shigeta, M.; Choi, S.; Watanabe, T. Formation Mechanism of Titanium Boride Nanoparticles by RF Induction Thermal Plasma. Chem. Eng. J. 2012, 183, 483–491. [Google Scholar] [CrossRef]
- Shigeta, M.; Watanabe, T. Effect of precursor fraction on silicide nanopowder growth under thermal plasma conditions: a computational study. Powder Technol. 2016, 288, 191–201. [Google Scholar] [CrossRef]
- Shigeta, M.; Watanabe, T. Numerical analysis for co-condensation processes in silicide nanoparticle synthesis using induction thermal plasma at atmospheric pressure conditions. J. Mater. Res. 2005, 20, 2801–2811. [Google Scholar] [CrossRef]
- The Japan Institute of Metals. Metal Data Book; Maruzen: Tokyo, Japan, 1993; pp. 10–91. (In Japanese) [Google Scholar]
- Wyslouzil, B.E.; Wilemski, G. Binary nucleation kinetics. II. Numerical solution of the birth–death equations. J. Chem. Phys. 1995, 103, 1137–1151. [Google Scholar] [CrossRef]
- Massalski, T.B. Binary Alloy Phase Diagrams, 2nd ed.; American Society for Metals: Materials Park, OH, USA, 1990; pp. 3367–3371. [Google Scholar]
- Wautelet, M.; Dauchot, J.P.; Hecq, M. Phase diagrams of small particles of binary systems: A theoretical approach. Nanotechnology 2000, 11, 6–9. [Google Scholar] [CrossRef]
- Friedlander, S.K. Smoke, Dust and Haze, Fundamentals of Aerosol Dynamics, 2nd ed.; Oxford University Press: New York, NY, USA, 2000; p. 280. [Google Scholar]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shigeta, M.; Watanabe, T. Effect of Saturation Pressure Difference on Metal–Silicide Nanopowder Formation in Thermal Plasma Fabrication. Nanomaterials 2016, 6, 43. https://doi.org/10.3390/nano6030043
Shigeta M, Watanabe T. Effect of Saturation Pressure Difference on Metal–Silicide Nanopowder Formation in Thermal Plasma Fabrication. Nanomaterials. 2016; 6(3):43. https://doi.org/10.3390/nano6030043
Chicago/Turabian StyleShigeta, Masaya, and Takayuki Watanabe. 2016. "Effect of Saturation Pressure Difference on Metal–Silicide Nanopowder Formation in Thermal Plasma Fabrication" Nanomaterials 6, no. 3: 43. https://doi.org/10.3390/nano6030043
APA StyleShigeta, M., & Watanabe, T. (2016). Effect of Saturation Pressure Difference on Metal–Silicide Nanopowder Formation in Thermal Plasma Fabrication. Nanomaterials, 6(3), 43. https://doi.org/10.3390/nano6030043





