Fe3+-Doped TiO2 Nanotube Arrays on Ti-Fe Alloys for Enhanced Photoelectrocatalytic Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphological Characterization
2.2. Crystalline Structure Characterization
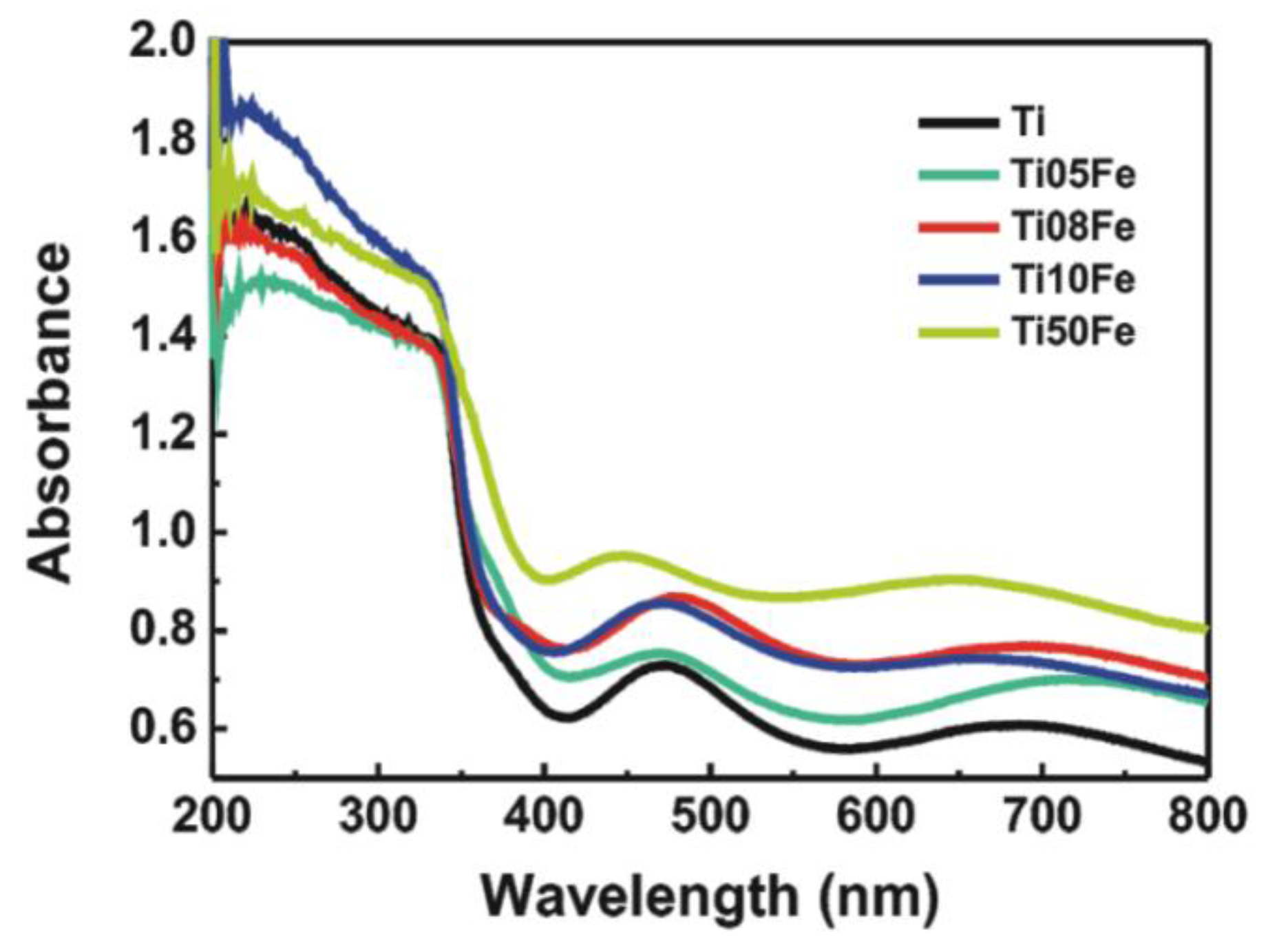
2.3. UV-Vis Diffuse Reflectance Spectra
2.4. Photoelectrochemical and Photoelectrocatalytic Activity
3. Experimental Section
3.1. Preparation of Fe-TNTs
3.2. Characterization of Fe-TNTs
3.3. Photoelectrochemical and Electrochemical Measurement
3.4. Photoelectrocatalytic Measurement
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Macdonald, T.J.; Tune, D.D.; Dewi, M.R.; Gibson, C.T.; Shapter, J.G.; Nann, T. A TiO2 Nanofiber–Carbon Nanotube–Composite Photoanode for Improved Efficiency in Dye–Sensitized Solar Cells. ChemSusChem 2015, 8, 3396–3400. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, T.J.; Xu, J.; Elmas, S.; Mange, Y.J.; Skinner, W.M.; Xu, H.; Nann, T. NiO Nanofibers as a Candidate for a Nanophotocathode. Nanomaterials 2014, 4, 256–266. [Google Scholar] [CrossRef]
- Tran, D.P.; Macdonald, T.J.; Wolfrum, B.; Stockmann, R.; Nann, T.; Offenhäusser, A.; Thierry, B. Photoresponsive properties of ultrathin silicon nanowires. Appl. Phys. Lett. 2014, 105. [Google Scholar] [CrossRef]
- Tune, D.D.; Flavel, B.S.; Krupke, R.; Shapter, J.G. Carbon Nanotube-Silicon Solar Cells. Adv. Energy Mater. 2012, 2, 1043–1055. [Google Scholar] [CrossRef]
- Macdonald, T.J.; Tune, D.D.; Dewi, M.R.; Bear, J.C.; McNaughter, P.D.; Mayes, A.G.; Skinner, W.M.; Parkin, I.P.; Shapter, J.G.; Nann, T. SWCNT photocathodes sensitised with InP/ZnS core–shell nanocrystals. J. Mater. Chem. C 2016, 4, 3379–3384. [Google Scholar] [CrossRef]
- Gong, D.; Grimes, C.A.; Varghese, O.K.; Hu, W.; Singh, R.; Chen, Z.; Dickey, E.C. Titanium oxide nanotube arrays prepared by anodic oxidation. J. Mater. Res. 2001, 16, 3331–3334. [Google Scholar] [CrossRef]
- Xie, K.; Sun, L.; Wang, C.; Lai, Y.; Wang, M.; Chen, H.; Lin, C. Photoelectrocatalytic properties of Ag nanoparticles loaded TiO2 nanotube arrays prepared by pulse current deposition. Electrochim. Acta 2010, 55, 7211–7218. [Google Scholar] [CrossRef]
- Jang, J.S.; Choi, S.H.; Kim, D.H.; Jang, J.W.; Lee, K.S.; Lee, J.S. Enhanced photocatalytic hydrogen production from water−methanol solution by nickel intercalated into titanate nanotube. J. Phys. Chem. C 2009, 113, 8990–8996. [Google Scholar] [CrossRef]
- Yang, Y.; Lee, K.; Kado, Y.; Schmuki, P. Nb-doping of TiO2/SrTiO3 nanotubular heterostructures for enhanced photocatalytic water splitting. Electrochem. Commun. 2012, 17, 56–59. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, Z. High photoelectrochemical water splitting performance on nitrogen doped double-wall TiO2 nanotube array electrodes. Int. J. Hydrogen Energy 2011, 36, 13481–13487. [Google Scholar] [CrossRef]
- Murdoch, M.; Waterhouse, G.; Nadeem, M.; Metson, J.; Keane, M.; Howe, R.; Llorca, J.; Idriss, H. The effect of gold loading and particle size on photocatalytic hydrogen production from ethanol over Au/TiO2 nanoparticles. Nat. Chem. 2011, 3, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Su, Y.; Yu, J.; Xiao, W.; Sun, L.; Lin, C. Enhanced photoelectrocatalytic hydrogen production activity of SrTiO3–TiO2 hetero-nanoparticle modified TiO2 nanotube arrays. Int. J. Hydrogen Energy 2015, 40, 9704–9712. [Google Scholar] [CrossRef]
- Yu, J.; Gong, C.; Wu, Z.; Wu, Y.; Xiao, W.; Su, Y.; Sun, L.; Lin, C. Efficient visible light-induced photoelectrocatalytic hydrogen production using CdS sensitized TiO2 nanorods on TiO2 nanotube arrays. J. Mater. Chem. A 2015, 3, 22218–22226. [Google Scholar] [CrossRef]
- Mor, G.K.; Shankar, K.; Paulose, M.; Varghese, O.K.; Grimes, C.A. Use of highly-ordered TiO2 nanotube arrays in dye-sensitized solar cells. Nano Lett. 2006, 6, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Mor, G.K.; Prakasam, H.E.; Varghese, O.K.; Shankar, K.; Grimes, C.A. Vertically oriented Ti-Fe-O nanotube array films: Toward a useful material architecture for solar spectrum water photoelectrolysis. Nano Lett. 2007, 7, 2356–2364. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Wu, Z.; Wu, Y.; Yu, J.; Sun, L.; Lin, C. Acid Orange II degradation through a heterogeneous Fenton-like reaction using Fe–TiO2 nanotube arrays as a photocatalyst. J. Mater. Chem. A 2015, 3, 8537–8544. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Böttcher, C.; Bahnemann, D.W.; Dohrmann, J.K. A comparative study of nanometer sized Fe (III)-doped TiO2 photocatalysts: Synthesis, characterization and activity. J. Mater. Chem. 2003, 13, 2322–2329. [Google Scholar] [CrossRef]
- Nair, P.B.; Justinvictor, V.; Daniel, G.P.; Joy, K.; Ramakrishnan, V.; Kumar, D.D.; Thomas, P. Structural, optical, photoluminescence and photocatalytic investigations on Fe doped TiO2 thin films. Thin Solid Films 2014, 550, 121–127. [Google Scholar] [CrossRef]
- Sun, L.; Li, J.; Wang, C.; Li, S.; Chen, H.; Lin, C. An electrochemical strategy of doping Fe3+ into TiO2 nanotube array films for enhancement in photocatalytic activity. Sol. Energy Mater. Sol. Cells 2009, 93, 1875–1880. [Google Scholar] [CrossRef]
- Wu, Q.; Ouyang, J.; Xie, K.; Sun, L.; Wang, M.; Lin, C. Ultrasound-assisted synthesis and visible-light-driven photocatalytic activity of Fe-incorporated TiO2 nanotube array photocatalysts. J. Hazard. Mater. 2012, 199, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Allam, N.K.; Alamgir, F.; El-Sayed, M.A. Enhanced photoassisted water electrolysis using vertically oriented anodically fabricated Ti–Nb–Zr–O mixed oxide nanotube arrays. ACS Nano 2010, 4, 5819–5826. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Liu, S.; Weng, L.; Liu, Y.; Liu, B. Growth, structure and photocatalytic properties of hierarchical Cu–Ti–O nanotube arrays by anodization. J. Alloys Compd. 2010, 501, 333–338. [Google Scholar] [CrossRef]
- Nah, Y.-C.; Ghicov, A.; Kim, D.; Berger, S.; Schmuki, P. TiO2–WO3 composite nanotubes by alloy anodization: Growth and enhanced electrochromic properties. J. Am. Chem. Soc. 2008, 130, 16154–16155. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, J.; Kang, Y.; Li, L.; Xu, X. Photoelectrochemical properties of Fe-doped TiO2 nanotube arrays fabricated by anodization. J. Appl. Electrochem. 2014, 44, 1–4. [Google Scholar] [CrossRef]
- Tu, Y.-F.; Huang, S.-Y.; Sang, J.-P.; Zou, X.-W. Preparation of Fe-doped TiO2 nanotube arrays and their photocatalytic activities under visible light. Mater. Res. Bull. 2010, 45, 224–229. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, F.; Zhang, J.; Chen, H.; Anpo, M. Fe3+-TiO2 photocatalysts prepared by combining sol–gel method with hydrothermal treatment and their characterization. J. Photochem. Photobiol. A 2006, 180, 196–204. [Google Scholar] [CrossRef]
- Tong, T.; Zhang, J.; Tian, B.; Chen, F.; He, D. Preparation of Fe3+-doped TiO2 catalysts by controlled hydrolysis of titanium alkoxide and study on their photocatalytic activity for methyl orange degradation. J. Hazard. Mater. 2008, 155, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Sun, L.; Chen, Y.; Zhuang, H.; Lin, C.; Chin, J.W. Effects of the structure of TiO2 nanotube array on Ti substrate on its photocatalytic activity. J. Electrochem. Soc. 2006, 153, D123–D127. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, J. Visible-light-induced photoelectrochemical behaviors of Fe-modified TiO2 nanotube arrays. Nanoscale 2011, 3, 3138–3144. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xiang, Q.; Zhou, M. Preparation, characterization and visible-light-driven photocatalytic activity of Fe-doped titania nanorods and first-principles study for electronic structures. Appl. Catal. B 2009, 90, 595–602. [Google Scholar] [CrossRef]
- Yang, Y.; Tian, C. Effects of calcining temperature on photocatalytic activity of Fe-doped sulfated titania. Photochem. Photobiol. 2012, 88, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Litter, M.; Navio, J.A. Photocatalytic properties of iron-doped titania semiconductors. J. Photochem Photobiol. A 1996, 98, 171–181. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, S.; Wu, M.; Wu, H.; Zhang, J.; Li, W.; Cao, C. Photoelectrocatalytic degradation of sulfosalicylic acid and its electrochemical impedance spectroscopy investigation. J. Phys. Chem. A 2000, 104, 7016–7020. [Google Scholar] [CrossRef]
- Liu, H.; Li, X.; Leng, Y.; Li, W. An alternative approach to ascertain the rate-determining steps of TiO2 photoelectrocatalytic reaction by electrochemical impedance spectroscopy. J. Phys. Chem. B 2003, 107, 8988–8996. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Tomkiewicz, M. Scaling properties in photocatalysis. Catal. Today 2000, 58, 115–123. [Google Scholar] [CrossRef]
- Harifi, T.; Montazer, M. Fe3+: Ag/TiO2 nanocomposite: Synthesis, characterization and photocatalytic activity under UV and visible light irradiation. Appl. Catal. A 2014, 473, 104–115. [Google Scholar] [CrossRef]
- Mizushima, K.; Tanaka, M.; Asai, A.; Iida, S.; Goodenough, J.B. Impurity levels of iron-group ions in TiO2 (II). J. Phys. Chem. Solids 1979, 40, 1129–1140. [Google Scholar] [CrossRef]
- Mizushima, K.; Tanaka, M.; Iida, S. Energy levels of iron group impurities in TiO2. J. Phys. Soc. Jap. 1972, 32, 1519–1524. [Google Scholar] [CrossRef]
- Yogi, C.; Kojima, K.; Wada, N.; Tokumoto, H.; Takai, T.; Mizoguchi, T.; Tamiaki, H. Photocatalytic degradation of methylene blue by TiO2 film and Au particles-TiO2 composite film. Thin Solid Films 2008, 516, 5881–5884. [Google Scholar] [CrossRef]
- Mo, S.-D.; Lin, L.; Lin, D. Electron states of iron group impurities in doped rutile (TiO2). J. Phys. Chem. Solids 1994, 55, 1309–1313. [Google Scholar]
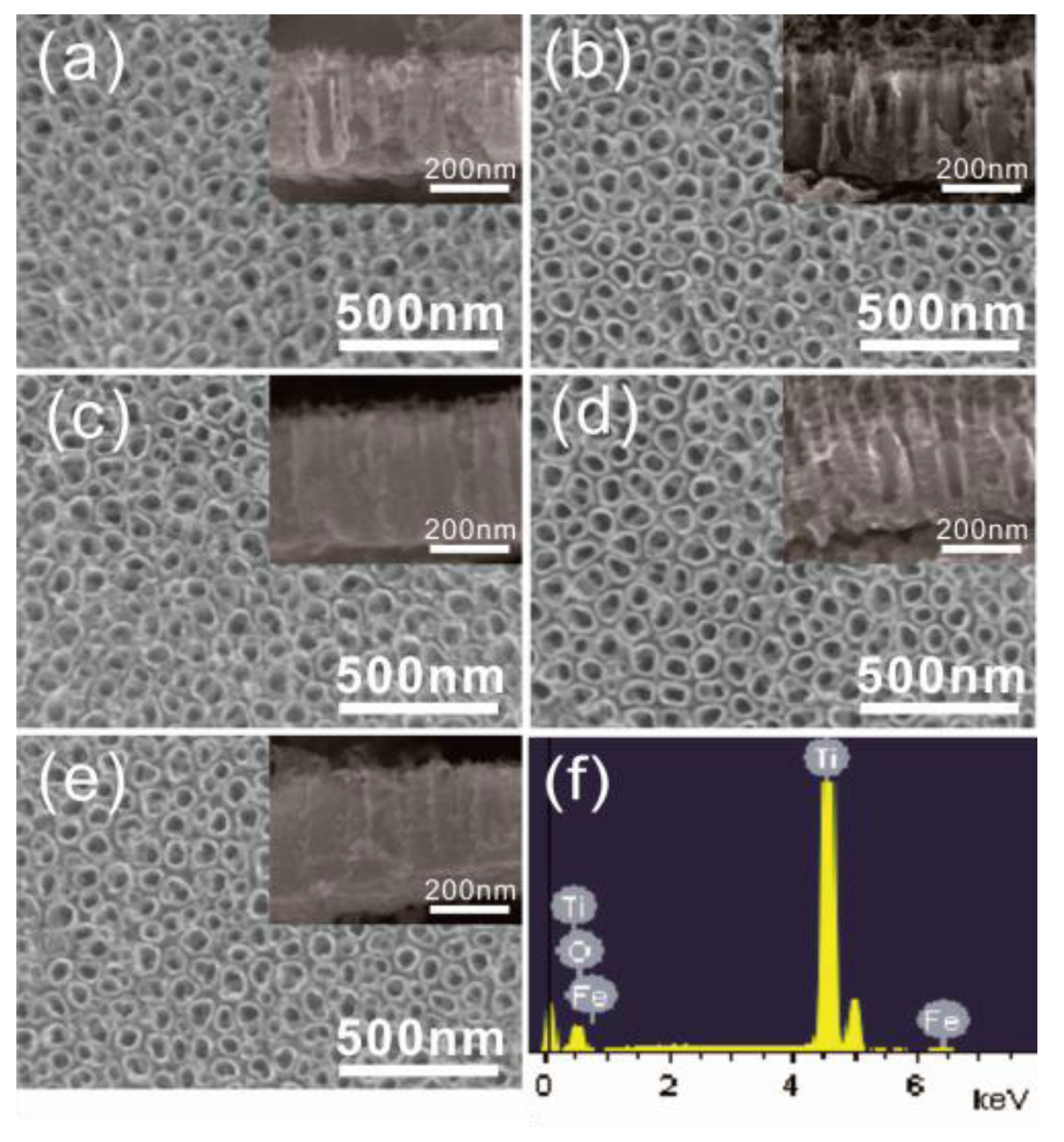








| Samples | C | F | Ti | O | Fe |
|---|---|---|---|---|---|
| Ti05Fe | 2.66 | 2.52 | 42.65 | 51.61 | 0.55 |
| Ti08Fe | 3.13 | 2.14 | 42.19 | 51.67 | 0.87 |
| Ti10Fe | 3.20 | 2.60 | 41.66 | 51.43 | 1.11 |
| Ti50Fe | 3.04 | 0.81 | 38.61 | 52.70 | 4.83 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Wu, Z.; Gong, C.; Xiao, W.; Sun, L.; Lin, C. Fe3+-Doped TiO2 Nanotube Arrays on Ti-Fe Alloys for Enhanced Photoelectrocatalytic Activity. Nanomaterials 2016, 6, 107. https://doi.org/10.3390/nano6060107
Yu J, Wu Z, Gong C, Xiao W, Sun L, Lin C. Fe3+-Doped TiO2 Nanotube Arrays on Ti-Fe Alloys for Enhanced Photoelectrocatalytic Activity. Nanomaterials. 2016; 6(6):107. https://doi.org/10.3390/nano6060107
Chicago/Turabian StyleYu, Jiangdong, Zhi Wu, Cheng Gong, Wang Xiao, Lan Sun, and Changjian Lin. 2016. "Fe3+-Doped TiO2 Nanotube Arrays on Ti-Fe Alloys for Enhanced Photoelectrocatalytic Activity" Nanomaterials 6, no. 6: 107. https://doi.org/10.3390/nano6060107





