Pregnancy Vaccination with Gold Glyco-Nanoparticles Carrying Listeria monocytogenes Peptides Protects against Listeriosis and Brain- and Cutaneous-Associated Morbidities
Abstract
:1. Introduction
2. Results
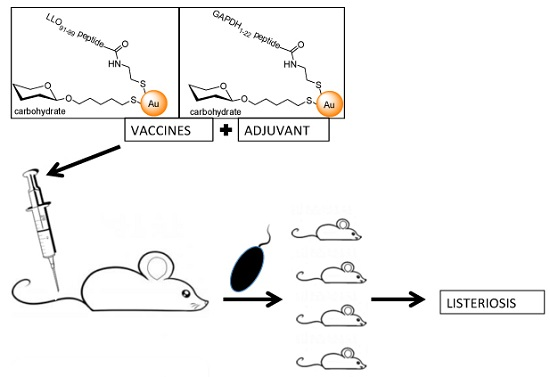
2.1. Nanoparticle Vaccine Effectiveness in Pregnancy Amilorates Listeriosis-Associated Morbidities
2.2. Nanoparticles Vaccine Reduced the Number of Viable Bacteria in Microglia and Pro-Inflammatory Cytokine Production
2.3. Nanoparticle Vaccines Shifted a Th2 Cytokine Pattern to Th1 Production
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| GFP | green fluorescence protein |
| CFU | colony forming unit |
| CNS | central nervous system |
| GAPDH | glyceraldehyde-3-phosphate-dehydrogenase |
| GNP | glyconanoparticle |
| IFN | interferon |
| i.v | intravenously |
| LLO | listeriolysin O |
| LM | Listeria monocytogenes |
References
- Mylonakis, E.; Paliou, M.; Hohmann, E.L.; Calderwood, S.B.; Wing, E.J. Listeriosis during pregnancy: A case series and review of 222 cases. Medicine 2002, 81, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Allerberger, F.; Wagner, M. Listeriosis: A resurgent foodborne infection. Clin. Microbiol. Infect. 2010, 16, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, R.; Almeida, G.; Ferreira, V.; Santos, I.; Silva, J.; Mendes, M.M.; Pita, J.; Mariano, G.; Mancio, I.; Sousa, M.M.; et al. Cheese-related listeriosis outbreak, Portugal, March 2009 to February 2012. Euro Surveill. 2015, 20, 1–6. [Google Scholar] [CrossRef]
- Pérez-Trallero, E.; Zigorraga, C.; Artieda, J.; Alkorta, M.; Marimón, J.M. Two outbreaks of Listeria monocytogenes infection, Northern Spain. Emerg. Infect. Dis. 2014, 20, 2155–2157. [Google Scholar] [CrossRef] [PubMed]
- Peña-Sagredo, J.L.; Hernández, M.V.; Fernandez-Llanio, N.; Giménez-Ubeda, E.; Muñoz-Fernandez, S.; Ortiz, A.; Gonzalez-Gay, M.A.; Fariñas, M.C.; Biobadaser, G. Listeria monocytogenes infection in patients with rheumatic diseases on TNF-alpha antagonist therapy: The spanish study group experience. Clin. Exp. Rheumatol. 2008, 26, 854–859. [Google Scholar] [PubMed]
- Fernandez-Sabe, N.; Cervera, C.; Lopez-Medrano, F.; Llano, M.; Sáez, E.; Len, O.; Fortún, J.; Blanes, M.; Laporta, R.; Torre-Cisneros, J. Risk factors, clinical features, and outcomes of listeriosis in solid-organ transplant recipients: a matched case-control study. Clin. Infect. Dis. 2009, 49, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Ariza-Miguel, J.; Fernández-Natal, M.I.; Soriano, F.; Hernández, M.; Stessl, B.; Rodríguez-Lázaro, D. Molecular epidemiology of invasive listeriosis due to Listeria monocytogenes in a spanish hospital over a nine-year study period, 2006-2014. Biomed. Res. Int. 2015. [Google Scholar] [CrossRef] [PubMed]
- Calderon-Gonzalez, R.; Frande-Cabanes, E.; Bronchalo-Vicente, L.; Lecea-Cuello, M.J.; Pareja, E.; Bosch-Martinez, A.; Fanarraga, M.L.; Yañez-Diaz, S.; Carrasco-Marin, E.; Alvarez-Dominguez, C. Cellular vaccines in listeriosis: Role of the Listeria antigen GAPDH. Front. Cell. Infect. Microbiol. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Calderon-Gonzalez, R.; Frande-Cabanes, E.; Tobes, R.; Pareja, E.; Alaez-Alvarez, L.; Alvarez-Dominguez, C. A dendritic cell targetted vaccine loaded with a glyceraldehyde-3-phosphate-dehydrogenase peptide proposed for individuals at high risk of listeriosis. J. Vaccines Vaccin. 2015, 6. [Google Scholar] [CrossRef]
- Calderon-Gonzalez, R.; Tobes, R.; Pareja, E.; Frande-Cabanes, E.; Alaez-Alvarez, L.; Petrovsky, N.; Alvarez-Dominguez, C. Identification and characterisation of T-cell epitopes for incorporation into dendritic cell-delivered Listeria vaccines. J. Immunol. Methods 2015, 9, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Del Rio, E.; Marradi, M.; Calderon-Gonzalez, R.; Frande-Cabanes, E.; Penades, S.; Petrovsky, N.; Alvarez-Dominguez, C. A gold-glyconanoparticle carrying a listeriolysin O peptide and formulated with Advax™ delta inulin adjuvant induces robust T-cell protection against Listeria infection. Vaccine 2015, 33, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.M.; Simon, J.K.; Baker, J.R. Applications of nanotechnology for immunology. Nat. Rev. Immunol. 2013, 13, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Irvine, D.J.; Hanso, M.C.; Rakhra, K.; Tokatlian, T. Synthetic nanoparticles for vaccines and immunotherapy. Chem. Rev. 2015, 115, 11109–11146. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Seth, A.; Wibowo, N.; Zhao, C.X.; Mitter, N.; Yu, C.Z.; Anton, P.J.M. Nanoparticle vaccines. Vaccine 2014, 32, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Dykman, L.A.; Staroverov, S.A.; Bogatyrev, V.A.; Shchyogolev, S.Y. Adjuvant properties of gold nanoparticles. Nanotechnol. Russia 2010, 5, 748–761. [Google Scholar] [CrossRef]
- Prashant, C.K.; Kumar, M.; Dinda, A.K. Nanoparticle based tailoring of adjuvant function: The role in vaccine development. J. Biomed. Nanotechnol. 2014, 10, 2317–2331. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.E.; Titball, R.; Williamson, D. Vaccine delivery using nanoparticles. Front. Cell. Infect. Microbiol. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.; Good, M.F.; Toth, I. Nanovaccines and their mode of action. Methods 2013, 60, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Brinas, R.P.; Sundgren, A.; Sahoo, P.; Morey, S.; Rittenhouse-Olson, K.; Wilding, G.E.; Deng, W.; Barchi, J.J. Design and Synthesis of Multifunctional Gold Nanoparticles Bearing Tumor-Associated Glycopeptide Antigens as Potential Cancer Vaccines. Bioconj. Chem. 2012, 23, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Parry, A.L.; Clemson, N.A.; Ellis, J.; Bernhard, S.S.R.; Davis, B.G.; Cameron, N.R. ‘Multicopy multivalent’ glycopolymer-stabilized gold nanoparticles as potential synthetic cancer vaccines. J. Am. Chem. Soc. 2013, 135, 9362–9365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safari, D.; Marradi, M.; Chiodo, F.; Dekker, H.A.; Shan, Y.; Adamo, R.; Oscarson, S.; Rijkers, G.T.; Lahmann, M.; Kamerling, J.P.; et al. Gold nanoparticles as carriers for a synthetic Streptococcus pneumoniae type 14 conjugate vaccine. Nanomedicine 2012, 7, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Ruiz, E.; Alvarez-García, G.; Aguado-Martínez, A.; Salman, H.; Irache, J.M.; Marugán-Hernández, V.; Ortega-Mora, L.M. Low efficacy of NcGRA7, NcSAG4, NcBSR4 and NcSRS9 formulated in poly-ε-caprolactone against neospora caninum infection in mice. Vaccine 2012, 30, 4983–4992. [Google Scholar] [CrossRef] [PubMed]
- Godshall, C.E.; Suh, G.; Lorber, B. Cutaneous listeriosis. J. Clin. Invest. 2013, 51, 3591–3596. [Google Scholar] [CrossRef] [PubMed]
- Drevets, D.A.; Bronze, M.S. Listeria monocytogenes: Epidemiology, human disease, and mechanisms of brain invasion. FEMS Immunol. Med. Microbiol. 2008, 53, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Frande-Cabanes, E.; Fernandez-Prieto, L.; Calderon-Gonzalez, R.; Rodriguez-Del Rio, E.; Yañez Diaz, S.; Lopez-Fanarraga, M.; Alvarez-Dominguez, C. Dissociation of innate immune responses in microglia infected with Listeria monocytogenes. Glia 2014, 62, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Disson, O.; Lecuit, M. In vitro and in vivo models to study human listeriosis: Mind the gap. Microbes Infect. 2013, 15, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Schlüter, D.; Back, C.; Reiter, S.; Meyer, T.; Hof, H.; Deckert-Schluter, M. Immune reactions to Listeria monocytogenes in the brain. Immunobiology 1999, 201, 188–195. [Google Scholar] [CrossRef]
- Unanue, E.R.; Carrero, J.A. Studies with Listeria monocytogenes lead the way. Adv. Immunol. 2012, 113, 1–5. [Google Scholar] [PubMed]
- Mukai, A.O.; Krebs, V.L.J.; Bertoli, C.J.; Okay, T.S. TNF-α and IL-6 in the diagnosis of bacterial and aseptic meningitis in children. Pediatr. Neurol. 2006, 34, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.R.; Chaturvedi, V.; Kinder, J.M.; Jiang, T.T.; Xin, L.; Erlet, J.M.; Way, S.S. Perinatal Listeria monocytogenes susceptibility despite preconceptual priming and maintenance of pathogen-specific CD8+ T cells during pregnancy. Cell. Mol. Immunol. 2014, 11, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ávila, O.; Hijazi, K.; Marradi, M.; Clavel, C.; Campion, C.; Kelly, C.; Spenades, S. Gold manno-glyconanoparticles: Multivalent systems to block HIV-1 gp120 binding to the lectin DC-SIGN. Chem. Eur. J. 2009, 15, 9874–9888. [Google Scholar] [CrossRef] [PubMed]
- Marradi, M.; Chiodo, F.; García, I.; Penadés, S. Glyconanoparticles as multifunctional and multimodal carbohydrate systems. Chem. Soc. Rev. 2013, 42, 4728–4745. [Google Scholar] [CrossRef] [PubMed]
- Calderon-Gonzalez, R.; Garcia, I.; Marradi, M.; Petrovsky, N.; Alvarez-Dominguez, C. Novel nanoparticles vaccines. Hum. Vaccin. Immunother. 2015, 11, 2501–2503. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Kirkendoll, B.; Zhao, H.; Pisani, L.; Luong, R.; Switzer, A.; McConnell, M.V.; Contag, C.H. Infection of pregnant mice with Listeria monocytogenes induces fetal bradicardia. Pediatr. Res. 2012, 71, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Fernandez-Suarez, D. Alternatively activated microglia and macrophages in the central nervous system. Prog. Neurobiol. 2015, 131, 65–86. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, J.M.; Penadés, S. Glyconanoparticles: Types, synthesis and applications in glycoscience, biomedicine and material science. Biochim. Biophys. Acta 2006, 1760, 636–651. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Fanarraga, M.; Carranza, G.; Bellido, J.; Kortazar, D.; Villegas, J.C.; Zabala, J.C. Tubulin cofactor B plays a role in the neuronal growth cone. J. Neurochem. 2007, 100, 1680–1687. [Google Scholar] [CrossRef] [PubMed]
- Ribes, S.; Ebert, S.; Regen, T.; Agarwal, A.M.; Tauber, S.C.; Czesnik, D.; Spreer, A.; Bunkowski, S.; Eiffert, S.; Hanischm, U.W.; et al. Toll-like receptor stimulation enhances phagocytosis and intracellular killing of nonencapsulated Streptococcus pneumonia by murine microglia. Infect. Immun. 2010, 78, 865–871. [Google Scholar] [CrossRef] [PubMed]


| Weight (mg) | Length (cm) | Coordination Movement b (cm) | Cutaneous Test c | |
|---|---|---|---|---|
| NV a | 170 ± 0.5 | 5.0 ± 0.1 | 0.2 ± 0.1 | grey-wrinkled |
| gold glyconanoparticles listeriolysin peptide 91–99 (GNP-LLO91–99) | 280 ± 0.4 | 4.0 ± 0.1 | 9.5 ± 0.8 | black-normal |
| gold glyconanoparticles glyceraldehyde-3-phosphate dehydrogenase 1–22 peptide (GNP-GAPDH1–22) | 295 ± 0.5 | 4.2 ± 0.1 | 10 ± 0.7 | black-normal |
| CONTROL | 300 ± 0.5 | 4.0 ± 0.1 | 10 ± 0.8 | black-normal |
| Relative Melanocytes a | Apoptotic Melanocytes b (%) | Blood Vessels c | |
|---|---|---|---|
| NV | 0.2 ± 0.1 | 70 ± 0.8 | Few |
| GNP-LLO91–99 | 1 ± 0.1 | 1 ± 0.1 | Normal |
| GNP-GAPDH1–22 | 1 ± 0.1 | 0.9 ± 0.1 | Normal |
| CONTROL | 1 ± 0.1 | 0.9 ± 0.1 | Normal |
| IL6 (pg/mL) a | IL-12 (pg/mL) | |
|---|---|---|
| NV | 1650 ± 0.9 | 17 ± 0.8 |
| GNP-LLO91–99 | 37 ± 0.1 | 27 ± 0.1 |
| GNP-GAPDH1–22 | 55 ± 0.1 | 40 ± 0.1 |
| CONTROL | 3 ± 0.1 | 1 ± 0.1 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calderón-Gonzalez, R.; Terán-Navarro, H.; Frande-Cabanes, E.; Ferrández-Fernández, E.; Freire, J.; Penadés, S.; Marradi, M.; García, I.; Gomez-Román, J.; Yañez-Díaz, S.; et al. Pregnancy Vaccination with Gold Glyco-Nanoparticles Carrying Listeria monocytogenes Peptides Protects against Listeriosis and Brain- and Cutaneous-Associated Morbidities. Nanomaterials 2016, 6, 151. https://doi.org/10.3390/nano6080151
Calderón-Gonzalez R, Terán-Navarro H, Frande-Cabanes E, Ferrández-Fernández E, Freire J, Penadés S, Marradi M, García I, Gomez-Román J, Yañez-Díaz S, et al. Pregnancy Vaccination with Gold Glyco-Nanoparticles Carrying Listeria monocytogenes Peptides Protects against Listeriosis and Brain- and Cutaneous-Associated Morbidities. Nanomaterials. 2016; 6(8):151. https://doi.org/10.3390/nano6080151
Chicago/Turabian StyleCalderón-Gonzalez, Ricardo, Héctor Terán-Navarro, Elisabet Frande-Cabanes, Eva Ferrández-Fernández, Javier Freire, Soledad Penadés, Marco Marradi, Isabel García, Javier Gomez-Román, Sonsoles Yañez-Díaz, and et al. 2016. "Pregnancy Vaccination with Gold Glyco-Nanoparticles Carrying Listeria monocytogenes Peptides Protects against Listeriosis and Brain- and Cutaneous-Associated Morbidities" Nanomaterials 6, no. 8: 151. https://doi.org/10.3390/nano6080151