Graphene-Enabled Electrodes for Electrocardiogram Monitoring
Abstract
:1. Introduction
2. Materials and Methods
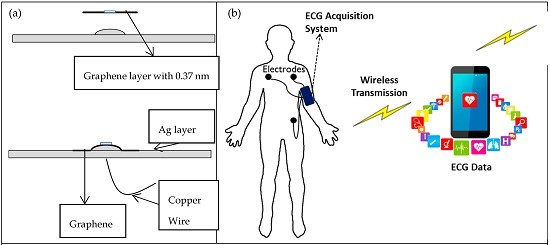
2.1. Fabrication Process
2.2. Raman Spectroscopy and SEM Images
2.3. Measurements of ECG Acquisition System
3. Experimental Results and Discussion
3.1. Simulation of Electroplating Graphene
3.2. Measurement of Skin-Electrode Contact Impedances
3.3. Measurements of ECG Signals
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yoo, J.; Yan, L.; Lee, S.; Kim, H.; Yoo, H.J. A wearable ECG acquisition system with compact planar-fashionable circuit board-based shirt. IEEE Trans. Inf. Technol. Biomed. Actions 2009, 13, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.G.; Clark, J.W. Medical Instrumentation; Wiley: New York, NY, USA, 1998. [Google Scholar]
- Oh, T.I.; Yoon, S.; Kim, T.E.; Wi, H.; Kim, K.J.; Woo, E.J.; Sadleir, R.J. Nanofiber web textile dry electrodes for long-term biopotential recording. IEEE Trans. Biomed. Circuits Syst. 2013, 7, 204–211. [Google Scholar] [PubMed]
- Meng, Y.; Li, Z.; Chen, J. A flexible dry electrode based on APTES-anchored PDMS substrate for portable ECG acquisition system. Microsyst. Technol. 2016, 22, 2027–2034. [Google Scholar] [CrossRef]
- Baek, J.Y.; An, J.H.; Choi, J.M.; Park, K.S.; Lee, S.H. Flexible polymeric dry electrodes for the long-term monitoring of ECG. Sens. Actuators A 2008, 143, 423–429. [Google Scholar] [CrossRef]
- Hoffmann, K.P.; Ruff, R. Flexible dry surface-electrodes for ECG long-term monitoring. In Proceedings of the 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 23–26 August 2007; pp. 5739–5742.
- Baek, H.J.; Lee, H.B.; Kim, J.S.; Choi, J.M.; Kim, K.K.; Park, K.S. Nonintrusive biological signal monitoring in a car to evaluate a driver’s stress and health state. Telemed. e-Health 2009, 15, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Sim, K.S.; Kim, K.K.; Lim, Y.G.; Park, K.S. Thin and flexible active electrodes with shield for capacitive electrocardiogram measurement. Med. Biol. Eng. Comput. 2010, 48, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Searle, A.; Kirkup, L. A direct comparison of wet, dry and insulating bioelectric recording electrodes. Physiol. Meas. 2000, 21. [Google Scholar] [CrossRef]
- Myers, A.C.; Huang, H.; Zhu, Y. Wearable silver nanowire dry electrodes for electrophysiological sensing. RSC Adv. 2015, 5, 11627–11632. [Google Scholar] [CrossRef]
- Jung, H.C.; Moon, J.H.; Baek, D.H.; Lee, J.H.; Choi, Y.Y.; Hong, J.S.; Lee, S.H. CNT/PDMS composite flexible dry electrodesfor long-term ECG monitoring. IEEE Trans. Biomed. Eng. 2012, 59, 1472–1479. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Mizukami, K. Well-ordered wrinkling patterns on chemically oxidized poly (dimethylsiloxane) surfaces. Macromolecules 2012, 45, 7128–7134. [Google Scholar] [CrossRef]
- Lee, S.K.; Kim, B.H.; Yoo, H.J. Planar fashionable circuit board technology and its applications. J. Semicond. Technol. Sci. 2009, 9, 174–180. [Google Scholar] [CrossRef]
- Thompson, C.S.; Abate, A.R. Adhesive-based bonding technique for PDMS microfluidic devices. Lab Chip 2013, 13, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Celik, N.; Balachandran, W.; Manivannan, N. Graphene-based biosensors: Methods, analysis and future perspectives. IET Circuits Devices Syst. 2015, 9, 434–445. [Google Scholar] [CrossRef]
- 2-DTech LTD. Available online: http://www.2-DTech.com (accessed on 14 April 2016).
- Mattevi, C.; Kim, H.; Chowalla, M. A Review of Chemical Vapor Deposition of Graphene on Copper. J. Mater. Chem. 2011, 21, 3324–3334. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-Area Synthesis of High-Quality and Uniform Graphene Films on Copper Foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef] [PubMed]
- Kidambi, P.R.; Ducati, C.; Dlubak, B.; Gardiner, D.; Weatherup, R.S.; Martin, M.-B.; Seneor, P.; Coles, H.; Hofmann, S. The Parameter Space of Graphene Chemical Vapor Deposition on Polycrystalline Cu. J. Phys. Chem. C 2012, 116, 22492–22501. [Google Scholar] [CrossRef]
- Reina, A.; Son, H.; Jiao, L.; Fan, B.; Dresselhaus, M.S.; Liu, Z.; Kong, J. Transferring and Identification of Single-and Few-Layer Graphene on Arbitrary Substrates. J. Phys. Chem. C 2008, 112, 17741–17744. [Google Scholar] [CrossRef]
- Strudwick, A.J.; Weber, N.E.; Schwab, M.G.; Kettner, M.; Weitz, R.T.; Wünsch, J.R.; Müllen, K.; Sachdev, H. Chemical Vapor Deposition of High Quality Graphene Films from Carbon Dioxide Atmospheres. ACS Nano 2014, 9, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Magnuson, C.W.; Venugopal, A.; Tromp, R.M.; Hannon, J.B.; Vogel, E.M.; Vogel, E.M.; Colombo, L.; Ruoff, R.S. Large-area graphene single crystals grown by low-pressure chemical vapor deposition of methane on copper. J. Am. Chem. Soc. 2011, 133, 2816–2819. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, C.; Reich, S. Double resonant Raman scattering in graphite. Phys. Rev. Lett. 2000, 85. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Chen, Z.; Ren, W.; Gao, L.; Liu, B.; Pei, S.; Cheng, H.M. Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition. Nat. Mater. 2011, 10, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Umer, D.; Ellahi, G.; Ellahi, W.; Ayub, S.; Din, Z.M.U. Bio-electrical Impedance Plethysmography-Designing an Efficient Non-invasive Electrode for Cardiac Rate Analysis. Int. J. Endorsing Health Sci. 2013, 1, 29–32. [Google Scholar]
- He, D.D.; Winokur, E.S.; Sodini, C.G. An Ear-Worn Vital Signs Monitor. IEEE Trans. Biomed. Eng. 2015, 62, 2547–2552. [Google Scholar] [CrossRef] [PubMed]
- KST Visualizing Data. Available online: https://kst-plot.kde.org/kst1/handbook/plugins-vector-processing.html (accessed on 12 March 2016).
- Yapici, M.K.; Alkhidir, T.; Samad, Y.A.; Liao, K. Graphene-clad textile electrodes for electrocardiogram monitoring. Sens. Actuators B 2015, 221, 1469–1474. [Google Scholar] [CrossRef]












| Layers of Modelling | Electrical Conductivity (S/m) | Relative Permittivity |
|---|---|---|
| Graphene (GN) | 1.18 × 109 | 11.5 |
| Ag-Silver | 61.6 × 106 | 3.4 |
| Stratum Corneum | 0.0005 | 1 |
| Epidermis | 0.95 | 1 |
| Dermis | 0.2 | 1 |
| Artery | 0.8 | 1 |
| Electrode Placement | Ambu Type Electrode | Covidien Type Electrode | ||
|---|---|---|---|---|
| GN-based | Ag/AgCl | GN-based | Ag/AgCl | |
| Chest-lead | 27.03 | 25.21 | 25.32 | 21.23 |
| Ear-lead | 22.96 | 17.27 | 21.74 | 15.34 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Celik, N.; Manivannan, N.; Strudwick, A.; Balachandran, W. Graphene-Enabled Electrodes for Electrocardiogram Monitoring. Nanomaterials 2016, 6, 156. https://doi.org/10.3390/nano6090156
Celik N, Manivannan N, Strudwick A, Balachandran W. Graphene-Enabled Electrodes for Electrocardiogram Monitoring. Nanomaterials. 2016; 6(9):156. https://doi.org/10.3390/nano6090156
Chicago/Turabian StyleCelik, Numan, Nadarajah Manivannan, Andrew Strudwick, and Wamadeva Balachandran. 2016. "Graphene-Enabled Electrodes for Electrocardiogram Monitoring" Nanomaterials 6, no. 9: 156. https://doi.org/10.3390/nano6090156
APA StyleCelik, N., Manivannan, N., Strudwick, A., & Balachandran, W. (2016). Graphene-Enabled Electrodes for Electrocardiogram Monitoring. Nanomaterials, 6(9), 156. https://doi.org/10.3390/nano6090156