SERS-Based Flavonoid Detection Using Ethylenediamine-β-Cyclodextrin as a Capturing Ligand
Abstract
:1. Introduction
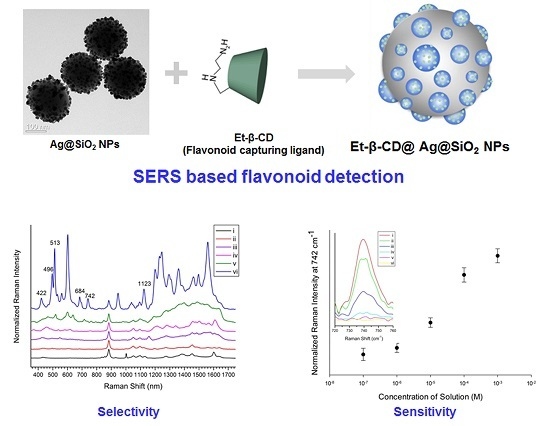
2. Results and Discussion
2.1. Preparation of Et-β-CD-Immobilized Ag-NP-Embedded Silica NPs (SiO2@Ag@Et-β-CD NPs)
2.1.1. Characterization of Et-β-CD
2.1.2. UV Absorption Spectra of the Inclusion Complex of Et-β-CD with Flavonoids
2.1.3. Preparation of SiO2@Ag@Et-β-CD
2.2. Detection of Flavonoids via SERS Using SiO2@Ag@Et-β-CD NPs
2.2.1. Detection of Various Flavonoids Using Et-β-CD-Immobilized Ag-NP-Assembled Silica NPs
2.2.2. Determination of the Detection Limit of SiO2@Ag@Et-β-CD NPs for Lut
2.2.3. Stability of the Flavonoid Captured by Et-β-CD-Immobilized Ag-NP-Assembled Silica NPs
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of Ethylenediamino-β-Cyclodextrin (Et-β-CD)
3.3. UV Absorption Measurement of the Inclusion Complex
3.4. Preparation of SiO2@Ag@Et-β-CD NPs
3.5. Interactions of SiO2@Ag@Et-β-CD NPs with Flavonoids and Organic Molecules
3.6. Raman Spectral Measurements
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Middleton, E. Effect of plant flavonoids on immune and inflammatory cell function. In Flavonoids in the Living System; Manthey, J.A., Buslig, B.S., Eds.; Springer: Boston, MA, USA, 1998; pp. 175–182. [Google Scholar]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Williams, R.J.; Spencer, J.P.E.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Ravishankar, D.; Rajora, A.K.; Greco, F.; Osborn, H.M.I. Flavonoids as prospective compounds for anti-cancer therapy. Int. J. Biochem. Cell Biol. 2013, 45, 2821–2831. [Google Scholar] [CrossRef] [PubMed]
- Burda, S.; Oleszek, W. Antioxidant and antiradical activities of flavonoids. J. Agric. Food Chem. 2001, 49, 2774–2779. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.R.; Sharma, S.; Mandal, S.; Goswami, A.; Mukhopadhyay, S.; Majumder, H.K. Luteolin, an emerging anti-cancer flavonoid, poisons eukaryotic DNA topoisomerase I. Biochem. J. 2002, 366, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Liu-Smith, F.; Meyskens, F.L. Molecular mechanisms of flavonoids in melanin synthesis and the potential for the prevention and treatment of melanoma. Mol. Nutr. Food Res. 2016, 60, 1264–1274. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-Y.; Kim, J.-M.; Kim, J.-S.; Choung, M.-G.; Sung, M.-K. Chemopreventive action of anthocyanin-rich black soybean fraction in APCMin/+ intestinal polyposis model. J. Cancer Prev. 2015, 20, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Nallasamy, P.; Liu, D.; Shah, H.; Li, J.Z.; Chitrakar, R.; Si, H.; McCormick, J.; Zhu, H.; Zhen, W. Luteolin protects against vascular inflammation in mice and TNF-alpha-induced monocyte adhesion to endothelial cells via suppressing IΚBα/NF-κB signaling pathway. J. Nutr. Biochem. 2015, 26, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Ma, L.; Wen, Y.; Wang, H.; Zhang, S. Studies of the in vitro antibacterial activities of several polyphenols against clinical isolates of methicillin-resistant Staphylococcus aureus. Molecules 2014, 19, 12630–12639. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.-M. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr. Cancer Drug Targets 2008, 8, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.G.; Oliveira, E.J. Solid-phase extraction and gas chromatography-mass spectrometry determination of kaempferol and quercetin in human urine after consumption of ginkgo biloba tablets. J. Chromatogr. B 1999, 723, 203–210. [Google Scholar] [CrossRef]
- Hasler, A.; Sticher, O.; Meier, B. High-performance liquid chromatographic determination of five widespread flavonoid aglycones. J. Chromatogr. A 1990, 508, 236–240. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, H.; Ye, J. Determination of rutin and quercetin in plants by capillary electrophoresis with electrochemical detection. Anal. Chim. Acta 2000, 423, 69–76. [Google Scholar] [CrossRef]
- He, C.; Cui, H.; Zhao, X.; Zhao, H.; Zhao, G. Determination of rutin by flow injection with inhibited chemiluminescence detection. Anal. Lett. 1999, 32, 2751–2759. [Google Scholar] [CrossRef]
- Nie, S.; Emory, S.R. Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Kneipp, K.; Wang, Y.; Kneipp, H.; Perelman, L.T.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Single molecule detection using surface-enhanced Raman scattering (SERS). Phys. Rev. Lett. 1997, 78, 1667. [Google Scholar] [CrossRef]
- Del Valle, E.M.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Rekharsky, M.V.; Inoue, Y. Complexation thermodynamics of cyclodextrins. Chem. Rev. 1998, 98, 1875–1918. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Choi, J.; Jung, S. Inclusion complexes of modified cyclodextrins with some flavonols. J. Incl. Phenom. Macrocycl. Chem. 2009, 64, 43–47. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.W.; Jung, S. Aqueous solubility enhancement of some flavones by complexation with cyclodextrins. Bull. Korean Chem. Soc. 2008, 29, 590–594. [Google Scholar]
- Kwon, Y.; Kim, H.; Park, S.; Jung, S. Enhancement of solubility and antioxidant activity of some flavonoids based on the inclusion complexation with sulfobutylether β-cyclodextrin. Bull. Korean Chem. Soc. 2010, 31, 3035–3037. [Google Scholar] [CrossRef]
- Kamphorst, A.O.; Mendes de Sa, I.; Faria, A.M.C.; Sinisterra, R.D. Association complexes between ovalbumin and cyclodextrins have no effect on the immunological properties of ovalbumin. Eur. J. Pharm. Biopharm. 2004, 57, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Patel, M. Solid-state characterization and in vitro dissolution behavior of lorazepam: Hydroxypropyl-beta-cyclodextrin inclusion complex. Drug. Discov. Ther. 2010, 4, 442–452. [Google Scholar] [PubMed]
- Uekama, K.; Hirayama, F.; Irie, T. Cyclodextrin drug carrier systems. Chem. Rev. 1998, 98, 2045–2076. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Brusseau, M.L. Solubilization of some low-polarity organic compounds by hydroxypropyl-β-cyclodextrin. Environ. Sci. Technol. 1993, 27, 2821–2825. [Google Scholar] [CrossRef]
- Ko, S.O.; Schlautman, M.A.; Carraway, E.R. Partitioning of hydrophobic organic compounds to hydroxypropyl-beta-cyclodextrin: Experimental studies and model predictions for surfactant-enhanced remediation applications. Environ. Sci. Technol. 1999, 33, 2765–2770. [Google Scholar] [CrossRef]
- Khan, A.R.; Forgo, P.; Stine, K.J.; D'Souza, V.T. Methods for selective modifications of cyclodextrins. Chem. Rev. 1998, 98, 1977–1996. [Google Scholar] [CrossRef] [PubMed]
- Torreggiani, A.; Trinchero, A.; Tamba, M.; Taddei, P. Raman and pulse radiolysis studies of the antioxidant properties of quercetin: Cu(II) chelation and oxidizing radical scavenging. J. Raman Spectrosc. 2005, 36, 380–388. [Google Scholar] [CrossRef]
- Cornard, J.P.; Merlin, J.C. Spectroscopic and structural study of complexes of quercetin with Al(III). J. Inorg. Biochem. 2002, 92, 19–27. [Google Scholar] [CrossRef]
- Cañamares, M.V.; Lombardi, J.R.; Leona, M. Raman and surface enhanced Raman spectra of 7-hydroxy flavone and 3′, 4′dihydroxy flavone. E-Preserv. Sci. 2009, 6, 81–88. [Google Scholar]
- Jurasekova, Z.; Garcia-Ramos, J.V.; Domingo, C.; Sanchez-Cortes, S. Surface-enhanced Raman scattering of flavonoids. J. Raman Spectrosc. 2006, 37, 1239–1241. [Google Scholar] [CrossRef]
- Teslova, T.; Corredor, C.; Livingstone, R.; Spataru, T.; Birke, R.L.; Lombardi, J.R.; Cañamares, M.V.; Leona, M. Raman and surface-enhanced Raman spectra of flavone and several hydroxy derivatives. J. Raman Spectrosc. 2007, 38, 802–818. [Google Scholar] [CrossRef]
- Cornard, J.P.; Boudet, A.C.; Merlin, J.C. Complexes of Al(III) with 3′,4′-dihydroxy-flavone: Characterization, theoretical and spectroscopic study. Spectrochim. Acta A 2001, 57, 591–602. [Google Scholar] [CrossRef]
- Jurasekova, Z.; Torreggiani, A.; Tamba, M.; Sanchez-Cortes, S.; Garcia-Ramos, J.V. Raman and surface-enhanced Raman scattering (SERS) investigation of the quercetin interaction with metals: Evidence of structural changing processes in aqueous solution and on metal nanoparticles. J. Mol. Struct. 2009, 918, 129–137. [Google Scholar] [CrossRef]
- Yuan, W.; He, J.M.; Hui, C.; Zhang, D.S.; Hua, C.; Shao, H.B. Analysis of flavones in rubus parvifolius linn by high performance liquid chromatography-electrospray ionization mass spectroscopy and thin layer chromatography-fourier transform surface enhanced Raman spectroscopy. Chin. J. Anal. Chem. 2006, 34, 1073–1077. [Google Scholar] [CrossRef]
- Hahm, E.; Jeong, D.; Cha, M.G.; Choi, J.M.; Pham, X.-H.; Kim, H.-M.; Kim, H.; Lee, Y.-S.; Jeong, D.H.; Jung, S. β-CD dimer-immobilized Ag assembly embedded silica nanoparticles for sensitive detection of polycyclic aromatic hydrocarbons. Sci. Rep. 2016, 6, 26082. [Google Scholar] [CrossRef]
- Ramasamy, M.; Yi, D.K.; An, S.S.A. Enhanced detection sensitivity of Escherichia coli O157: H7 using surface-modified gold nanorods. Int. J. Nanomed. 2015, 10, 179–190. [Google Scholar]
- Kim, J.-H.; Kim, J.-S.; Choi, H.; Lee, S.-M.; Jun, B.-H.; Yu, K.-N.; Kuk, E.; Kim, Y.-K.; Jeong, D.H.; Cho, M.-H. Nanoparticle probes with surface enhanced Raman spectroscopic tags for cellular cancer targeting. Anal. Chem. 2006, 78, 6967–6973. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Bharti, N.; Madan, J.; Hiremath, S. Characterization of cyclodextrin inclusion complexes—A review. J. Pharm. Sci. Technol. 2010, 2, 171–183. [Google Scholar]
- Uekama, K.; Fujinaga, T.; Hirayama, F.; Otagiri, M.; Yamasaki, M. Inclusion complexations of steroid hormones with cyclodextrins in water and in solid phase. Int. J. Pharm. 1982, 10, 1–15. [Google Scholar]
- Rajagopalan, N.; Chen, S.C.; Chow, W.-S. A study of the inclusion complex of amphotericin-B with γ-cyclodextrin. Int. J. Pharm. 1986, 29, 161–168. [Google Scholar] [CrossRef]
- Al-Marzouqi, A.H.; Shehatta, I.; Jobe, B.; Dowaidar, A. Phase solubility and inclusion complex of itraconazole with β-cyclodextrin using supercritical carbon dioxide. J. Pharm. Sci. 2006, 95, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, G.S.; Vavia, P.R. Physicochemical, in silico and in vivo evaluation of a danazol–β-cyclodextrin complex. Int. J. Pharm. 2008, 352, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Chow, D.D.; Karara, A.H. Characterization, dissolution and bioavailability in rats of ibuprofen-β-cyclodextrin complex system. Int. J. Pharm. 1986, 28, 95–101. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Szejtli, J. Selectivity/structure correlation in cyclodextrin chemistry. Supramol. Chem. 1995, 6, 217–223. [Google Scholar] [CrossRef]
- Byun, H.-S.; Zhong, N.; Bittman, R. 6A-O-p-Toluenesulfonyl-β-cyclodextrin. Org. Synth. 2000, 77, 225–230. [Google Scholar]
- Bonomo, R.P.; Cucinotta, V.; Allessandro, F.D.; Impellizzeri, G.; Maccarrone, G.; Rizzarelli, E.; Vecchio, G. Coordination properties of 6-deoxy-6-[1-(2-amino) ethylamino]-β-cyclodextrin and the ability of its copper(II) complex to recognize and separate amino acid enantiomeric pairs. J. Incl. Phenom. Macrocycl. Chem. 1993, 15, 167–180. [Google Scholar] [CrossRef]






© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.M.; Hahm, E.; Park, K.; Jeong, D.; Rho, W.-Y.; Kim, J.; Jeong, D.H.; Lee, Y.-S.; Jhang, S.H.; Chung, H.J.; et al. SERS-Based Flavonoid Detection Using Ethylenediamine-β-Cyclodextrin as a Capturing Ligand. Nanomaterials 2017, 7, 8. https://doi.org/10.3390/nano7010008
Choi JM, Hahm E, Park K, Jeong D, Rho W-Y, Kim J, Jeong DH, Lee Y-S, Jhang SH, Chung HJ, et al. SERS-Based Flavonoid Detection Using Ethylenediamine-β-Cyclodextrin as a Capturing Ligand. Nanomaterials. 2017; 7(1):8. https://doi.org/10.3390/nano7010008
Chicago/Turabian StyleChoi, Jae Min, Eunil Hahm, Kyeonghui Park, Daham Jeong, Won-Yeop Rho, Jaehi Kim, Dae Hong Jeong, Yoon-Sik Lee, Sung Ho Jhang, Hyun Jong Chung, and et al. 2017. "SERS-Based Flavonoid Detection Using Ethylenediamine-β-Cyclodextrin as a Capturing Ligand" Nanomaterials 7, no. 1: 8. https://doi.org/10.3390/nano7010008