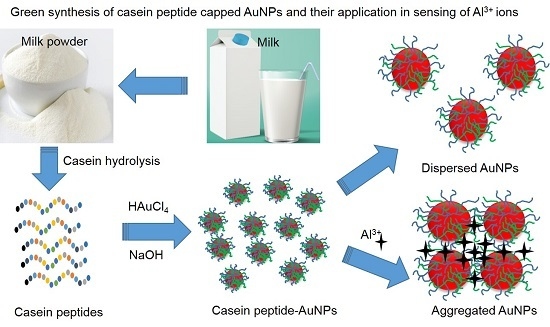
A Spectral Probe for Detection of Aluminum (III) Ions Using Surface Functionalized Gold Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of the Gold Nanoparticles
2.3. Characterization of the AuNPs
2.4. Selectivity of the AuNPs
2.5. Determination of the Al3+ Standard Solution
2.6. Effect of pH and Ionic Strength
2.7. Real-time Response of the AuNPs toward Al3+
2.8. Interference from other Metal Ions
2.9. UV-vis Response toward Al3+ in Real Samples
3. Results and Discussion
3.1. Synthesis of the AuNPs
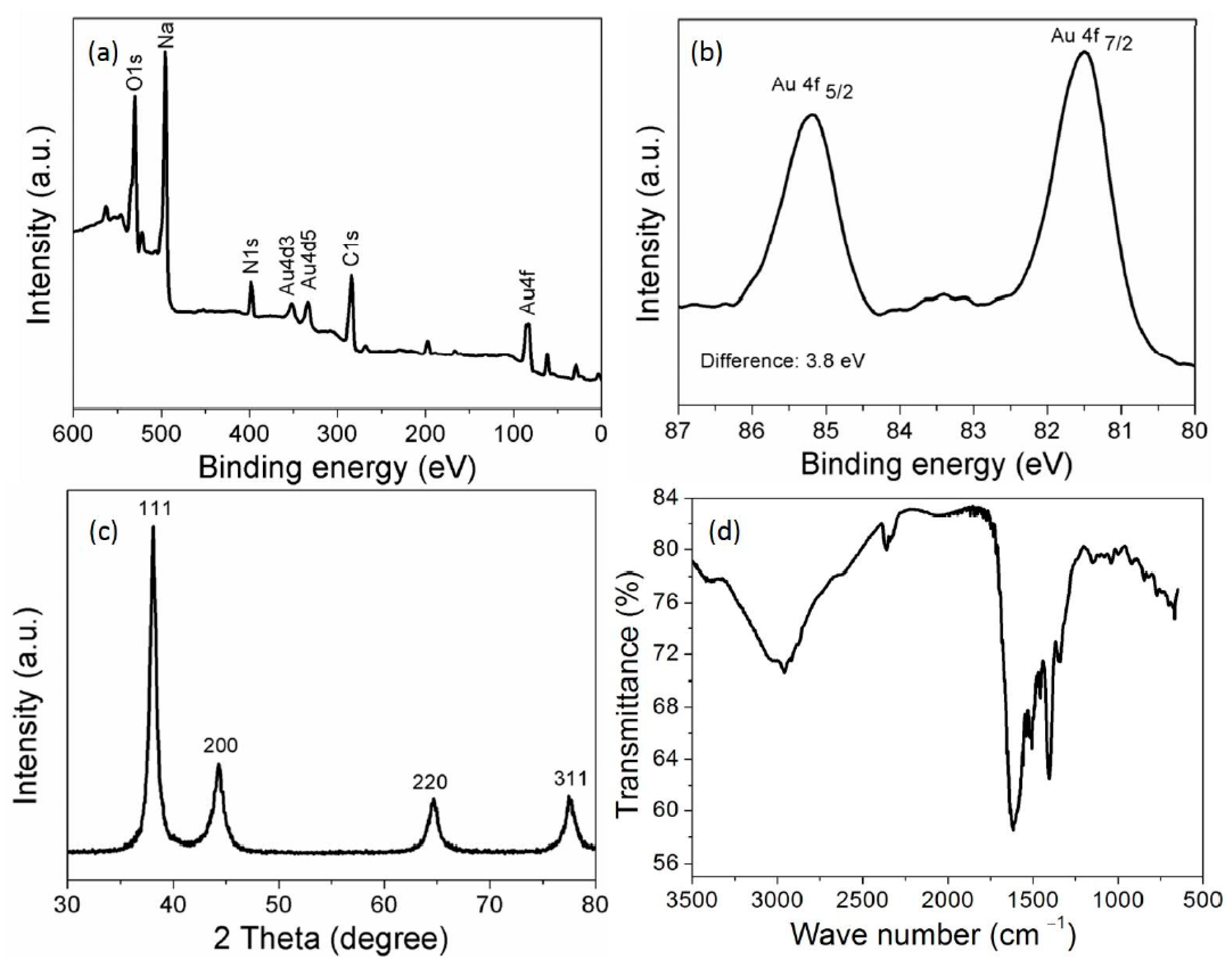
3.2. Characterization of the AuNPs
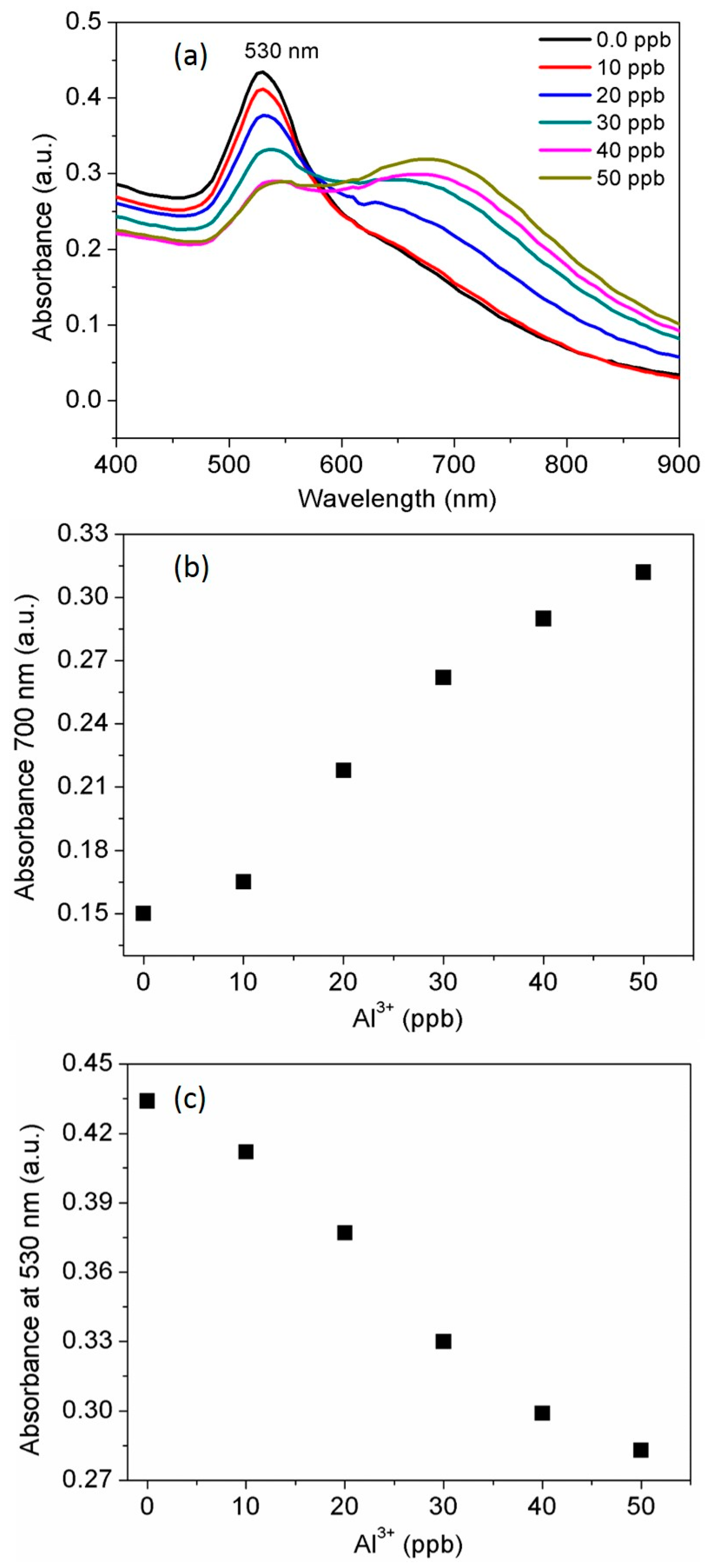
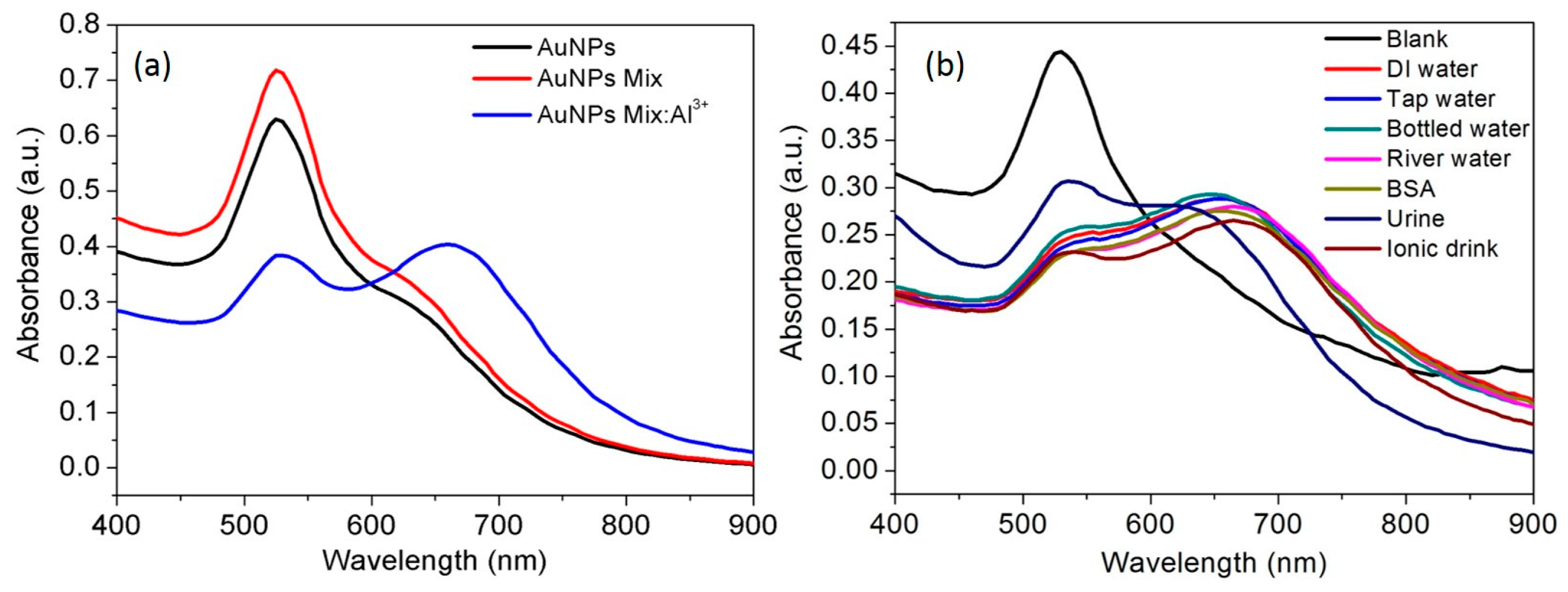
3.3. Selectivity of the AuNPs toward Al3+
3.4. Determination of the Standard Al3+ Concentration
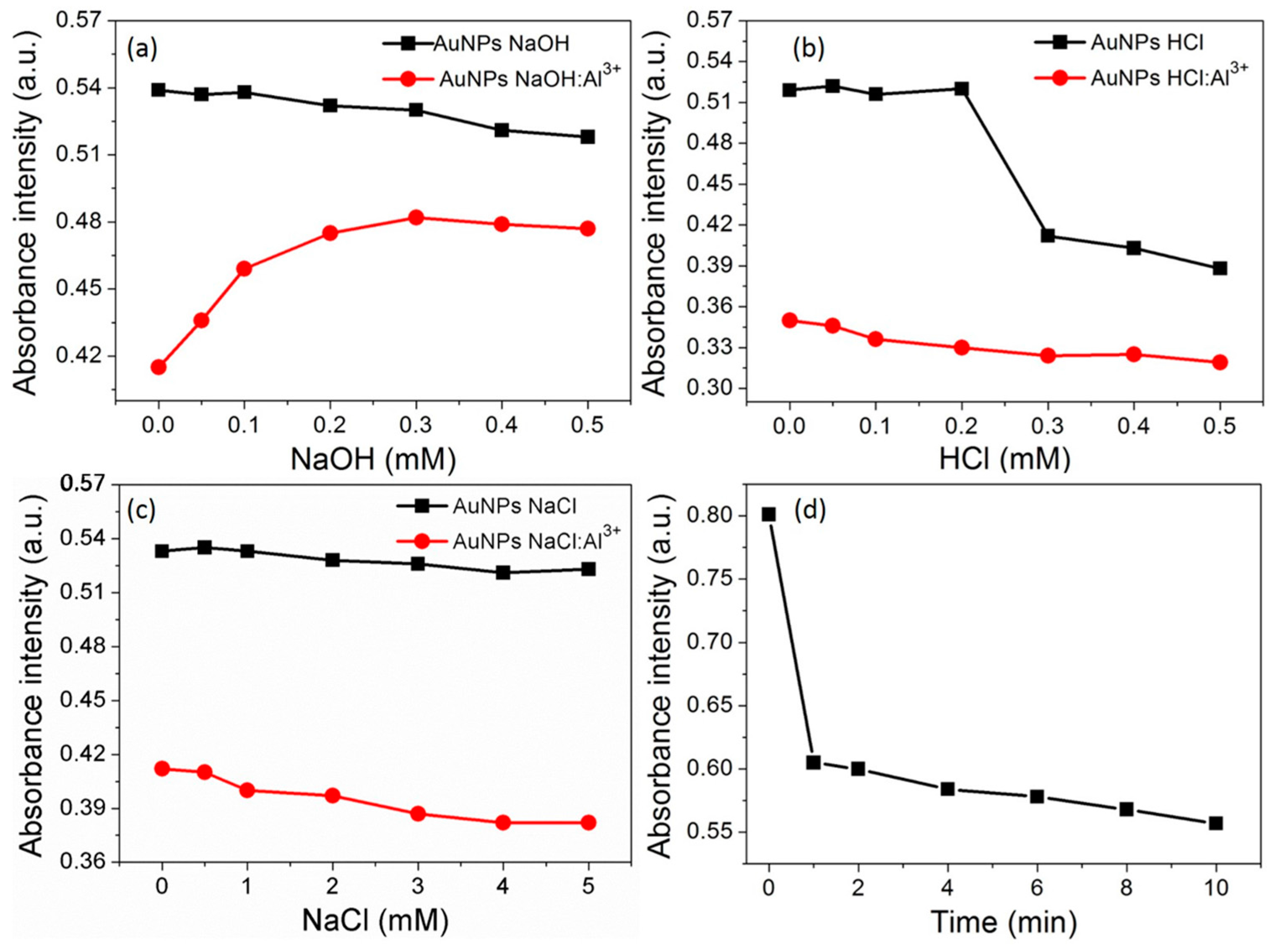
3.5. Effect of pH and Ionic Strength
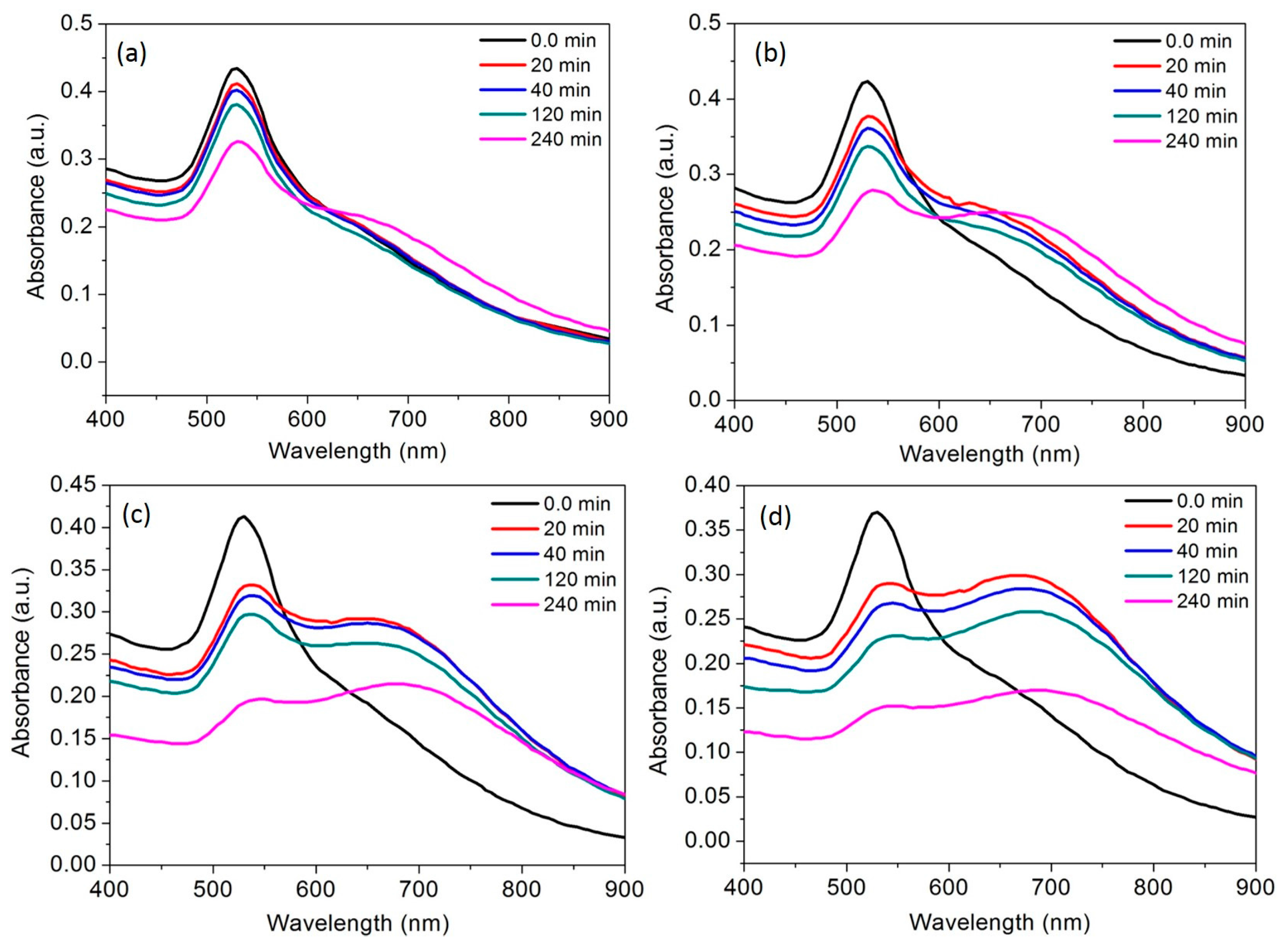
3.6. Time Course of the AuNPs toward Al3+
3.7. Plausible Coordination of Al3+ with Casein Peptide Ligands
3.8. Interference from Other Metal Ions
3.9. Spectral Response of Al3+ in Real Samples, Water, Urine and Ionic Drink
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mavhungu, S.T.; Akinlabi, E.T.; Onitiri, M.A.; Varachia, F.M. Aluminum matrix composites for industrial use: advances and trends. Procedia Manuf. 2017, 7, 178–182. [Google Scholar] [CrossRef]
- Ding, W.-H.; Cao, W.; Zheng, X.-J.; Fang, D.-C.; Wong, W.-T.; Jin, L.-P. A Highly Selective fluorescent chemosensor for AlIII Ion and fluorescent species formed in the solution. Inorg. Chem. 2013, 52, 7320–7322. [Google Scholar] [CrossRef] [PubMed]
- Valeur, B.; Leray, I. Design principles of fluorescent molecular sensors for cation recognition. Coord. Chem. Rev. 2000, 205, 3–40. [Google Scholar] [CrossRef]
- Tennakone, K.; Wickramanayake, S.; Fernando, C.A.N. Aluminium contamination from fluoride assisted dissolution of metallic aluminium. Environ. Pollut. 1988, 49, 133–143. [Google Scholar] [CrossRef]
- Vallejos, S.; Muñoz, A.; Ibeas, S.; Serna, F.; García, F.C.; García, J.M. Forced solid-state interactions for the selective “Turn-On” fluorescence sensing of aluminum ions in water using a sensory polymer substrate. ACS Appl. Mater. Interfaces 2015, 7, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Olivi, L.; Cheong, J.H.; Maertens, A.; Bressler, J.P. Aluminum stimulates uptake of non-transferrin bound iron and transferrin bound iron in human glial cells. Toxicol. Appl. Pharm. 2007, 220, 349–356. [Google Scholar] [CrossRef]
- Corain, B.; Bombi, G.G.; Tapparo, A.; Nicolini, M.; Zatta, P.; Perazzolo, M.; Favarato, M. Alzheimer’s disease and aluminum toxicology. Environ. Health Perspect. 1990, 89, 233–235. [Google Scholar] [PubMed]
- Maity, D.; Govindaraju, T. Pyrrolidine constrained bipyridyl-dansyl click fluoroionophore as selective Al3+ sensor. Chem. Commun. 2010, 46, 4499–4501. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, K.K.; Kumar, A. Pyrimidine based highly sensitive fluorescent receptor for Al3+ showing dual signalling mechanism. Org. Biomol. Chem. 2010, 8, 4892–4897. [Google Scholar] [CrossRef]
- Dhara, A.; Jana, A.; Konar, S.; Ghatak, S.K.; Ray, S.; Das, K.; Bandyopadhyay, A.; Guchhait, N.; Kar, S.K. A novel rhodamine-based colorimetric chemodosimeter for the rapid detection of Al3+ in aqueous methanol: fluorescent ‘OFF-ON’ mechanism. Tetrahedron Lett. 2013, 54, 3630–3634. [Google Scholar] [CrossRef]
- Cui, S.; Tang, Y.; Lu, R.; Pu, S. A multi-addressable diarylethene for the selective detection of Al3+ and the construction of a logic circuit. RSC Adv. 2016, 6, 107475–107482. [Google Scholar] [CrossRef]
- Wang, G.; Sun, W. Optical limiting of gold nanoparticle aggregates induced by electrolytes. J. Phys. Chem. B 2006, 110, 20901–20905. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Wang, Y.; Li, X.; Jiang, X.; Gao, P.; Sun, K.; Zhou, J.; Zhang, Z.; Jiang, Q. An optical sensor with polyaniline-gold hybrid nanostructures for monitoring pH in saliva. Nanomaterials 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Lermé, J.; Bonnet, C.; Lebeault, M.-A.; Pellarin, M.; Cottancin, E. Surface plasmon resonance damping in spheroidal metal particles: Quantum confinement, shape, and polarization dependences. J. Phys. Chem. C 2017, 121, 5693–5708. [Google Scholar] [CrossRef]
- Irshad, M.; Iqbal, N.; Mujahid, A.; Afzal, A.; Hussain, T.; Sharif, A.; Ahmad, E.; Athar, M. Molecularly imprinted nanomaterials for sensor applications. Nanomaterials 2013, 3, 615–637. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cheng, Y.; Xu, L.; Du, H.; Zhang, P.; Wen, Y.; Zhang, X. A Nanostructured SERS switch based on molecular beacon-controlled assembly of gold nanoparticles. Nanomaterials 2016, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Schluesener, H. J.; Xu, S. Gold nanoparticle-based biosensors. Gold Bull. 2010, 43, 29–41. [Google Scholar] [CrossRef]
- Singha, D.K.; Mahata, P. Highly selective and sensitive luminescence Turn-On-based sensing of Al3+ ions in aqueous medium using a MOF with free functional sites. Inorg. Chem. 2015, 54, 6373–6379. [Google Scholar] [CrossRef] [PubMed]
- Ghodake, G.; Kim, D.-Y.; Jo, J.H.; Jang, J.; Lee, D.S. One-step green synthesis of gold nanoparticles using casein hydrolytic peptides and their anti-cancer assessment using the DU145 cell line. J. Ind. Eng. Chem. 2016, 33, 185–189. [Google Scholar] [CrossRef]
- Ji, X.; Song, X.; Li, J.; Bai, Y.; Yang, W.; Peng, X. Size control of gold nanocrystals in citrate reduction: The third role of citrate. J. Am. Chem. Soc. 2007, 129, 13939–13948. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lévy, R.; Fernig, D.G.; Brust, M. The peptide route to multifunctional gold nanoparticles. Bioconjug. Chem. 2005, 16, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Malin, E.L.; Alaimo, M.H.; Brown, E.M.; Aramini, J.M.; Germann, M.W.; Farrell, H.M.; McSweeney, P.L.H.; Fox, P.F. Solution structures of casein peptides: NMR, FTIR, CD, and molecular modeling studies of αs1-casein, 1–23. J. Protein Chem. 2001, 20, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Nune, S.K.; Chanda, N.; Katti, K.; Mekapothula, S.; Kulkarni, R.R.; Welshons, W.V.; Kannan, R.; Katti, K.V. Soybeans as a phytochemical reservoir for the production and stabilization of biocompatible gold nanoparticles. Small 2008, 4, 1425–1436. [Google Scholar] [CrossRef] [PubMed]
- Doty, R.C.; Tshikhudo, T.R.; Brust, M.; Fernig, D.G. Extremely stable water-soluble Ag nanoparticles. Chem. Mater. 2005, 17, 4630–4635. [Google Scholar] [CrossRef]
- Pezzato, C.; Maiti, S.; Chen, J.L.Y.; Cazzolaro, A.; Gobbo, C.; Prins, L.J. Monolayer protected gold nanoparticles with metal-ion binding sites: Functional systems for chemosensing applications. Chem. Commun. 2015, 51, 9922–9931. [Google Scholar] [CrossRef] [PubMed]
- Lévy, R.; Thanh, N.T.K.; Doty, R.C.; Hussain, I.; Nichols, R.J.; Schiffrin, D.J.; Brust, M.; Fernig, D.G. Rational and combinatorial design of peptide capping ligands for gold nanoparticles. J. Am. Chem. Soc. 2004, 126, 10076–10084. [Google Scholar] [CrossRef]
- Neda, I.; Vlazan, P.; Pop, R.O.; Sfarloaga, P.; Grozescu, I.; Segneanu, A.-E. Peptide and amino acids separation and identification from natural products. In Analytical Chemistry; Krull, I.S., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Selvakannan, P.R.; Swami, A.; Srisathiyanarayanan, D.; Shirude, P.S.; Pasricha, R.; Mandale, A.B.; Sastry, M. Synthesis of aqueous Au core–Ag shell nanoparticles using tyrosine as a pH-dependent reducing agent and assembling phase-transferred silver nanoparticles at the air-water interface. Langmuir 2004, 20, 7825–7836. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-B.; Kong, R.-M.; Lu, Y. Metal ion sensors based on dnazymes and related DNA molecules. Annu. Rev. Anal. Chem. 2011, 4, 105–128. [Google Scholar] [CrossRef]
- Xiang, Y.; Lu, Y. DNA as sensors and imaging agents for metal ions. Inorg. Chem. 2014, 53, 1925–1942. [Google Scholar] [CrossRef] [PubMed]
- Sovago, I.; Osz, K. Metal ion selectivity of oligopeptides. Dalton Trans. 2006, 32, 3841–3854. [Google Scholar] [CrossRef] [PubMed]
- Slocik, J.M.; Zabinski, J.S.; Phillips, D.M.; Naik, R.R. Colorimetric response of peptide-functionalized gold nanoparticles to metal ions. Small 2008, 4, 548–551. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhou, Y.; Huang, D.; Su, M.; Wang, K.; Hong, M. A Highly sensitive and selective fluorescent sensor for detection of Al3+ using a europium(III) quinolinecarboxylate. Inorg. Chem. 2014, 53, 6497–6499. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Sun, Y.; Zhang, L.; Sun, D.; Liu, F.; Meng, Q.; Wang, R.; Sun, D. A tubular europium-organic framework exhibiting selective sensing of Fe3+ and Al3+ over mixed metal ions. Chem. Commun. 2013, 49, 11557–11559. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.; Majumdar, A.; Sarkar, T. Selective sensing of Al3+ by naphthyridine coupled rhodamine chemosensors. RSC Adv. 2014, 4, 23428–23432. [Google Scholar] [CrossRef]
- Hasni, I.; Yaakoubi, H.; Hamdani, S.; Tajmir-Riahi, H.-A.; Carpentier, R. Mechanism of interaction of Al3+ with the proteins composition of photosystem II. PLoS ONE 2015, 10, e0120876. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Song, J.; Ma, Q.; Zhou, Y.; Yang, J.; Shuang, S.; Dong, C. A colorimetric probe for the detection of aluminum ions based on 11-mercaptoundecanoic acid functionalized gold nanoparticles. Anal. Methods 2016, 8, 7232–7236. [Google Scholar] [CrossRef]
- Zhang, M.; Han, J.; Wu, H.; Wei, Q.; Xie, G.; Chen, S.; Gao, S. Tb-MOF: A naked-eye and regenerable fluorescent probe for selective and quantitative detection of Fe3+ and Al3+ ions. RSC Adv. 2016, 6, 94622–94628. [Google Scholar] [CrossRef]
- Guo, L.; Jackman, J.A.; Yang, H.-H.; Chen, P.; Cho, N.-J.; Kim, D.-H. Strategies for enhancing the sensitivity of plasmonic nanosensors. Nano Today 2015, 10, 213–239. [Google Scholar] [CrossRef]
- Gómez-Graña, S.; Fernández-López, C.; Polavarapu, L.; Salmon, J.-B.; Leng, J.; Pastoriza-Santos, I.; Pérez-Juste, J. Gold nanooctahedra with tunable size and microfluidic-induced 3D assembly for highly uniform SERS-active supercrystals. Chem. Mater. 2015, 27, 8310–8317. [Google Scholar] [CrossRef]
- Lee, J. T.Y.; Leng, Y.; Chow, K.L.; Ren, F.; Ge, X.; Wang, K.; Lu, X. Cell culture medium as an alternative to conventional simulated body fluid. Acta Biomater. 2011, 7, 2615–2622. [Google Scholar] [CrossRef]
- Sato, J.D.; Kan, M. Media for culture of mammalian cells. In Current Protocols in Cell Biology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001. [Google Scholar]
- Kang, K. A.; Wang, J.; Jasinski, J.B.; Achilefu, S. Fluorescence manipulation by gold nanoparticles: From complete quenching to extensive enhancement. J. Nanobiotechnol. 2011, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, J.; Liu, X.; Gao, Y.; Ye, Z.; Li, G. Colorimetric copper(II) ion sensor based on the conformational change of peptide immobilized onto the surface of gold nanoparticles. Anal. Methods 2014, 6, 2580–2585. [Google Scholar] [CrossRef]
- Sener, G.; Uzun, L.; Denizli, A. Colorimetric sensor array based on gold nanoparticles and amino acids for identification of toxic metal ions in water. ACS Appl. Mater. Interfaces 2014, 6, 18395–18400. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, Z.; Zhou, X.; Hu, J. Colorimetric response of peptide modified gold nanoparticles: An original assay for ultrasensitive silver detection. Biosens. Bioelectron. 2017, 92, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, H.M.; Shah, A.; Konieczny, M.; Hoffmann, J.A.; Nijdam, A.J.; Reeves, M.E. Small molecule- and amino acid-induced aggregation of gold nanoparticles. Langmuir 2013, 29, 7661–7673. [Google Scholar] [CrossRef] [PubMed]
- Manjunath, R.; Hrishikesan, E.; Kannan, P. A selective colorimetric and fluorescent sensor for Al3+ ion and its application to cellular imaging. Spectrochim. Acta A 2015, 140, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Ku, K.-S.; Muthukumar, P.; Angupillai, S.; Son, Y.-A. A new rhodamine 6 G based chemosensor for trace level Al3+ and its thin film application in 100% aqueous medium. Sensor. Actuators B-Chem. 2016, 236, 184–191. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Sun, L.; Wang, Z. Gold nanoparticle-based colorimetric assay for selective detection of aluminium cation on living cellular surfaces. Chem. Commun. 2010, 46, 988–990. [Google Scholar] [CrossRef]
- Polavarapu, L.; Xu, Q.-H. Water-soluble conjugated polymer-induced self-assembly of gold nanoparticles and its application to SERS. Langmuir 2008, 24, 10608–10611. [Google Scholar] [CrossRef] [PubMed]
- Phelan, M.; Aherne, A.; FitzGerald, R.J.; O’Brien, N.M. Casein-derived bioactive peptides: Biological effects, industrial uses, safety aspects and regulatory status. Int. Dairy J. 2009, 19, 643–654. [Google Scholar] [CrossRef]
- Nehete, J.Y.; Bhambar, R.S.; Narkhede, M.R.; Gawali, S.R. Natural proteins: Sources, isolation, characterization and applications. Pharmacogn. Rev. 2013, 7, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Dallas, D.C.; Guerrero, A.; Parker, E.A.; Robinson, R.C.; Gan, J.; German, J.B.; Barile, D.; Lebrilla, C.B. Current peptidomics: Applications, purification, identification, quantification, and functional analysis. Proteomics 2015, 15, 1026–1038. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Fang, M.; Cao, C.-S.; Qiao, W.-Z.; Zhao, B. Unique (3,4,10)-connected lanthanide–organic framework as a recyclable chemical sensor for detecting Al3+. Inorg. Chem. 2016, 55, 4790–4794. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.M.; Nie, S.M. Single-molecule and single-nanoparticle SERS: From fundamental mechanisms to biomedical applications. Chem. Soc. Rev. 2008, 37, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Shiohara, A.; Langer, J.; Polavarapu, L.; Liz-Marzan, L.M. Solution processed polydimethylsiloxane/gold nanostar flexible substrates for plasmonic sensing. Nanoscale 2014, 6, 9817–9823. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Gao, Y.; Zhang, Y.; Shen, Z.; Wu, A. A new rapid colorimetric detection method of Al3+ with high sensitivity and excellent selectivity based on a new mechanism of aggregation of smaller etched silver nanoparticles. Talanta 2014, 122, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Alam, R.; Mistri, T.; Bhowmick, R.; Katarkar, A.; Chaudhuri, K.; Ali, M. Dual channel selective fluorescent detection of Al3+ and PPI in mixed aqueous media: DFT studies and cell imaging applications. RSC Adv. 2015, 5, 53940–53948. [Google Scholar] [CrossRef]
- Sinha, S.; Chowdhury, B.; Ghosh, P. A Highly sensitive ESIPT-based ratiometric fluorescence sensor for selective detection of Al3+. Inorg. Chem. 2016, 55, 9212–9220. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Fang, Y.-M.; Xiao, Q.; Li, J.; Li, S.-B.; Chen, H.-J.; Sun, J.-J.; Yang, H.-H. Rapid visual detection of aluminium ion using citrate capped gold nanoparticles. Analyst 2012, 137, 2021–2023. [Google Scholar] [CrossRef] [PubMed]
- Bothra, S.; Kumar, R.; Sahoo, S.K. Pyridoxal derivative functionalized gold nanoparticles for colorimetric determination of zinc(II) and aluminium(III). RSC Adv. 2015, 5, 97690–97695. [Google Scholar] [CrossRef]
- Chen, W.; Jia, Y.; Feng, Y.; Zheng, W.; Wang, Z.; Jiang, X. Colorimetric detection of Al(III) in vermicelli samples based on ionic liquid group coated gold nanoparticles. RSC Adv. 2015, 5, 62260–62264. [Google Scholar] [CrossRef]
- Huang, P.; Li, J.; Liu, X.; Wu, F. Colorimetric determination of aluminum(III) based on the aggregation of Schiff base-functionalized gold nanoparticles. Microchim. Acta 2016, 183, 863–869. [Google Scholar] [CrossRef]
- Kumar, A.; Bhatt, M.; Vyas, G.; Bhatt, S.; Paul, P. Sunlight induced preparation of functionalized gold nanoparticles as recyclable colorimetric dual sensor for aluminum and fluoride in water. ACS Appl. Mater. Interfaces 2017, 9, 17359–17368. [Google Scholar] [CrossRef] [PubMed]


| Method | Surface Chemistry | Limit of Detection (LOD) of Al3+ | Solvent | Reference |
|---|---|---|---|---|
| Colorimetric | Citrate | 1.0 μM | Water | [61] |
| Colorimetric | Pyridoxal derivative | 0.51 μM | Water | [62] |
| Colorimetric | Ionic liquid | 1.0 μM | Water | [63] |
| Colorimetric | Schiff base | 0.29 μM | Water | [64] |
| Colorimetric | Polyacrylate | 2.0 μM | Water | [65] |
| Spectral | Casein peptides | 0.067 μM | Water | This work |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shinde, S.; Kim, D.-Y.; Saratale, R.G.; Syed, A.; Ameen, F.; Ghodake, G. A Spectral Probe for Detection of Aluminum (III) Ions Using Surface Functionalized Gold Nanoparticles. Nanomaterials 2017, 7, 287. https://doi.org/10.3390/nano7100287
Shinde S, Kim D-Y, Saratale RG, Syed A, Ameen F, Ghodake G. A Spectral Probe for Detection of Aluminum (III) Ions Using Surface Functionalized Gold Nanoparticles. Nanomaterials. 2017; 7(10):287. https://doi.org/10.3390/nano7100287
Chicago/Turabian StyleShinde, Surendra, Dae-Young Kim, Rijuta Ganesh Saratale, Asad Syed, Fuad Ameen, and Gajanan Ghodake. 2017. "A Spectral Probe for Detection of Aluminum (III) Ions Using Surface Functionalized Gold Nanoparticles" Nanomaterials 7, no. 10: 287. https://doi.org/10.3390/nano7100287