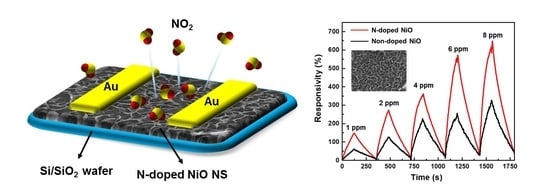
Three-Dimensional Porous Nitrogen-Doped NiO Nanostructures as Highly Sensitive NO2 Sensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the NiO Nanostructure
2.2. Chracterization of the N-Doped NiO Nanostructure
3. Results and Discussion
3.1. Morphology of the N-Doped NiO-NS Nanostructure
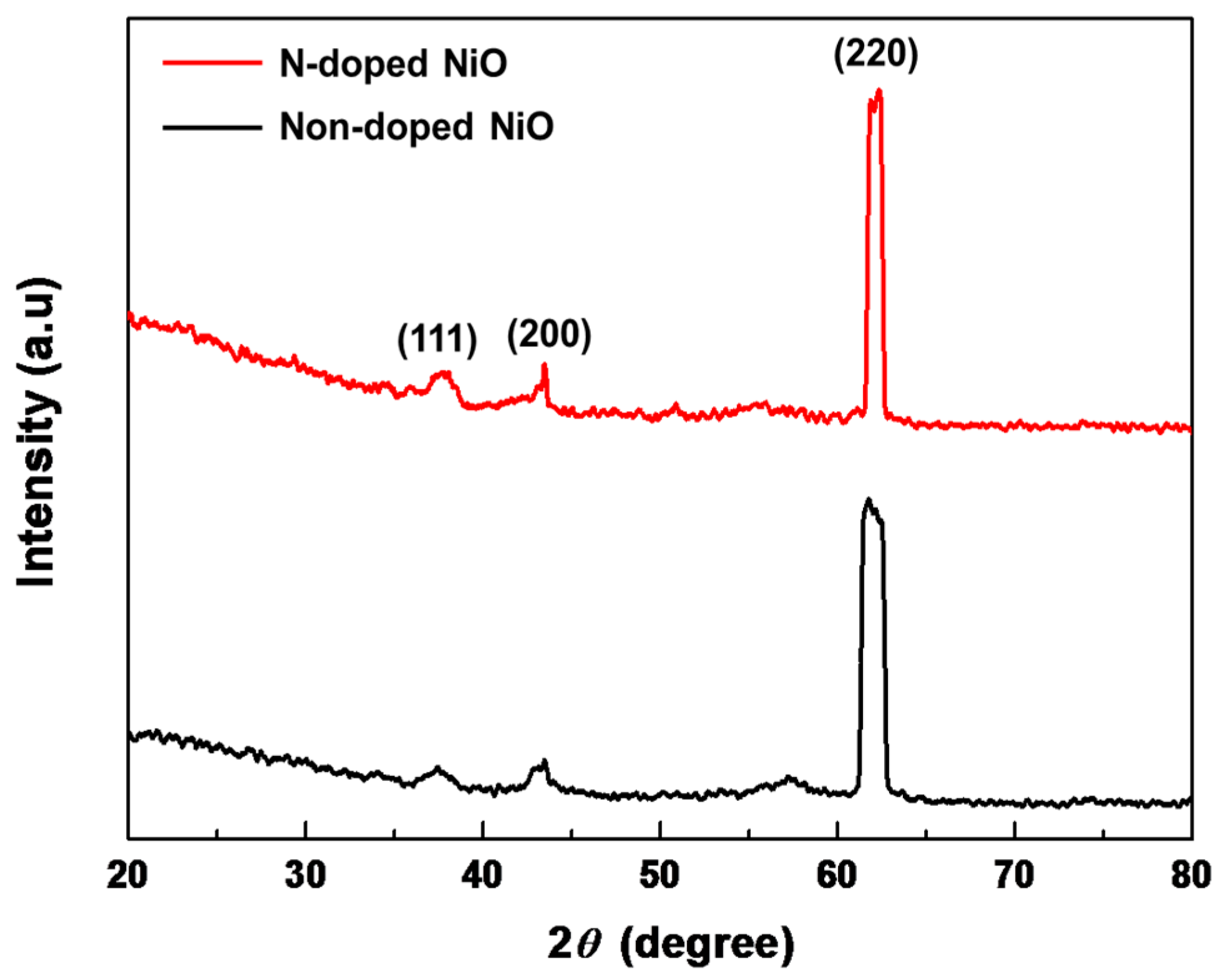
3.2. Crystallinity of the N-Doped NiO NS
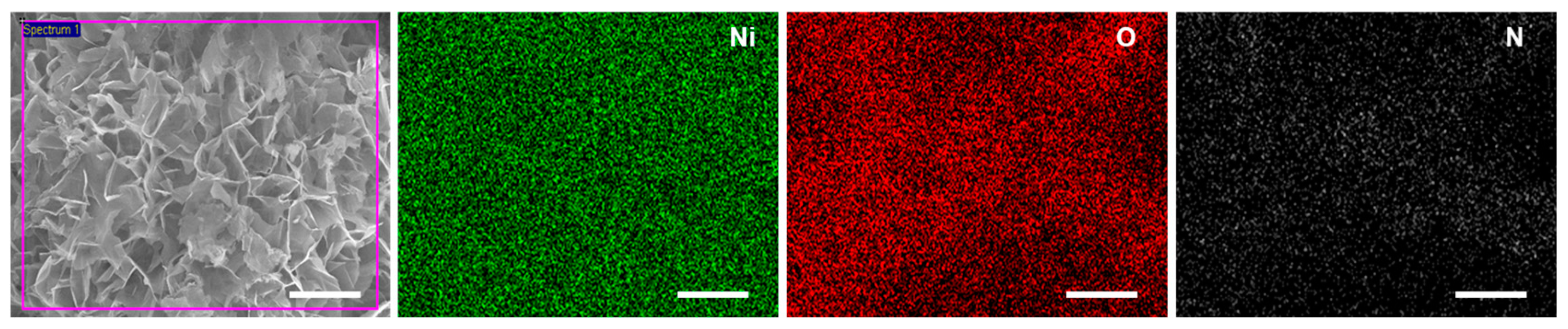
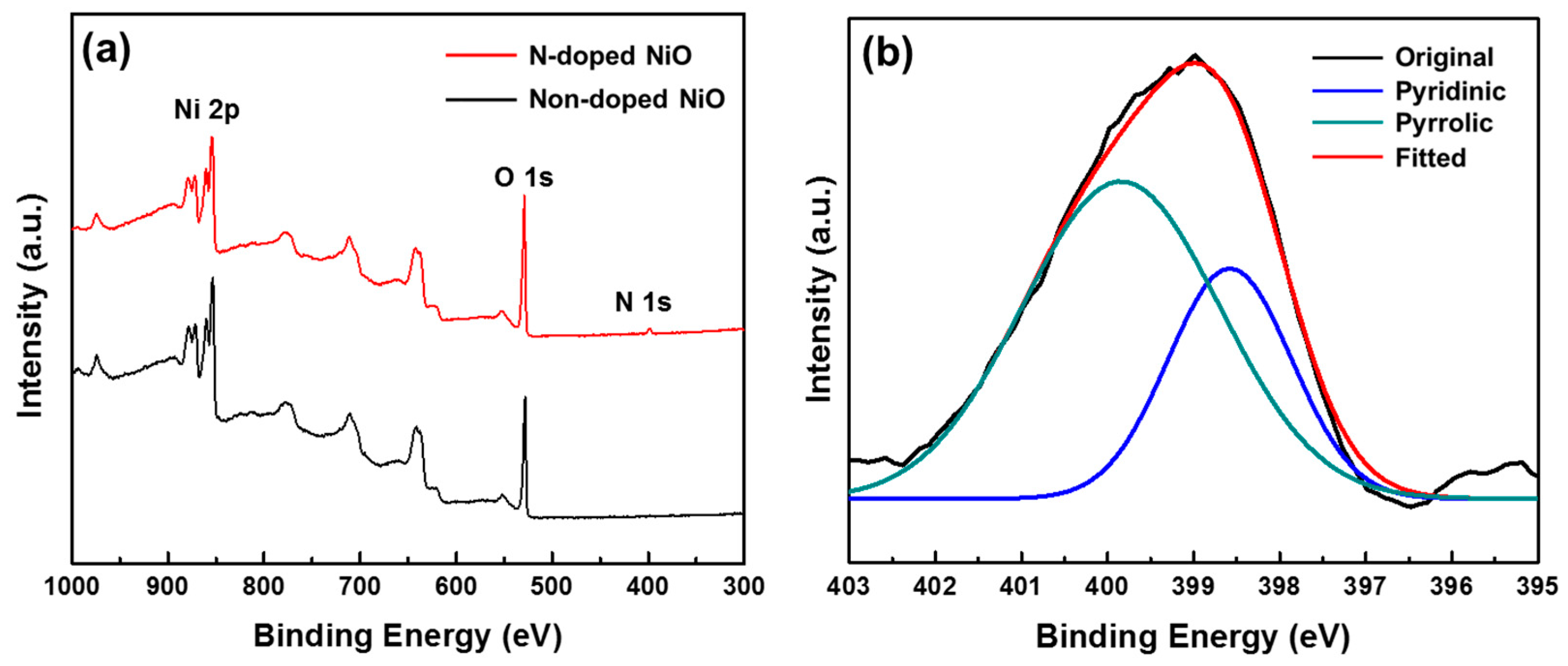
3.3. Chemical Characterization of the N-Doped NiO NSs
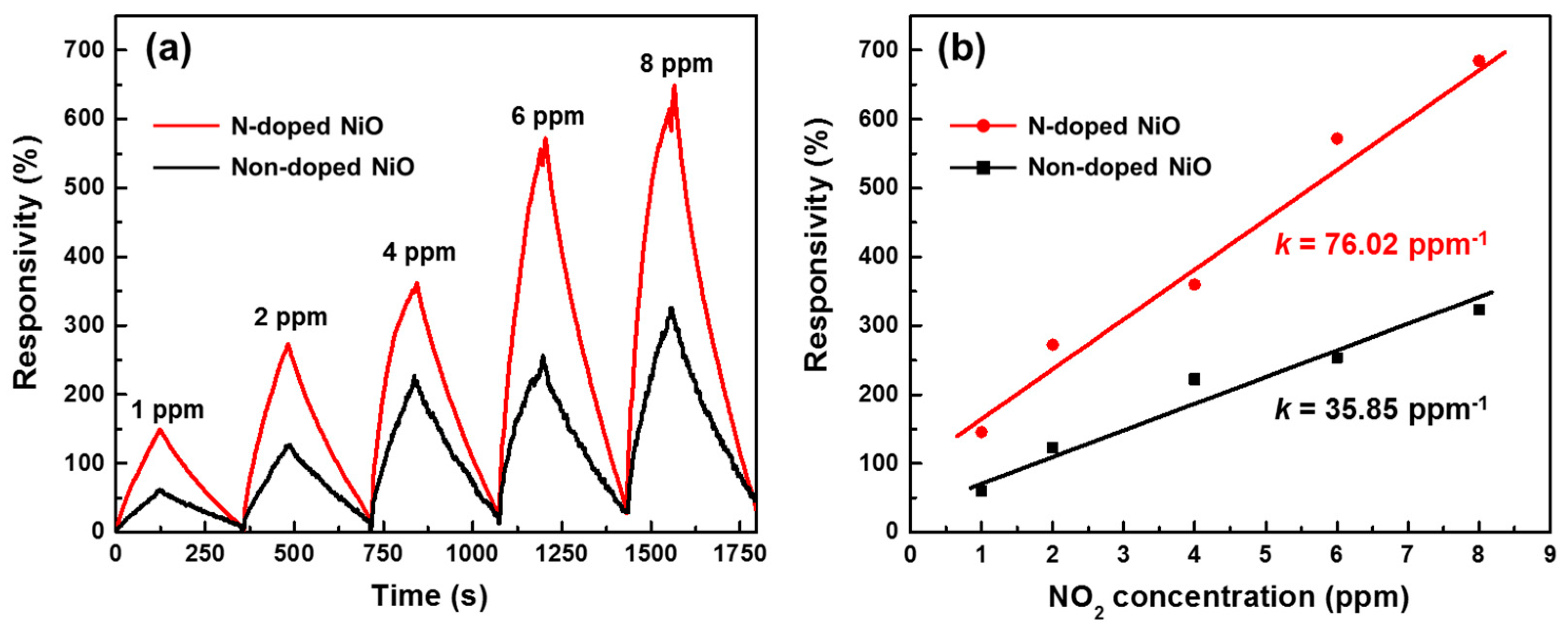
3.4. NO2 Sensing Performance of the N-Doped NiO NSs Nanostructure
3.5. N-Doping Effect on the NiO NSs
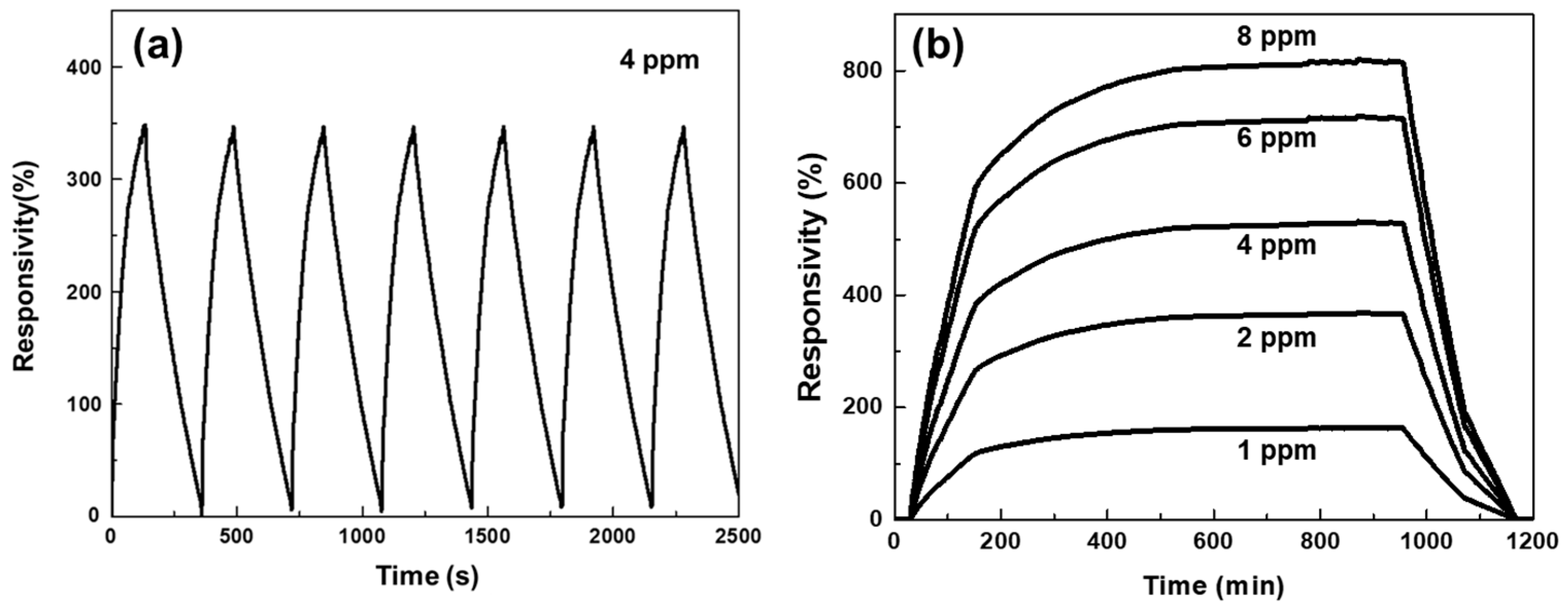
3.6. Repetitive and Saturate Responses of the N-Doped NiO-NSs-Based NO2 Sensor
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hjiri, M.; El Mir, L.; Leonardi, S.G.; Donato, N.; Neri, G. CO and NO2 selective monitoring by ZnO-based sensors. Nanomaterials 2013, 3, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Duvall, R.M.; Long, R.W.; Beaver, M.R.; Kronmiller, K.G.; Wheeler, M.L.; Szykman, J.J. Performance evaluation and community application of low-cost sensors for ozone and nitrogen dioxide. Sensors 2016, 16, 1698. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Hang, N.T.; Zhang, Z.; Yue, H.; Yang, W. Preparation of g-C3N4/graphene composite for detecting NO2 at room temperature. Nanomaterials 2017, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Capone, S.; Rella, R.; Siciliano, P.; Vasanelli, L. A comparison between V2O5 and WO3 thin films as sensitive elements for NO detection. Thin Solid Films 1999, 350, 264–268. [Google Scholar] [CrossRef]
- Diéguez, A.; Romano-Rodríguez, A.; Alay, J.L.; Morante, J.R.; Bârsan, N. Parameter optimisation in SnO2 gas sensors for NO2 detection with low cross-sensitivity to CO: Sol–gel preparation, film preparation, powder calcination, doping and grinding. Sens. Actuators B Chem. 2000, 65, 166–168. [Google Scholar]
- Cheng, J.P.; Wang, J.; Li, Q.Q.; Liu, H.G.; Li, Y. A review of recent developments in tin dioxide composites for gas sensing application. J. Ind. Eng. Chem. 2016, 44, 1–22. [Google Scholar] [CrossRef]
- Luan, V.H.; Chung, J.S.; Hur, S.H. Preparation of a reduced graphene oxide hydrogel by Ni ions and its use in a supercapacitor electrode. RSC Adv. 2015, 5, 22753–22758. [Google Scholar] [CrossRef]
- Xia, X.H.; Tu, J.P.; Zhang, J.; Wang, X.L.; Zhang, W.K.; Huang, H. Electrochromic properties of porous NiO thin films prepared by a chemical bath deposition. Sol. Energy Mater. Sol. Cells 2008, 92, 628–633. [Google Scholar] [CrossRef]
- Varghese, B.; Reddy, M.V.; Yanwu, Z.; Lit, C.S.; Hoong, T.C.; Subba Rao, G.V.; Chowdari, B.V.R.; Wee, A.T.S.; Lim, C.T.; Sow, C.H. Fabrication of NiO nanowall electrodes for high performance lithium ion battery. Chem. Mater. 2008, 20, 3360–3367. [Google Scholar] [CrossRef]
- Garcia-Miquel, J.L.; Zhang, Q.; Allen, S.J.; Rougier, A.; Blyr, A.; Davies, H.O.; Jones, A.C. Nickel oxide sol-gel films from nickel diacetate for electrochromic applications. Thin Solid Films 2003, 424, 165–170. [Google Scholar] [CrossRef]
- Kuang, D.B.; Lei, B.X.; Pan, Y.P.; Yu, X.Y.; Su, C.Y. Fabrication of novel hierarchical β-Ni(OH)2 and NiO microspheres via an easy hydrothermal process. J. Phys. Chem. C 2009, 113, 5508–5513. [Google Scholar] [CrossRef]
- Vučinić-Vasić, M.; Antic, B.; Kremenović, A.; Nikolic, A.S.; Stoiljkovic, M.; Bibic, N.; Spasojevic, V.; Colomban, P. Zn, Ni ferrite/NiO nanocomposite powder obtained from acetylacetonato complexes. Nanotechnology 2006, 17, 4877–4884. [Google Scholar]
- Kwak, B.S.; Choi, B.H.; Ji, M.J.; Park, S.M.; Kang, M. Synthesis of spherical NiO nanoparticles using a solvothermal treatment with acetone solvent. J. Ind. Eng. Chem. 2012, 18, 11–15. [Google Scholar] [CrossRef]
- Meng, T.; Xu, Q.Q.; Li, Y.T.; Chang, J.L.; Ren, T.Z.; Yuan, Z.Y. Nickle nanoparticles highly dispersed on reduced graphene oxide for ammonia decomposition to hydrogen. J. Ind. Eng. Chem. 2015, 32, 373–379. [Google Scholar] [CrossRef]
- Minegishi, K.; Koiwai, Y.; Kikuchi, Y.; Yano, K.; Kasuga, M.; Shimizu, A. Growth of p-type zinc oxide films by chemical vapor deposition. Jpn. J. Appl. Phys. 1997, 36, L1453–L1455. [Google Scholar] [CrossRef]
- Joseph, M.; Tabata, H.; Kawai, T. p-Type electrical conduction in ZnO thin films by Ga and N codoping. Jpn. J. Appl. Phys. 1999, 38, L1205–L1207. [Google Scholar] [CrossRef]
- Look, D.C.; Reynolds, D.; Litton, C.; Jones, R.; Eason, D.; Cantwell, G. Characterization of homoepitaxial p-type ZnO grown by molecular beam epitaxy. Appl. Phys. Lett. 2002, 81, 1830–1832. [Google Scholar] [CrossRef]
- Liu, G.; Li, F.; Wang, D.W.; Tang, D.M.; Liu, C.; Ma, X.; Lu, G.Q.; Cheng, H.M. Electron field emission of a nitrogen-doped TiO2 nanotube array. Nanotechnology 2008, 19, 025606–025611. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Dong, L.; Guo, W.; Liang, T.; Lai, W. First principles study of nitrogen doping at the anatase TiO2 (101) surface. J. Phys. Chem. C 2013, 117, 13037–13044. [Google Scholar] [CrossRef]
- Abbasi, A.; Sardroodi, J.J. N-doped TiO2 anatase nanoparticles as a highly sensitive gas sensor for NO2 detection: Insights from DFT computations. Environ. Sci. Nano 2016, 3, 1153–1164. [Google Scholar] [CrossRef]
- Livraghi, S.; Paganini, M.C.; Giamello, E.; Selloni, A.; Di Valentin, C.; Pacchioni, G. Origin of photoactivity of nitrogen-doped titanium dioxide under visible light. J. Am. Chem. Soc. 2006, 128, 15666–15671. [Google Scholar] [CrossRef] [PubMed]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.X.; Hui, K.S.; Hui, K.N.; Hwang, D.H.; Lee, S.K.; Zhou, W.; Cho, Y.R.; Kwon, S.H.; Wang, Q.M.; Son, Y.G. A facile synthesis method of hierarchically porous NiO nanosheets. Mater. Lett. 2012, 69, 69–71. [Google Scholar] [CrossRef]
- Hoa, L.T.; Tien, H.N.; Luan, V.H.; Chung, J.S.; Hur, S.H. Fabrication of a novel 2D-graphene/2D-NiO nanosheet-based hybrid nanostructure and its use in highly sensitive NO2 sensors. Sens. Actuators B Chem. 2013, 185, 701–705. [Google Scholar] [CrossRef]
- Zhang, G.; Yu, L.; Hoster, H.E.; Lou, X.W. Synthesis of one-dimensional hierarchical NiO hollow nanostructures with enhanced supercapacitive performance. Nanoscale 2013, 5, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.D.; Servaites, J.D.; Buchholz, D.B.; Leever, B.J.; Liu, J.; Emery, J.D.; Zhang, M.; Song, J.J.; Durstock, M.F.; Freeman, A.J.; et al. Structural and electrical functionality of NiO interfacial films in bulk heterojunction organic solar cells. Chem. Mater. 2011, 23, 2218–2226. [Google Scholar] [CrossRef]
- Atay, F.; Bilgin, V.; Akyuz, I.; Kose, S. The effect of in doping on some physical properties of CdS films. Mater. Sci. Semicond. Process. 2003, 6, 197–203. [Google Scholar] [CrossRef]
- Haranath, D.; Sahai, S.; Joshi, A.G.; Gupta, B.K.; Shanker, V. Investigation of confinement effects in ZnO quantum dots. Nanotechnology 2009, 20, 425701–425707. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Lu, C.H.; Chang, F.C.; Kuo, S.W. Supramolecular ionic strength-modulating microstructures and properties of nacre-like biomimetic nanocomposites containing high loading clay. RSC Adv. 2012, 2, 6295–6305. [Google Scholar] [CrossRef]
- Chen, H.L.; Lu, Y.M.; Hwang, W.S. Effect of film thickness on structural and electrical properties of sputter-deposited nickel oxide films. Mater. Trans. 2005, 46, 872–879. [Google Scholar] [CrossRef]
- Biju, V.; Khadar, M.A. Electronic structure of nanostructured nickel oxide using Ni 2p XPS analysis. J. Nanopart. Res. 2002, 4, 247–253. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Lau, L.W.M.; Gerson, A.; St Smart, R.C. X-ray photoelectron spectroscopic chemical state quantification of mixed nickel metal, oxide and hydroxide systems. Surf. Interface Anal. 2009, 41, 324–332. [Google Scholar] [CrossRef]
- Zhuang, Z.; Giles, S.A.; Zheng, J.; Jenness, G.R.; Caratzoulas, S.; Vlachos, D.G.; Yan, Y. Nickel supported on nitrogen-doped carbon nanotubes as hydrogen oxidation reaction catalyst in alkaline electrolyte. Nat. Commun. 2016, 7, 10141. [Google Scholar] [CrossRef] [PubMed]
- Delegan, N.; Daghrir, R.; Drogui, P.; El Khakani, M.A. Bandgap tailoring of in-situ nitrogen-doped TiO2 sputtered films intended for electrophotocatalytic applications under solar light. J. Appl. Phys. 2014, 116, 153510–153518. [Google Scholar] [CrossRef]
- Biju, V.; Khadar, M. Dielectric properties of nanostructured nickel oxide. J. Mater. Sci. 2003, 38, 4055–4063. [Google Scholar] [CrossRef]
- Vasu, K.; Sreedhara, M.B.; Ghatak, J.; Rao, C.N.R. Atomic layer deposition of p-type epitaxial thin films of undoped and N-doped anatase TiO2. ACS Appl. Mater. Interfaces 2016, 8, 7897–7901. [Google Scholar] [CrossRef] [PubMed]

| Sample | β (Deg) | D (nm) |
|---|---|---|
| N-doped NiO | 0.65 ± 0.02 | 26.3 ± 0.8 |
| Non-doped NiO | 1.42 ± 0.05 | 12.0 ± 0.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luan, V.H.; Tien, H.N.; Hur, S.H.; Han, J.H.; Lee, W. Three-Dimensional Porous Nitrogen-Doped NiO Nanostructures as Highly Sensitive NO2 Sensors. Nanomaterials 2017, 7, 313. https://doi.org/10.3390/nano7100313
Luan VH, Tien HN, Hur SH, Han JH, Lee W. Three-Dimensional Porous Nitrogen-Doped NiO Nanostructures as Highly Sensitive NO2 Sensors. Nanomaterials. 2017; 7(10):313. https://doi.org/10.3390/nano7100313
Chicago/Turabian StyleLuan, Van Hoang, Huynh Ngoc Tien, Seung Hyun Hur, Jong Hun Han, and Wonoh Lee. 2017. "Three-Dimensional Porous Nitrogen-Doped NiO Nanostructures as Highly Sensitive NO2 Sensors" Nanomaterials 7, no. 10: 313. https://doi.org/10.3390/nano7100313