1. Introduction
As a stable, low-cost and environmentally benign material, nanoscaled titanium dioxide (TiO
2) with unique structural and functional properties has become a widely used semiconductor photocatalyst for various solar-driven clean energy technologies [
1]. Tailoring the morphology of TiO
2 photoanode is a preferred route to achieving high performance in solar cells due to its enhanced properties, such as high surface area, faster electron transport, lower electron-hole recombination rate and good light-harvesting features [
2,
3]. Nevertheless, the wide optical bandgap of TiO
2, which seriously limits its light harvesting capability, leaving about 96% of the solar light energy wasted [
4]. Compared with the solution of generating donor or acceptor states in the band gap by adding impurities, rationally designing and constructing the surface heterostructures would be a more efficient strategy for achieving an excellent photocatalyst [
5].
Recently, Sn
3O
4, a novel non-stoichiometric oxide, has raised particular interest in the field of photocatalysis—especially in terms of its catalytic behavior under visible light irradiation—due to a suitable band-gap inside visible light (2.2–2.9 eV) and a distinct surface structure composed of both valences of tin [
6,
7]. Several studies have shown its great potential as an auspicious photocatalyst under visible light, both for generating hydrogen and degrading dyes [
8]. However, some drawbacks have hindered its performance in practice. As a semiconductor with a relatively narrow band gap, pure Sn
3O
4 generally leads to a fast recombination of photoexcited electron-hole pairs, which ultimately decreases its degradation rate [
9].
Discussing these problems together, it proposes an intriguing idea that Sn
3O
4, as the second component, attaches to the surface of TiO
2 nanostructures, for an exquisite TiO
2/Sn
3O
4 heterostructure. On the one hand, a theoretical analysis indicates that the interface between TiO
2 and Sn
3O
4 is to be a perfect type-II heterojunction (both the potentials of valence band (VB) and conduction band (CB) of Sn
3O
4 are higher than that of TiO
2) [
10], which is actually conducive to the separation of photoexcited electron-hole pairs. Furthermore, latest reports have exhibited the superiority of the heterogeneous composite of this kind [
11,
12]. On the other hand, increased photoactive facets can effectively facilitate the efficiency of photo-absorption and oxygen chemisorption, and bring about a fast rate of surface reactions [
13]. Therefore, highly dispersive anatase TiO
2 mesopore nanospheres, which possess a large number of active surfaces, would likely be an amazing matrix in TiO
2/Sn
3O
4 nanocomposites.
The two-step self-assembly approach is a feasible strategy for the refined design of hierarchical nanostructures with complex morphologies, and has been proven to be an effective way to design multiscale nanostructures, since the morphology and composition obtained from the first step can be further tuned and adjusted by a subsequent second process. Moreover, this approach also allows the combination of multiple synthetic techniques, and the synthesis of complex nanostructures with hierarchical multiscale structures compared with the conventional one-step self-assembling method [
14]. Recently, a lot of complex nanostructures with high photocatalytic performance for both visible light and ultraviolet has been acquired by the two-step synthesis method. Usually, these methods can be classified into two categories: (1) synthesis under two continuous identical methods, such as in [
9,
15,
16]; and (2) synthesis under two different methods, such as in [
17,
18]. In 2015, TiO
2/Sn
3O
4 nanobelts [
9] were successfully produced by first synthesizing the TiO
2 nanobelts, and then assembling Sn
3O
4 onto the TiO
2 nanobelts in a subsequent hydrothermal procedure; in the same year, hierarchical Sn
3O
4/N-TiO
2 nanotubes [
17] were synthesized by first weaving N-doped TiO
2 nanotube via electro-spinning, and then modifying them with Sn
3O
4 via hydrothermal reaction. In 2017, a range of heterojunction WO
3/TiO
2 thin films were deposited via a two-step process using chemical vapor deposition (CVD) methods [
15]. Generally, electro-spinning and chemical vapor deposition is associated with at least one of the following factors: expensive equipment, high voltage, hazardous by-products, or toxic chemicals, rendering the method less environmental friendly and much more complicated than the hydrothermal method or sol-gel synthesis. Hence, synthesizing complex nanostructures via a combination of sol-gel and hydrothermal methods would be a low-cost, scalable, easy to control, and eco-friendly strategy in terms of preparing high-quality, uniform, catalysts.
Herein, we developed a scalable two-step route, combining the sol-gel method and hydrothermal progress to achieve excellent visible and ultraviolet photocatalytic activity by uniformly synthesizing the Sn3O4 nanoparticles on the surface of TiO2 nanospheres. As expected, an enormous enhancement of photocatalytic efficiency was achieved by the distinctive TiO2/Sn3O4 nanocomposites.
3. Results and Discussion
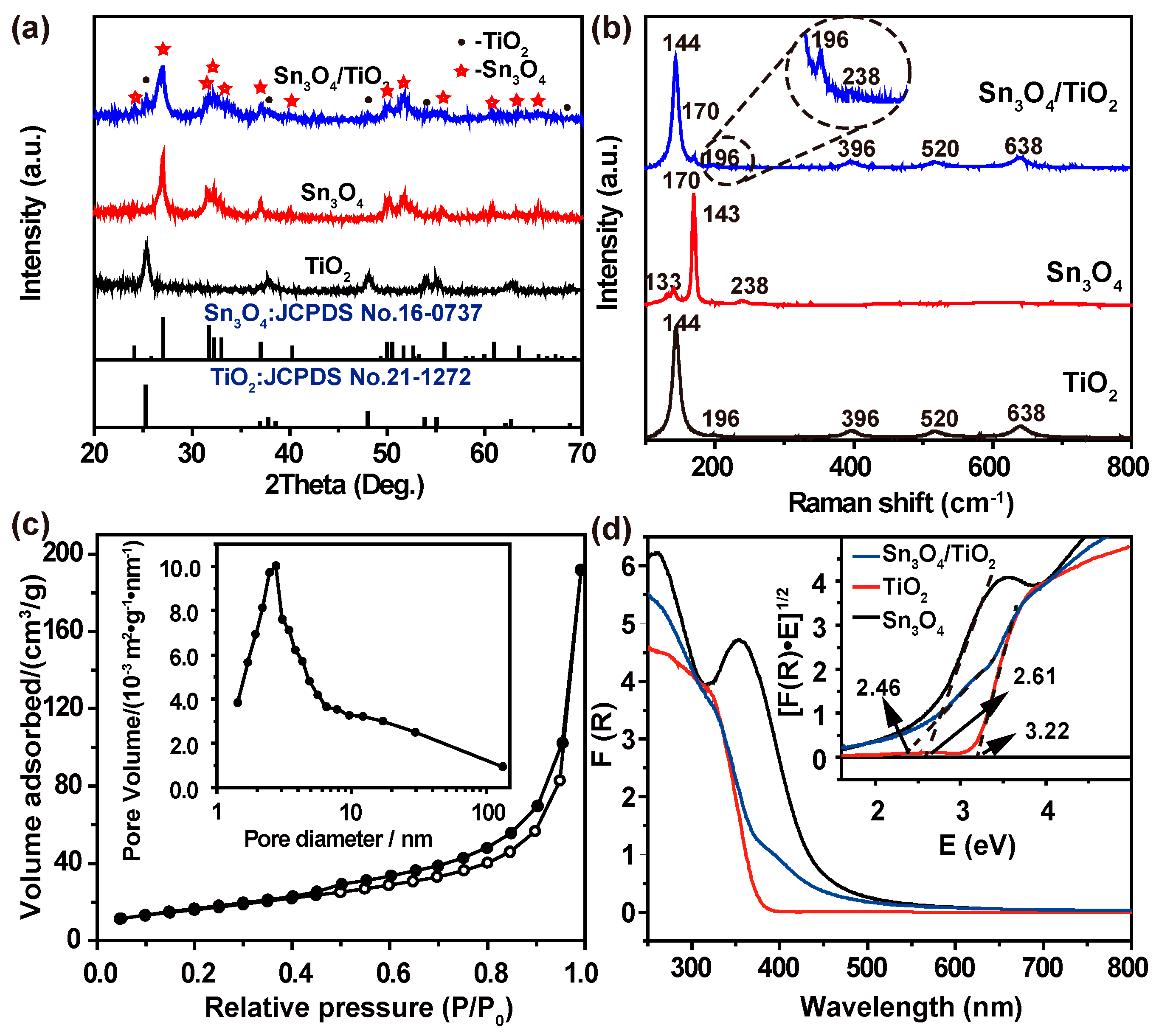
Figure 1a illustrates the XRD pattern of the TiO
2/Sn
3O
4 nanocomposites. All the diffraction peaks can be indexed to the anatase TiO
2 (JCPDS 21-1272, marked with black •) and triclinic Sn
3O
4 (JCPDS 16-0737, marked with red ☆), validating the high purity of the synthesized TiO
2/Sn
3O
4 composite phase. Compared with the single-phase TiO
2 and Sn
3O
4, all the diffraction peaks are broad and weak, which indicates that the crystallinities are slightly reduced [
21]. This result may be attributed to lattice distortion induced by interfacial strain because of different lattice parameters between Sn
3O
4 and TiO
2 [
22]. In addition, Raman spectroscopy results (
Figure 1b) further confirm the purity of the synthesized TiO
2/Sn
3O
4 composite phase. Specifically, Raman activities of 144, 196, 396, 520 and 638 cm
−1 were assigned to the anatase TiO
2 [
23], and the 133, 143, 170 and 238 cm
−1 Raman peaks could be attributed to the Sn
3O
4, in accordance with previous reports [
7,
24]. The textures of the as-synthesized TiO
2/Sn
3O
4, TiO
2, Sn
3O
4 and P25 were characterized by N
2 physisorption experiments. The Brunauer–Emmett–Teller (BET) surface area data of samples are provided in
Table 1. The N
2 adsorption-desorption isotherm and pore-size distribution of TiO
2/Sn
3O
4 nanocomposites are shown in
Figure 1c. The results display that the TiO
2/Sn
3O
4 nanocomposites possess an average pore diameter of 2.733 nm and a larger surface area of 68.1 m
2/g than the as-prepared TiO
2 (0.04 m
2/g), Sn
3O
4 (35.2 m
2/g), and the reported TiO
2/Sn
3O
4 nanobelt heterostructure (51.5 m
2/g) [
9]. Such a high surface-to-volume ratio for the TiO
2/Sn
3O
4 nanocomposites might be of extreme good value in photocatalytic processes, as they would provide more active sites for the adsorption of reactant molecules, and their optical absorbance would increase at visible wavelengths [
25].
Figure 1d shows the UV-visible diffusion reflectance spectra (DRS) and plots of (F(R)
hv)
1/2 versus photo energy (
hv) of the TiO
2/Sn
3O
4 along with spectra of the pristine TiO
2 and Sn
3O
4 for comparison. The absorption spectra of the TiO
2/Sn
3O
4 nanocomposites exhibit the mixed absorption properties of both the components. In particular, the absorption edge for TiO
2/Sn
3O
4 nanocomposites is clearly shifted towards visible region (near 505 nm). The optical band gap determined from the plot of the Kubelka-Munk function was found to be 2.46 eV, compared to the observed values of 3.22 eV and 2.61 eV for TiO
2 and Sn
3O
4, respectively. These data reveal the Sn
3O
4/TiO
2 nanocomposites have a lower band gap than the pure Sn
3O
4 and TiO
2 nanoparticles, which is consistent with the published literature [
9], and can be explained by the reduced crystallinity of both materials [
1], as shown by XRD analysis.
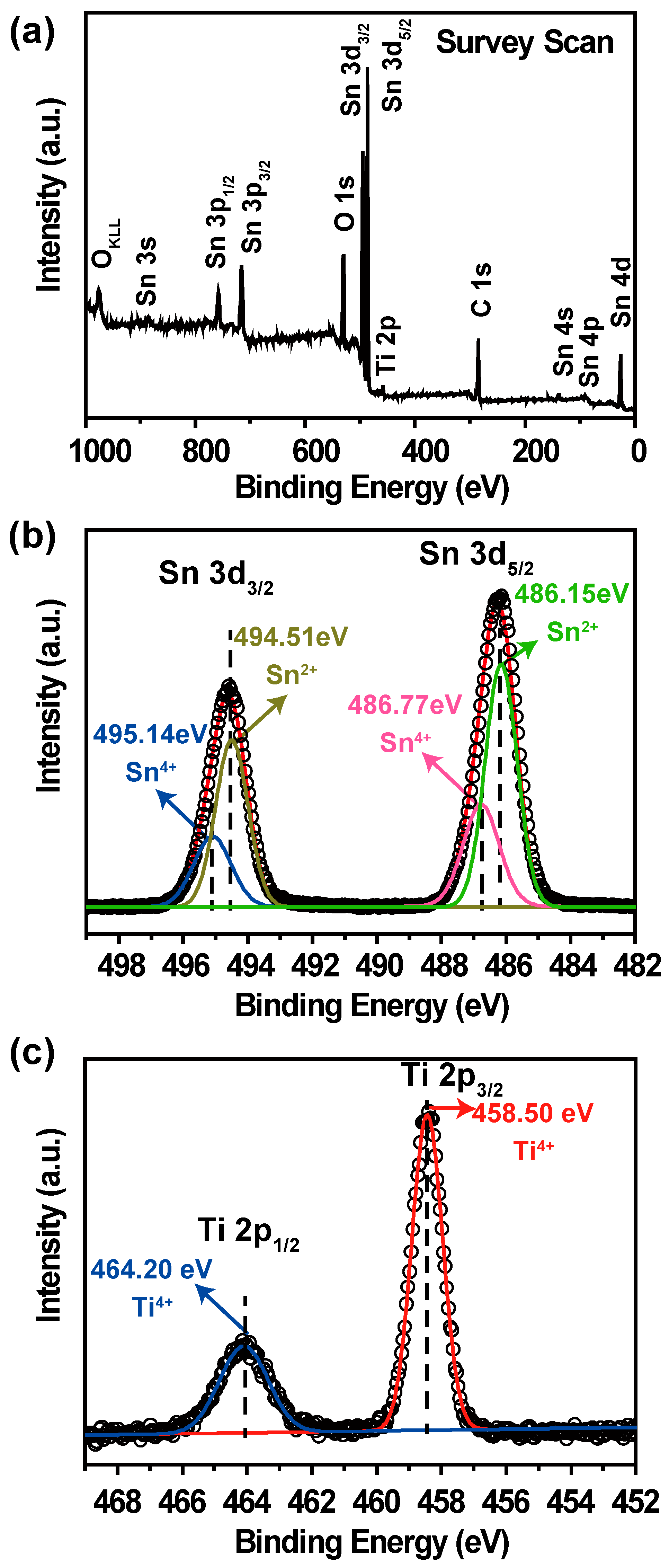
The chemical composition and valence state were characterized by X-ray photoelectron spectroscopy (XPS). The full range of XPS spectra, ranging from 0 to 1000 eV, of TiO
2/Sn
3O
4 nanocomposites are shown in
Figure 2a. No impurities were observed in the spectra, which is consistent with the results of XRD and Raman.
Figure 2b shows the curve fitting data of the Sn 3d core-level spectra. Moreover, the Sn 3d doublet characterized by Sn 3d
3/2–Sn 3d
5/2 splitting peak can be clearly observed. The prominent peak of Sn 3d
5/2 level is dissolved into two peaks centered at 486.77 and 486.15 eV, which can be attributed to Sn(IV) and Sn(II) configurations [
26], respectively. The Sn 3d
3/2 spectra exhibit two peaks at 495.14 and 494.51 eV, which are assigned to Sn
2+ and Sn
4+ [
26]. As shown in
Figure 2c, the binding energies (BE) of Ti 2p
3/2 and Ti 2p
1/2 are 458.5 and 464.2 eV respectively, which are ascribed to the Ti
4+ oxidation states [
27]. On the basis of the above discussion, it can be concluded that the TiO
2/Sn
3O
4 sample is composed of Ti(IV), Sn(II and IV), and O, which is in good agreement with the XRD and Raman results. In addition, the calculated Ti/Sn ratio is 0.20, indicating that most of the surface of the TiO
2 nanocrystals is covered by Sn
3O
4 nanocrystals (SEM and TEM experiments further confirm this result, and will be discussed later).
The morphology and microstructure of the as-prepared TiO
2/Sn
3O
4 nanocomposites were carefully analyzed by microscopy. Generally, lots of interleaved Sn
3O
4 nanoplates are able to self-assemble into an ordinary flower-like nanostructure, as shown in
Figure 3a. Similarly, by introducing highly monodispersed TiO
2 nanospheres of ~130 nm diameter (
Figure 3b) into the growth environment, the homogeneous Sn
3O
4 nanoparticles started to grow on the surface of each individual TiO
2 core with intimate contact, thus forming an interface of two different semiconductors that would facilitate photo-excited electron transfer and photon-generated carrier separation (
Figure 3c,d). Noticeably, the composites inherit a favorable dispersion in the solution, and fully contact with the absorbate, which is positive for the outstanding photocatalytic performance. However, it should be noted that the TiO
2/Sn
3O
4 nanocomposites are not completely covered by Sn
3O
4 nanocrystals. Furthermore, these advantageous heterojunctions with 10–20 nm sizes wrapping uniformly onto the surface of TiO
2 nanospheres were verified by TEM observation, as shown in
Figure 1e,f. The well-resolved lattice fringes from the core and shell regions manifestly correspond to the (101) planes of anatase TiO
2 and the (
10) planes of Sn
3O
4, respectively, clearly revealing the phase distribution of the TiO
2/Sn
3O
4 nanocomposites again.
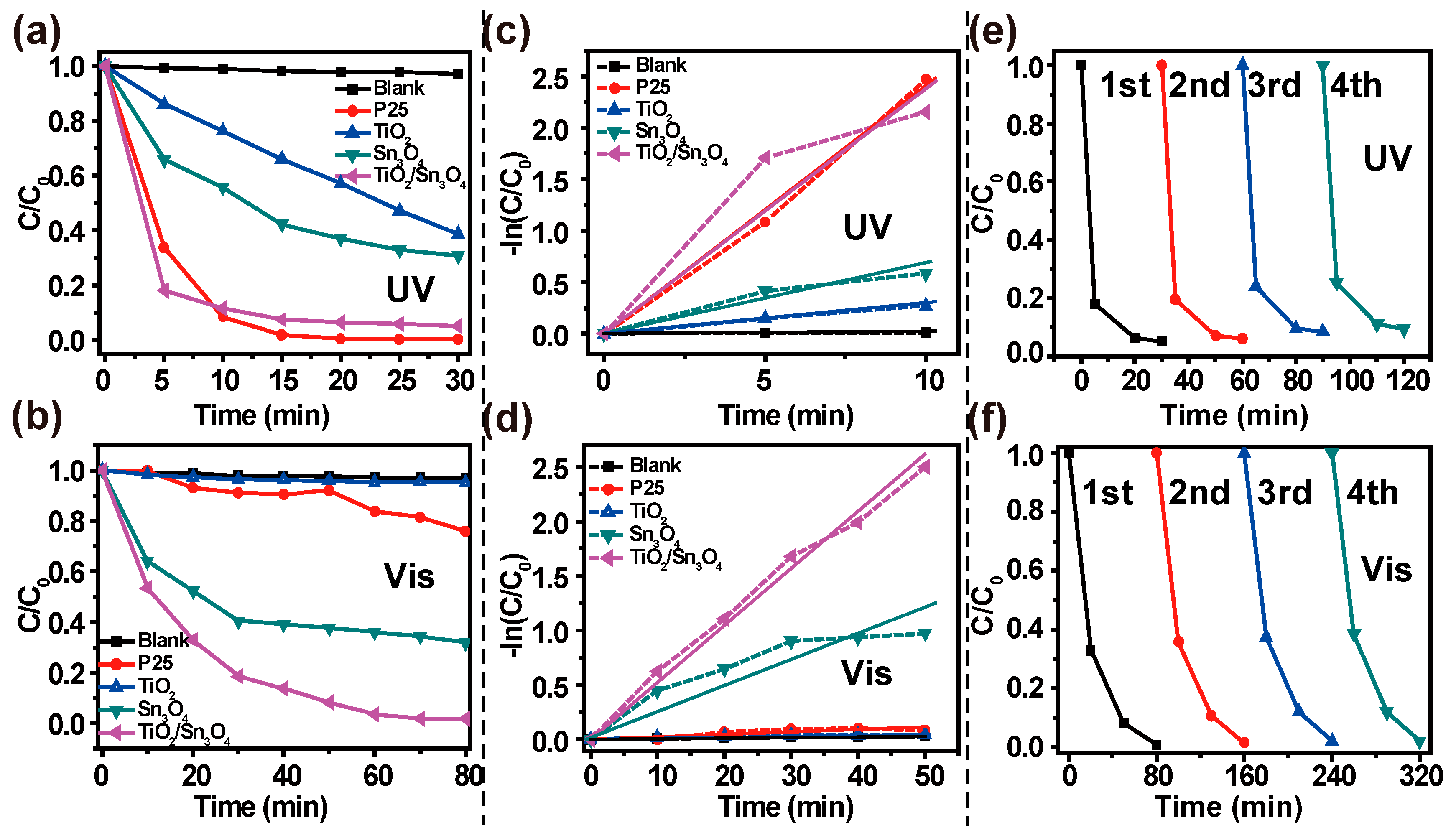
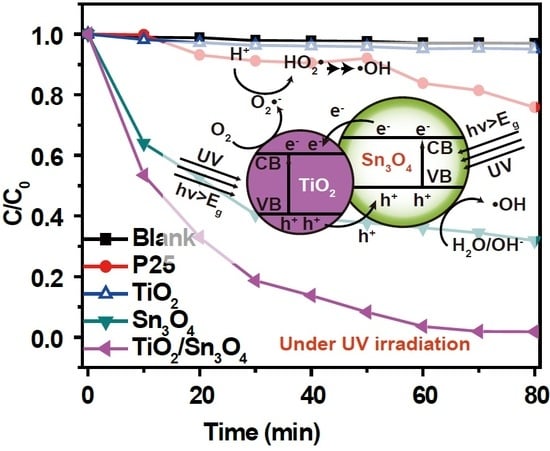
The photocatalytic activities of P25, TiO
2, Sn
3O
4 and TiO
2/Sn
3O
4 heterostructures were evaluated by the degradation of MO in water under UV- and visible-light irradiation (
Figure 4a,b).The degradation of the MO solution under identical experimental conditions, but with no photocatalyst, is provided for comparison. The degradation efficiency of the as-synthesized TiO
2/Sn
3O
4 heterostructures was defined as C/C
0, where C
0 is the initial concentration of MO after equilibrium adsorption, and C is the concentration during the reaction. Both blank experiment results showed that MO could not be decomposed without photocatalyst under UV- or visible-light irradiation. In contrast, the photodegradation efficiency of TiO
2/Sn
3O
4 nanocomposites was 95% within 30 min under UV-light irradiation, which is superior to the as-prepared TiO
2 nanospheres (62%) and Sn
3O
4 nanoplates (70%). Furthermore, the MO decomposition efficiency found for the TiO
2/Sn
3O
4 photocatalyst was comparable to that determined under the same experimental conditions for the reference P25 catalyst; that is, 99% after 10 min. Additionally, in the visible-light irradiation experiment (
Figure 4b), the TiO
2/Sn
3O
4 nanocomposites (81%) exhibited significantly higher photocatalytic activity than P25 (9%), TiO
2 (0.4%) and Sn
3O
4 (60%) at 30 min. Finally, MO was completely degraded within 80 min. The results show that the TiO
2/Sn
3O
4 heterostructures exhibited improved photocatalytic activity.
For a better understanding, the photocatalytic kinetics of the samples was analyzed using the Langmuir-Hinshelwood model, as shown in
Figure 4c,d. All of the data follow a first-order reaction model, and the calculated apparent kinetic rate constants (κ) are summarized in
Table 1. We found that, under UV irradiation, the TiO
2/Sn
3O
4 exhibited a much faster photo-decomposition activity (κ = 0.24 min
−1) than the pure TiO
2 (0.028 min
−1) and Sn
3O
4 (0.064 min
−1), and was as fast as the P25 (0.24 min
−1). Furthermore, under visible-light irradiation, the calculated value of κ for the TiO
2/Sn
3O
4 sample (κ = 0.052 min
−1) was twice as high as that for the neat Sn
3O
4 (0.024 min
−1), and more than a dozen times higher than that for the single TiO
2 (0.0010 min
−1) and the P25 (κ = 0.0023 min
−1). In addition, the TiO
2/Sn
3O
4 heterostructures could be recycled and reused at least four times without significant loss of efficiency (
Figure 4e,f), which demonstrates its great potential as an efficient and stable photocatalytic material. These remarkably good performances can be attributed to the improved UV- and visible-light absorption efficiency, and the high photo-excited carrier-separation rate resulting from the novel TiO
2/Sn
3O
4 heterostructures.
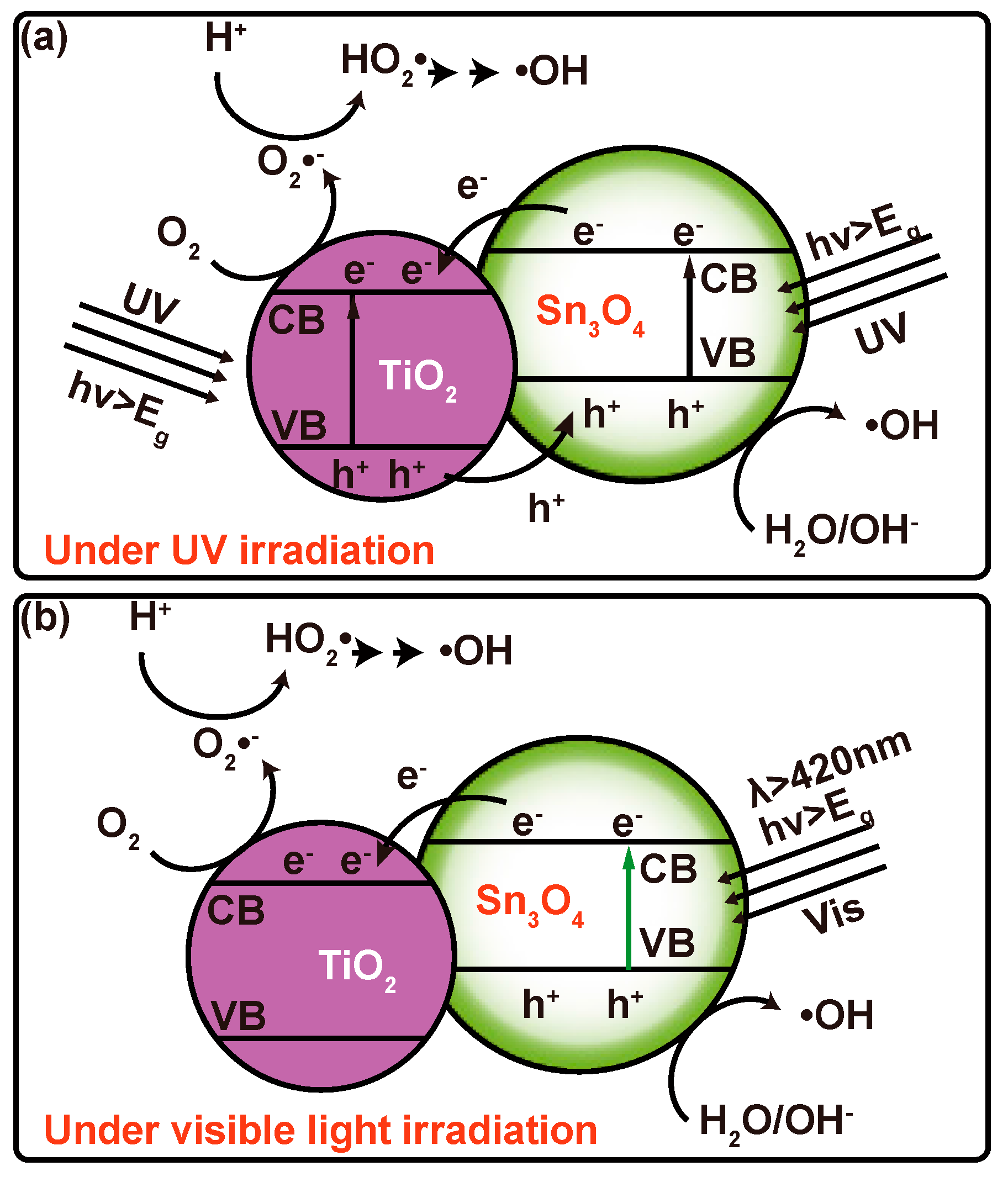
Based on all of the results above, a possible mechanism for charge transfer and photocatalytic process can be proposed (
Scheme 1). As illustrated in
Figure 1d, the diffusion reflectance spectra (DRS) and plots of (F(R)
hv)
1/2 versus photo energy (
hv) indicate that the bandgap of Sn
3O
4 (2.61 eV) is smaller than that of TiO
2 (3.22 eV). Additionally, the potentials of the valence band (VB) and conduction band (CB) of Sn
3O
4 are higher than those of TiO
2, so the heterostructure of TiO
2/Sn
3O
4 belongs to typical type-II heterojunction [
9]. When Sn
3O
4 contacts TiO
2 cores to form a heterojunction, the difference in chemical potential causes band bending at the interface of the junction [
28], which drives photoexcited electrons to transfer from Sn
3O
4 to TiO
2, and photoexcited holes to migrate in the opposite direction, until the Fermi levels of TiO
2 and Sn
3O
4 reach equilibrium. The possible mechanisms for charge transfer and hydroxyl radical (•OH) generation under UV- and visible-light irradiation will be discussed separately. (1) Upon UV illumination, electrons in the VB could be excited to the CB of both oxides, simultaneously forming the same number of holes in the VB. This is due to the fact that the Sn
3O
4 nanoparticles were not fully coated as a shell onto the TiO
2 nanospheres (
Figure 3c,d) and that the suitable bandgap of TiO
2 and Sn
3O
4 is lower than the energy of ultraviolet photons. Next, the photo-generated electrons were collected by the TiO
2 particles and the holes by the Sn
3O
4 particles; that is, electrons transferred from Sn
3O
4 to TiO
2, and holes migrated from TiO
2 to Sn
3O
4 (compare
Scheme 1a with
Figure 1d). The unique behavior that electrons and holes preferentially accumulate on different materials would result in a great separation of photo-generated carriers, and thus reduce the charge recombination rate, ultimately increasing carrier lifetime. As a consequence, the formation efficiency of hydroxyl radicals (•OH)—a strong oxidant for most pollutants [
9,
20,
29]—by the reaction of holes with surface hydroxyl groups or physisorbed water molecules at the Sn
3O
4 surface and the production rates of •OH and superoxide radicals (O
2−) radicals resulting from the reactions of electrons with dissolved oxygen molecules and water molecules will be massively enhanced; this will increase the volume of oxidant inside the system. (2) Under visible-light irradiation (
Scheme 1b), electrons in the VB could be exclusively excited to the CB of Sn
3O
4, with a concomitant formation of the same number of holes in the VB. Due to the type II band alignment of the as-prepared sample, the photoexcited electrons in the Sn
3O
4 CB will be easily injected into the TiO
2 CB, where the electrons could reduce surface-absorbed O
2 over TiO
2 active sites to form superoxide radicals (O
2−), and the new species can further yield •OH by reacting with water or oxidize MO. On the other hand, holes remaining in Sn
3O
4 could react with surface-absorbed H
2O to generate more •OH. Hydroxyl radicals (•OH) and superoxide radicals (O
2−) stemming from the above procedure will degrade MO into colorless chemicals, and even CO
2 and H
2O, which is similar under UV illumination. All in all, the enhanced charge separation related to the TiO
2/Sn
3O
4 heterojunction favors the interfacial charge transfer to physisorbed species, forming •OH radicals and reducing possible back reactions, and therefore accounts for the higher activity of the TiO
2/Sn
3O
4 nanocomposites.
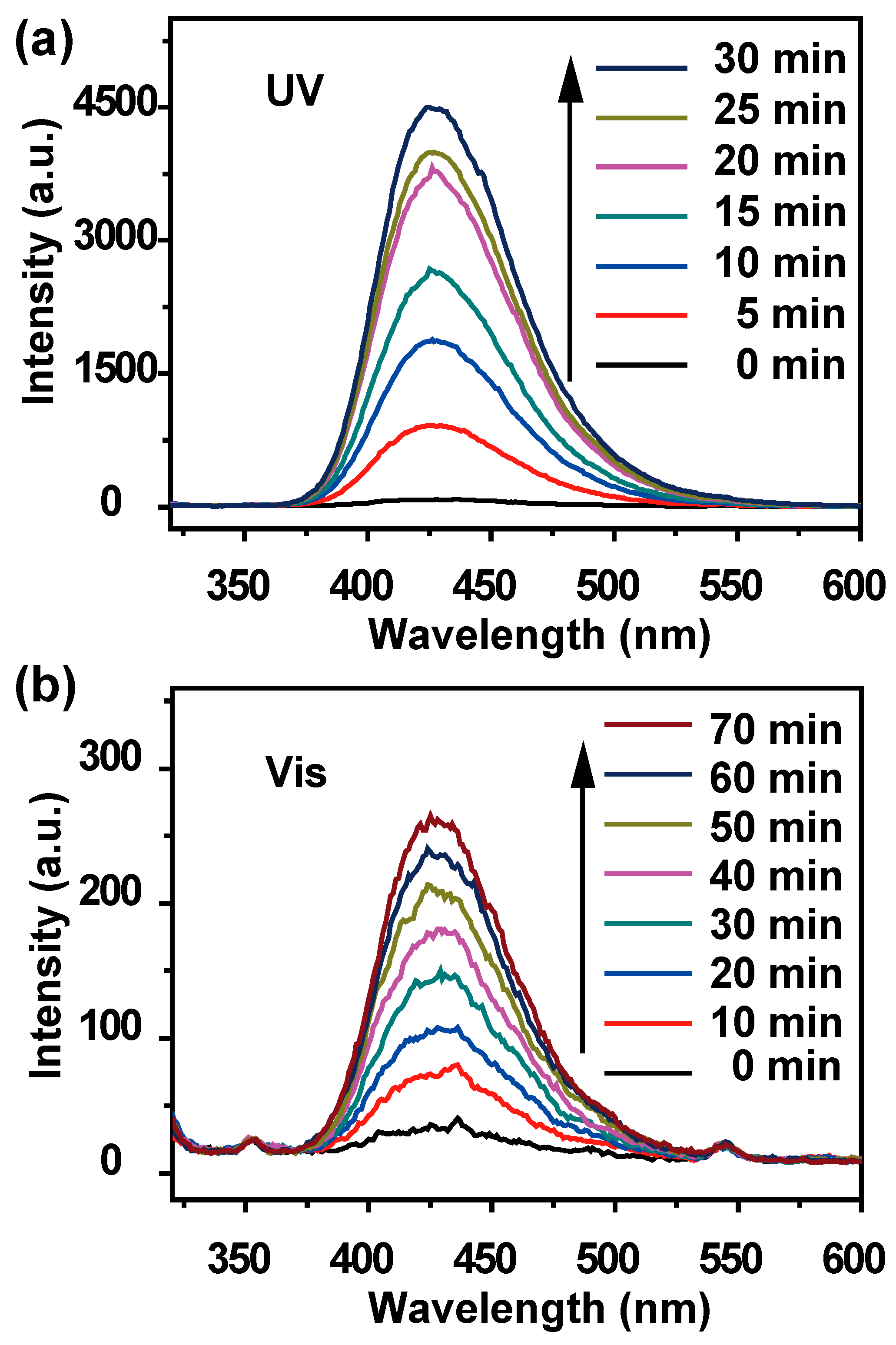
The photocatalytic oxidation of dyes occurs through the reactive species, which came into being after the light absorption and electron-hole formation by the photocatalyst [
30]. Terephthalic acid photoluminescence probing technique (TAPL) was employed to examine the generation of active •OH radicals [
31].
Figure 5a,b gives the •OH-trapping photoluminescent spectra of TiO
2/Sn
3O
4 nanocomposites in TA solution with UV- and visible-light irradiation, respectively. The increased photoluminescence intensity confirms that the •OH radicals are mainly responsible for the photodegradation process, and it also verifies the photocatalytic activity of the TiO
2/Sn
3O
4 nanocomposites.