Flotation Assembly of Large-Area Ultrathin MWCNT Nanofilms for Construction of Bioelectrodes
Abstract
:1. Introduction
2. Results
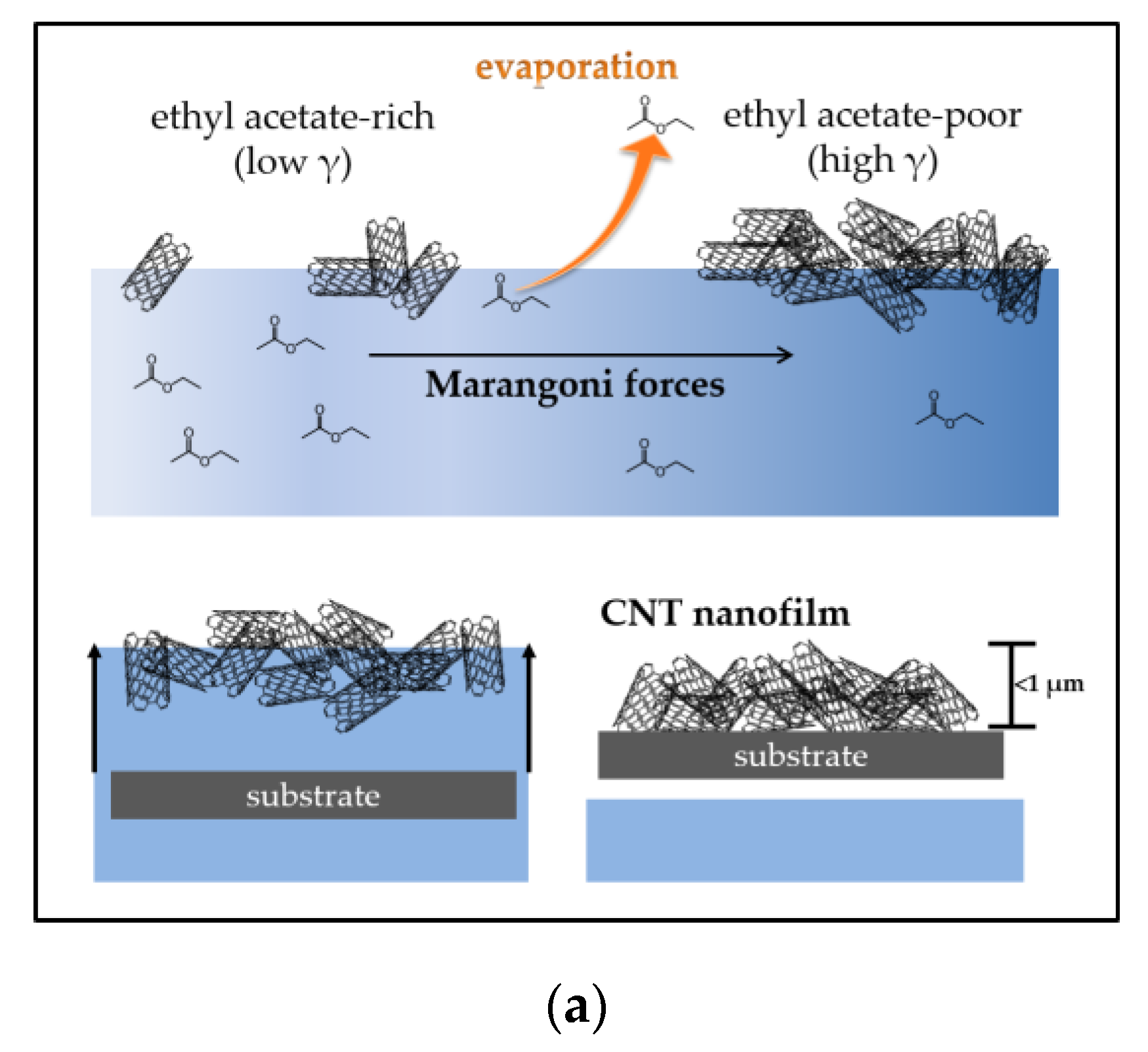
2.1. Flotation Assembly of Thin and Thick MWCNT Films
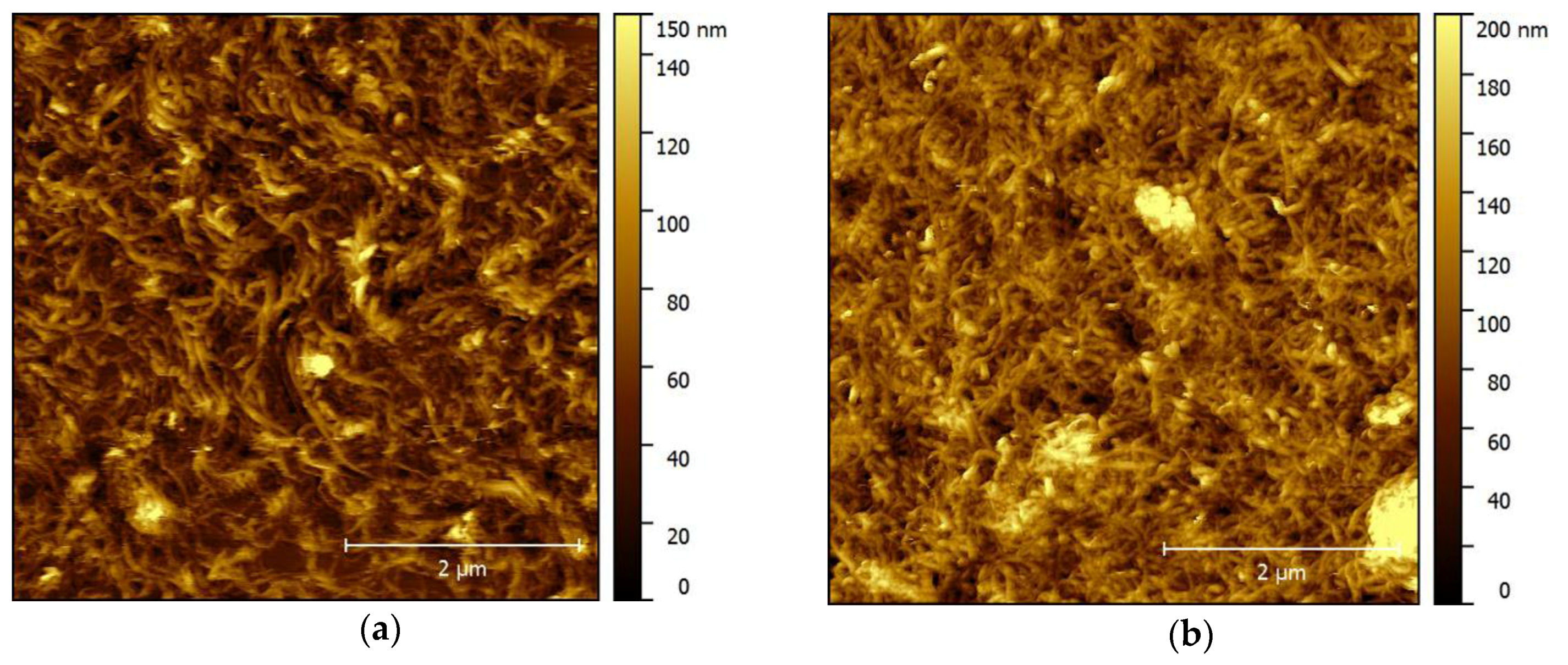
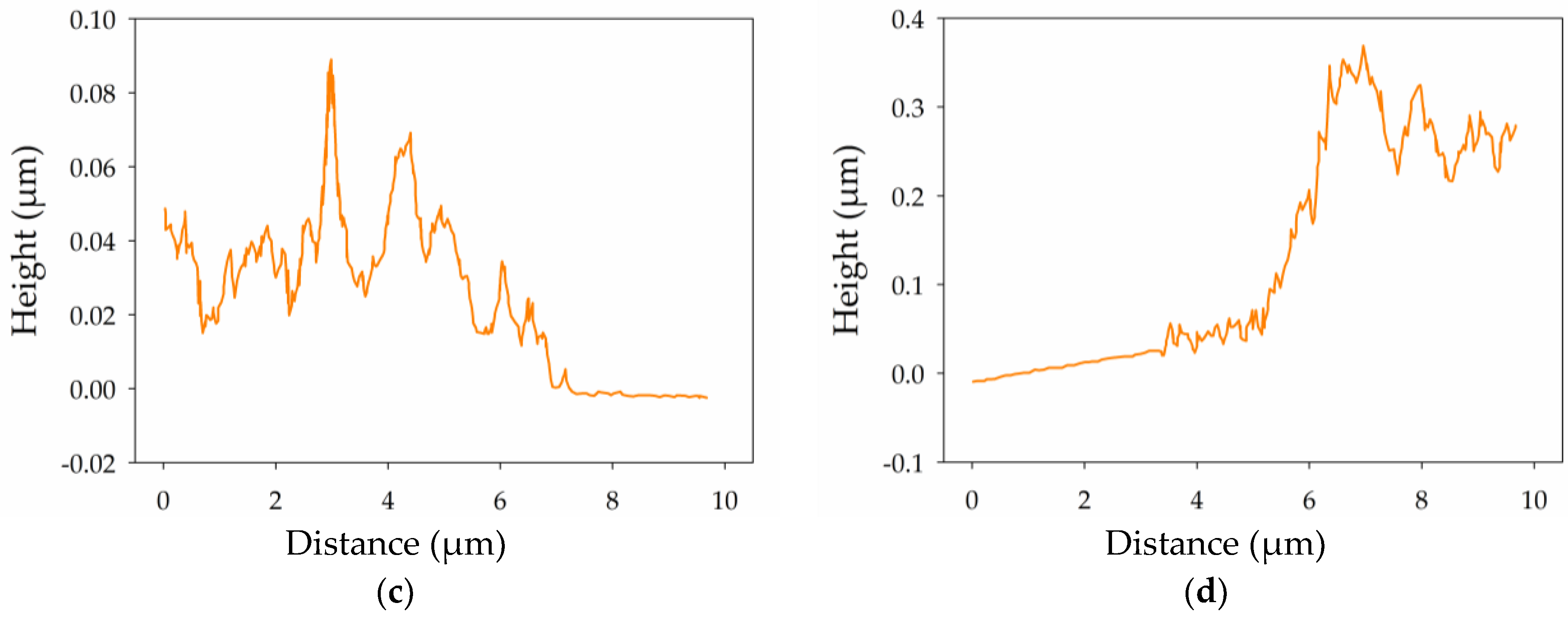
2.2. Surface Microscopy of Thin and Thick MWCNT Films
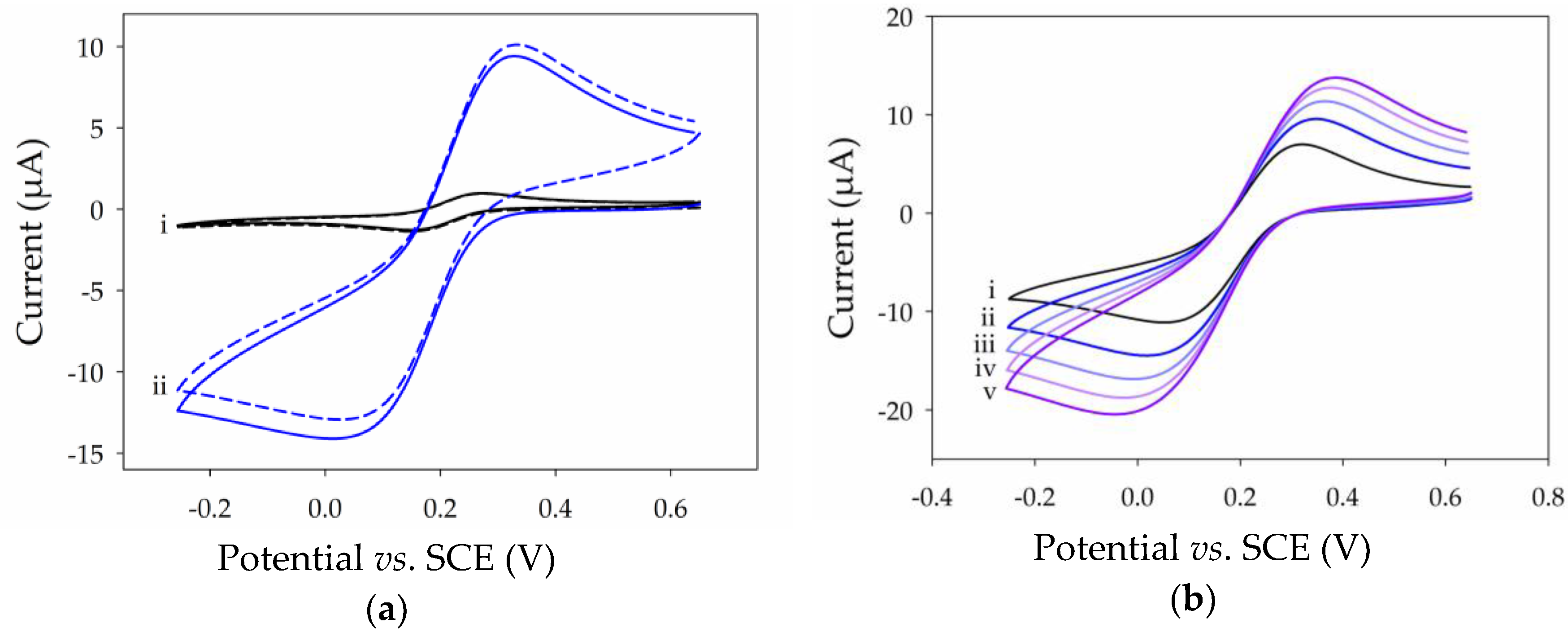
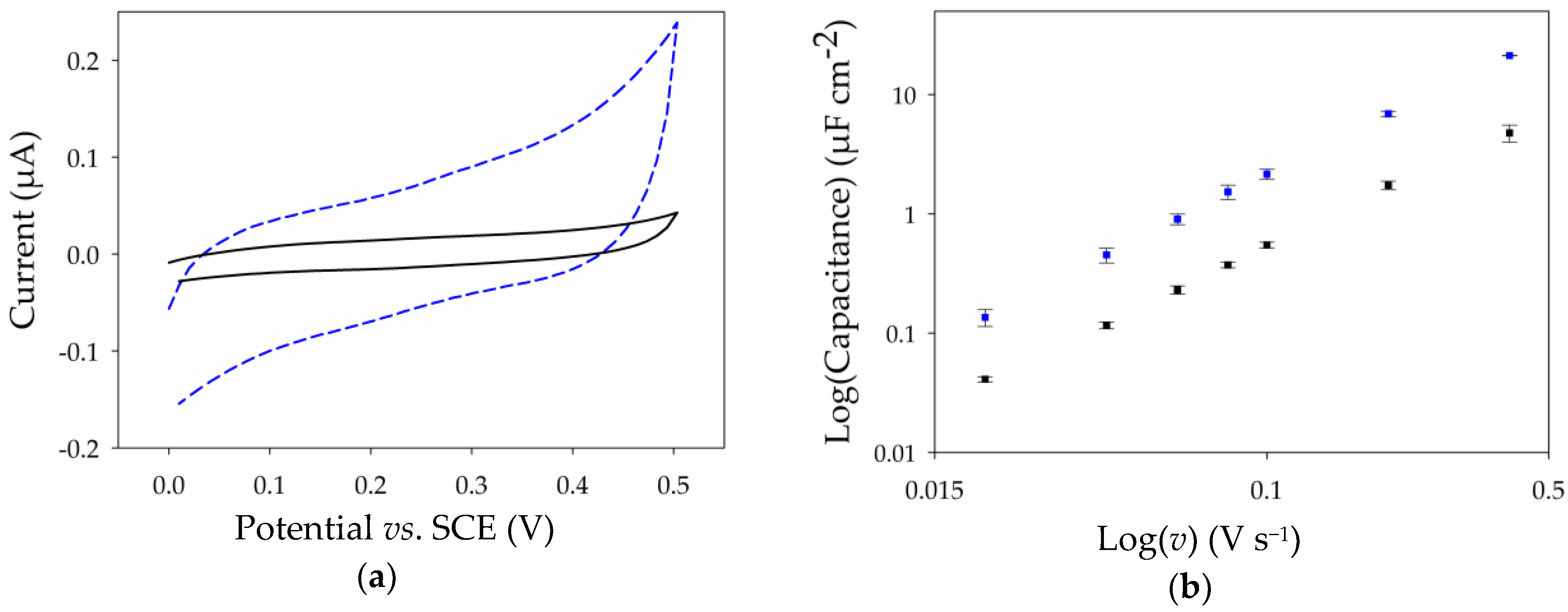
2.3. Electrochemical Properties of Thin MWCNT Film Electrode
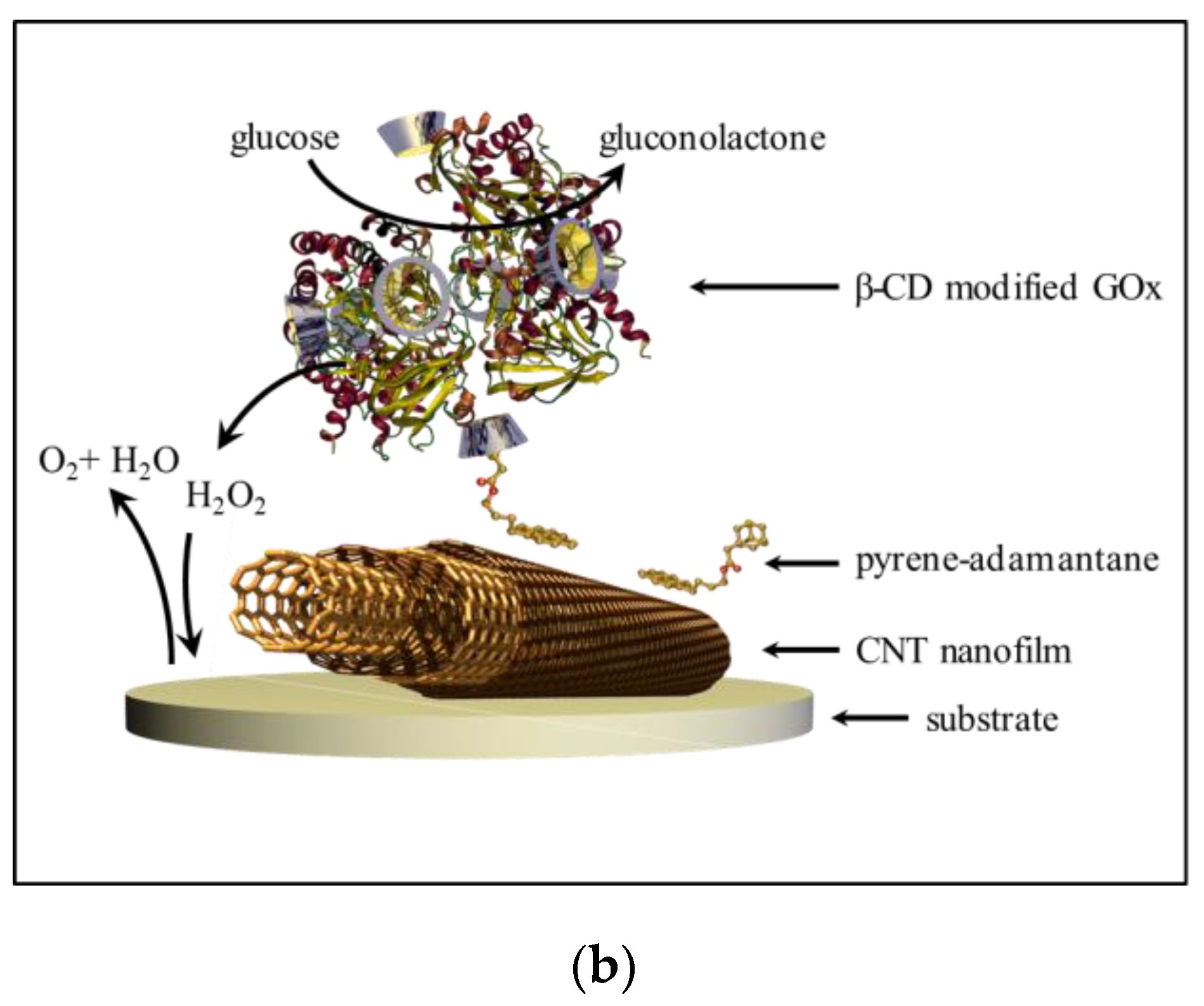
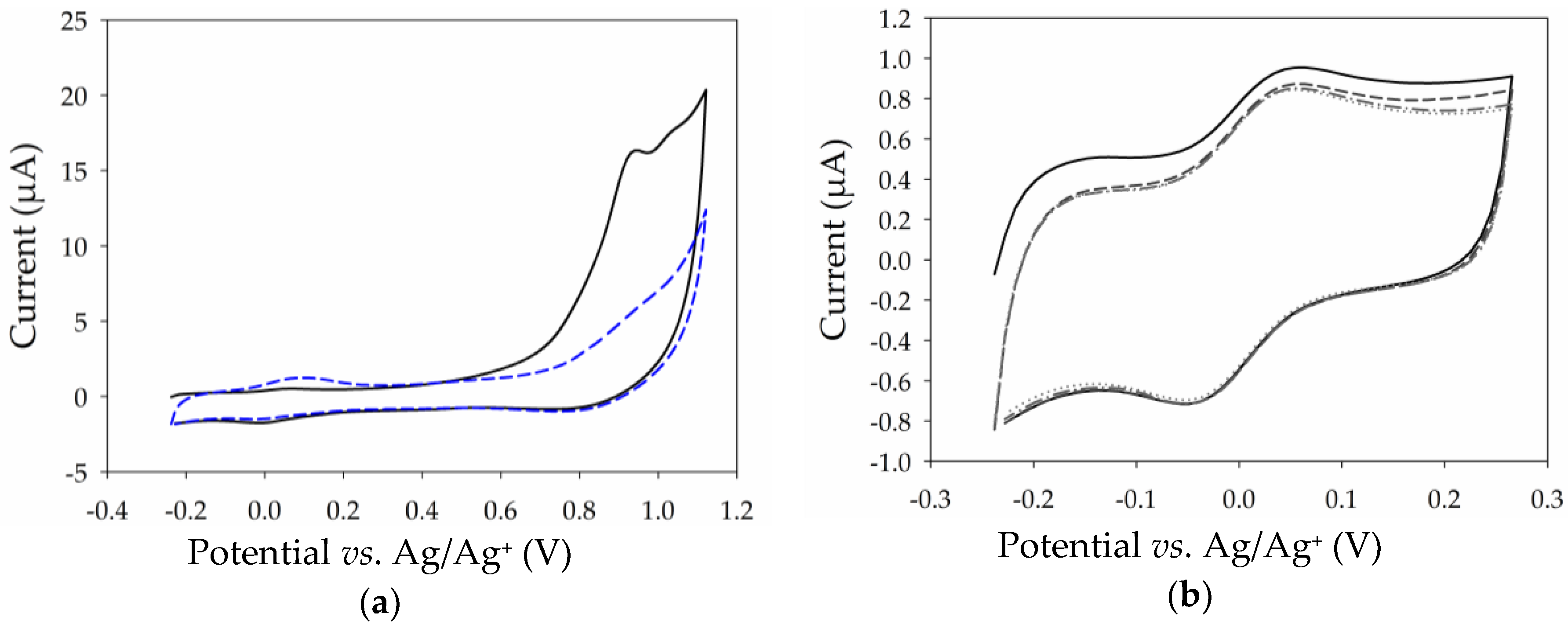
2.4. Surface Modification of Thin MWCNT Film Electrode with Pyrene-Adamantane
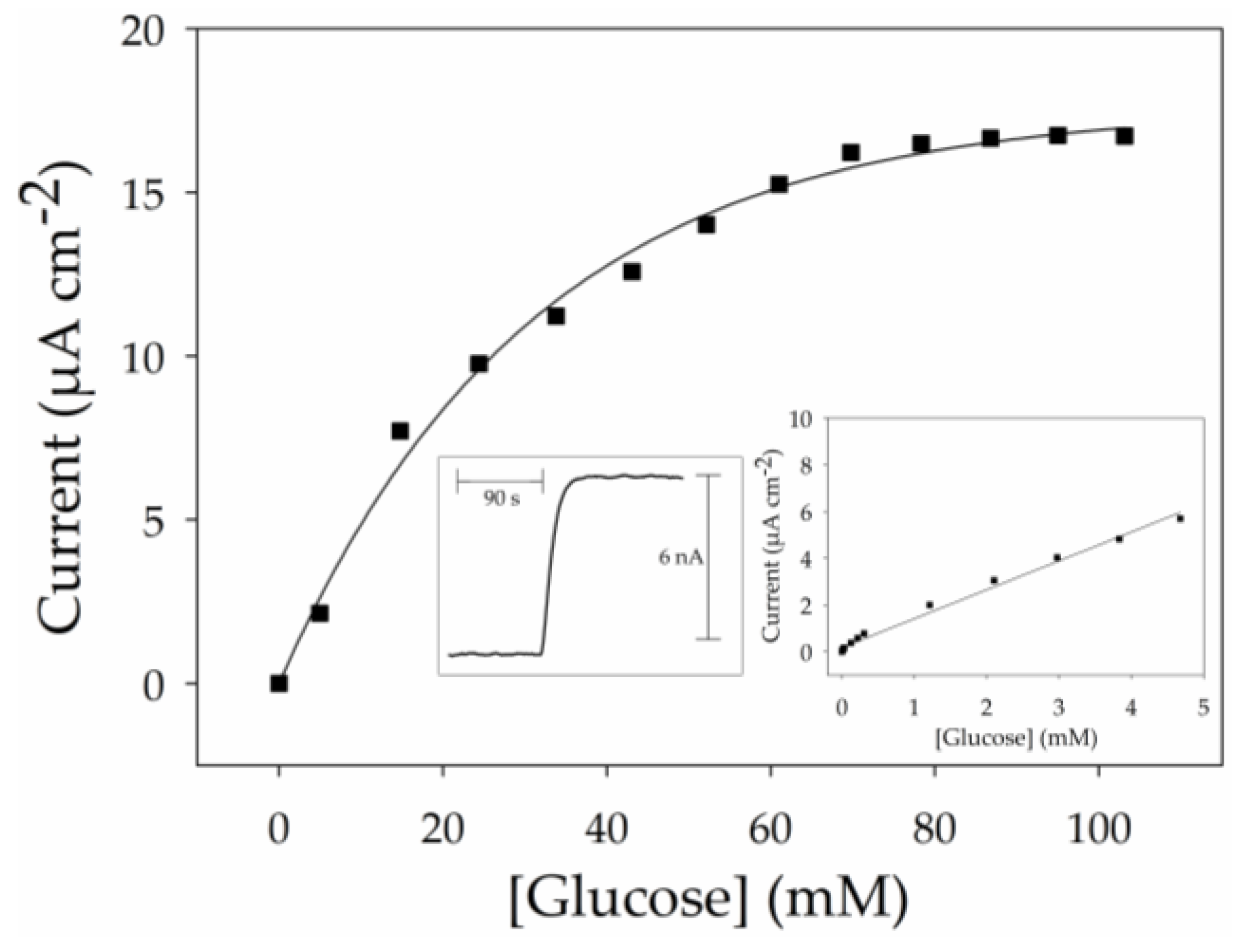
2.5. GOx-Modified Thin MWCNT Film Electrode
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Preparation of MWCNT Films by Flotation Assembly
4.2.2. GOx-β-CD Immobilization
4.2.3. Electrochemistry
4.2.4. Microscopy and Spectroscopy Imaging
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dai, H. Carbon Nanotubes: Synthesis, Integration, and Properties. Acc. Chem. Res. 2002, 35, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Salvetat, J.-P.; Bonard, J.-M.; Thomson, N.H.; Kulik, A.J.; Forró, L.; Benoit, W.; Zuppiroli, L. Mechanical properties of carbon nanotubes. Appl. Phys. A 1999, 69, 255–260. [Google Scholar] [CrossRef]
- Smart, S.K.; Cassady, A.I.; Lu, G.Q.; Martin, D.J. The biocompatibility of carbon nanotubes. Carbon 2006, 44, 1034–1047. [Google Scholar] [CrossRef]
- Peigney, A.; Laurent, C.; Flahaut, E.; Bacsa, R.R.; Rousset, A. Specific surface area of carbon nanotubes and bundles of carbon nanotubes. Carbon 2001, 39, 507–514. [Google Scholar] [CrossRef] [Green Version]
- Georgakilas, V.; Kordatos, K.; Prato, M.; Guldi, D.M.; Holzinger, M.; Hirsch, A. Organic Functionalization of Carbon Nanotubes. J. Am. Chem. Soc. 2002, 124, 760–761. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A. Functionalization of Single-Walled Carbon Nanotubes. Angew. Chem. Int. Ed. 2002, 41, 1853–1859. [Google Scholar] [CrossRef]
- Cosnier, S.; Holzinger, M.; Le Goff, A. Recent Advances in Carbon Nanotube-Based Enzymatic Fuel Cells. Front. Bioeng. Biotechnol. 2014, 2, 45. [Google Scholar] [CrossRef] [PubMed]
- Cang-Rong, J.T.; Pastorin, G. The influence of carbon nanotubes on enzyme activity and structure: Investigation of different immobilization procedures through enzyme kinetics and circular dichroism studies. Nanotechnology 2009, 20, 255102. [Google Scholar] [CrossRef] [PubMed]
- Saifuddin, N.; Raziah, A.Z.; Junizah, A.R. Carbon Nanotubes: A Review on Structure and Their Interaction with Proteins. J. Chem. 2013, 18. [Google Scholar] [CrossRef]
- Cosnier, S.; Gross, A.J.; Le Goff, A.; Holzinger, M. Recent advances on enzymatic glucose/oxygen and hydrogen/oxygen biofuel cells: Achievements and limitations. J. Power Sources 2016, 325, 252–263. [Google Scholar] [CrossRef]
- Gross, A.J.; Chen, X.; Giroud, F.; Abreu, C.; Le Goff, A.; Holzinger, M.; Cosnier, S. A High Power Buckypaper Biofuel Cell: Exploiting 1,10-Phenanthroline-5,6-dione with FAD-Dependent Dehydrogenase for Catalytically-Powerful Glucose Oxidation. ACS Catal. 2017, 7, 4408–4416. [Google Scholar] [CrossRef]
- Zebda, A.; Cosnier, S.; Alcaraz, J.-P.; Holzinger, M.; Goff, A.L.; Gondran, C.; Boucher, F.; Giroud, F.; Gorgy, K.; Lamraoui, H.; et al. Single Glucose Biofuel Cells Implanted in Rats Power Electronic Devices. Sci. Rep. 2013, 3, srep01516. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Electrochemical Glucose Biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Special Issue for Wearable Electrochemical Sensors. Electroanalysis 2016, 28, 1148. [Google Scholar] [CrossRef]
- Zhou, Y.; Azumi, R. Carbon nanotube based transparent conductive films: Progress, challenges, and perspectives. Sci. Technol. Adv. Mater. 2016, 17, 493–516. [Google Scholar] [CrossRef] [PubMed]
- Feifel, S.C.; Lokstein, H.; Hejazi, M.; Zouni, A.; Lisdat, F. Unidirectional Photocurrent of Photosystem I on π-System-Modified Graphene Electrodes: Nanobionic Approaches for the Construction of Photobiohybrid Systems. Langmuir 2015, 31, 10590–10598. [Google Scholar] [CrossRef] [PubMed]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- José, Y.M.; Miki, Y.M.; Rendón, L.; Santiesteban, J.G. Catalytic growth of carbon microtubules with fullerene structure. Appl. Phys. Lett. 1993, 62, 657–659. [Google Scholar] [CrossRef]
- Guo, T.; Nikolaev, P.; Thess, A.; Colbert, D.T.; Smalley, R.E. Catalytic growth of single-walled manotubes by laser vaporization. Chem. Phys. Lett. 1995, 243, 49–54. [Google Scholar] [CrossRef]
- De Volder, M.F.L.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon nanotubes: Present and future commercial applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.W.; Kitt, A.; Magnuson, C.W.; Hao, Y.; Ahmed, S.; An, J.; Swan, A.K.; Goldberg, B.B.; Ruoff, R.S. Transfer of CVD-Grown Monolayer Graphene onto Arbitrary Substrates. ACS Nano 2011, 5, 6916–6924. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gong, X.-L.; Gai, J.-G. Progress and Challenges in Transfer of Large-Area Graphene Films. Adv. Sci. 2016, 3, 1500343–1500358. [Google Scholar] [CrossRef] [PubMed]
- Yuksel, R.; Sarioba, Z.; Cirpan, A.; Hiralal, P.; Unalan, H.E. Transparent and Flexible Supercapacitors with Single Walled Carbon Nanotube Thin Film Electrodes. ACS Appl. Mater. Interfaces 2014, 6, 15434–15439. [Google Scholar] [CrossRef] [PubMed]
- Palomar, Q.; Gondran, C.; Holzinger, M.; Marks, R.; Cosnier, S. Controlled carbon nanotube layers for impedimetric immunosensors: High performance label free detection and quantification of anti-cholera toxin antibody. Biosens. Bioelectron. 2017, 97, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Giroud, F.; Sawada, K.; Taya, M.; Cosnier, S. 5,5-Dithiobis(2-nitrobenzoic acid) pyrene derivative-carbon nanotube electrodes for NADH electrooxidation and oriented immobilization of multicopper oxidases for the development of glucose/O2 biofuel cells. Biosens. Bioelectron. 2017, 87, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Chen, Z.; Du, X.; Logan, J.M.; Sippel, J.; Nikolou, M.; Kamaras, K.; Reynolds, J.R.; Tanner, D.B.; Hebard, A.F.; et al. Transparent, Conductive Carbon Nanotube Films. Science 2004, 305, 1273–1276. [Google Scholar] [CrossRef] [PubMed]
- Putzbach, W.; Ronkainen, N.J. Immobilization Techniques in the Fabrication of Nanomaterial-Based Electrochemical Biosensors: A Review. Sensors 2013, 13, 4811–4840. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, J.; Gao, L.; Wang, Y.; Zhang, J.; Kajiura, H.; Li, Y.; Noda, K. Removal of the Residual Surfactants in Transparent and Conductive Single-Walled Carbon Nanotube Films. J. Phys. Chem. C 2009, 113, 17685–17690. [Google Scholar] [CrossRef]
- Chen, L.; Xie, H.; Yu, W. Functionalization Methods of Carbon Nanotubes and Its Applications. In Carbon Nanotubes Applications on Electron Devices; IN TECH: London, UK, 2011; pp. 213–232. ISBN 978-953-307-496-2. [Google Scholar]
- Shim, J.; Yun, J.M.; Yun, T.; Kim, P.; Lee, K.E.; Lee, W.J.; Ryoo, R.; Pine, D.J.; Yi, G.-R.; Kim, S.O. Two-Minute Assembly of Pristine Large-Area Graphene Based Films. Nano Lett. 2014, 14, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Keeley, G.P.; Lyons, M.E. The effects of thin layer diffusion at glassy carbon electrodes modified with porous films of single-walled carbon nanotubes. Int. J. Electrochem. Sci. 2009, 4, 794–809. [Google Scholar]
- Pan, H.; Li, J.; Feng, Y. Carbon Nanotubes for Supercapacitor. Nanoscale Res. Lett. 2010, 5, 654–668. [Google Scholar] [CrossRef] [PubMed]
- Frackowiak, E.; Metenier, K.; Bertagna, V.; Beguin, F. Supercapacitor electrodes from multiwalled carbon nanotubes. Appl. Phys. Lett. 2000, 77, 2421–2423. [Google Scholar] [CrossRef]
- Haddad, R.; Holzinger, M.; Villalonga, R.; Neumann, A.; Roots, J.; Maaref, A.; Cosnier, S. Pyrene-adamantane-β-cyclodextrin: An efficient host-guest system for the biofunctionalization of SWCNT electrodes. Carbon 2011, 49, 2571–2578. [Google Scholar] [CrossRef]
- Waltman, R.J.; Diaz, A.F.; Bargon, J. The Electrochemical Oxidation and Polymerization of Polycyclic Hydrocarbons. J. Electrochem. Soc. 1985, 132, 631–634. [Google Scholar] [CrossRef]
- Granadero, D.; Bordello, J.; Pérez-Alvite, M.J.; Novo, M.; Al-Soufi, W. Host-Guest Complexation Studied by Fluorescence Correlation Spectroscopy: Adamantane-Cyclodextrin Inclusion. Int. J. Mol. Sci. 2010, 11, 173–188. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, M.; Singh, M.; Cosnier, S. Biotin-β-Cyclodextrin: A New Host–Guest System for the Immobilization of Biomolecules. Langmuir 2012, 28, 12569–12574. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, M.; Bouffier, L.; Villalonga, R.; Cosnier, S. Adamantane/β-cyclodextrin affinity biosensors based on single-walled carbon nanotubes. Biosens. Bioelectron. 2009, 24, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gross, A.J.; Hammond, J.L.; Holzinger, M.; Cosnier, S. Flotation Assembly of Large-Area Ultrathin MWCNT Nanofilms for Construction of Bioelectrodes. Nanomaterials 2017, 7, 342. https://doi.org/10.3390/nano7100342
Gross AJ, Hammond JL, Holzinger M, Cosnier S. Flotation Assembly of Large-Area Ultrathin MWCNT Nanofilms for Construction of Bioelectrodes. Nanomaterials. 2017; 7(10):342. https://doi.org/10.3390/nano7100342
Chicago/Turabian StyleGross, Andrew J., Jules L. Hammond, Michael Holzinger, and Serge Cosnier. 2017. "Flotation Assembly of Large-Area Ultrathin MWCNT Nanofilms for Construction of Bioelectrodes" Nanomaterials 7, no. 10: 342. https://doi.org/10.3390/nano7100342





