Preparation of Nano-TiO2-Coated SiO2 Microsphere Composite Material and Evaluation of Its Self-Cleaning Property
Abstract
:1. Introduction
2. Methods
2.1. Raw Materials and Reagents
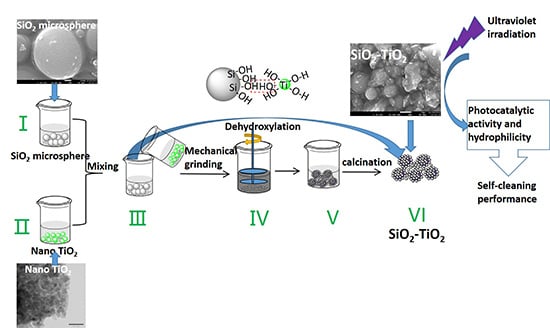
2.2. Preparation Method
2.2.1. Depolymerization of SiO2 Microspheres
2.2.2. Preparation of Nano-TiO2 Soliquid
2.2.3. Preparation of SiO2–TiO2
2.3. Characterization
2.3.1. Evaluation of Self-Cleaning Performance
Photocatalytic Activity
Hydrophilicity
2.3.2. Characterization of Structure and Morphology
3. Results and Discussion
3.1. Properties of SiO2–TiO2
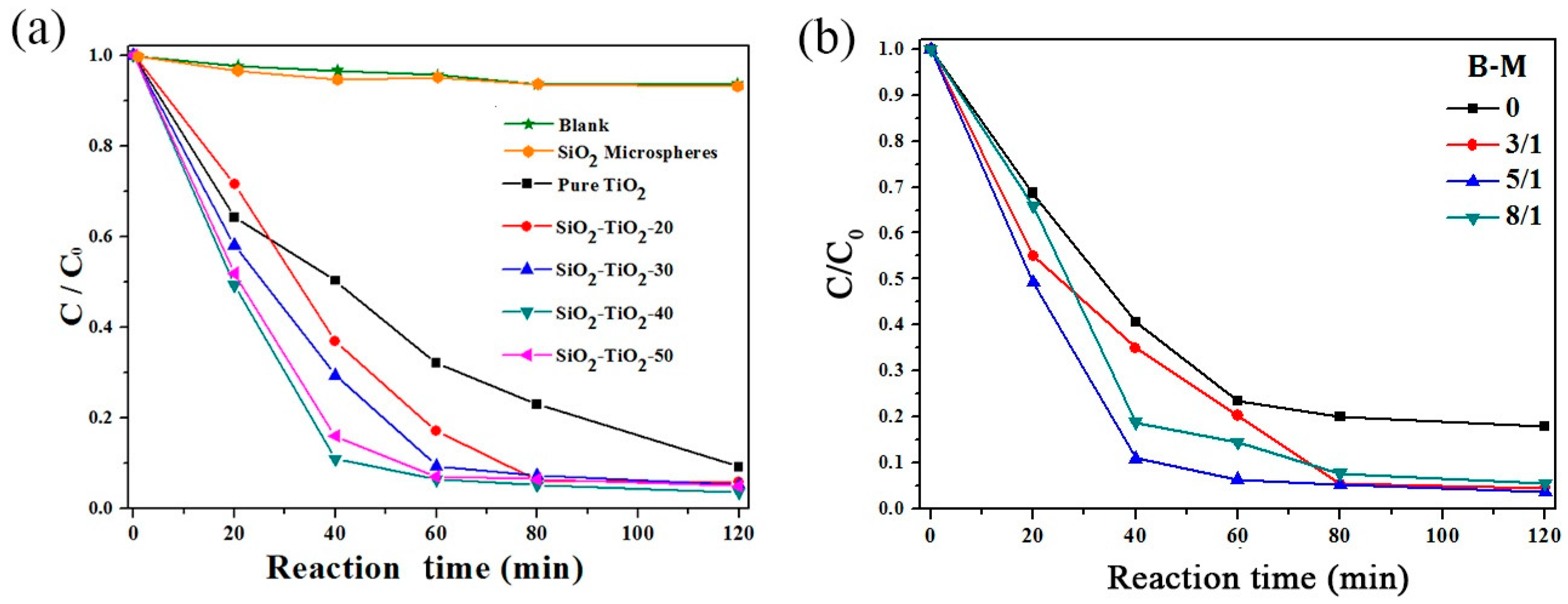
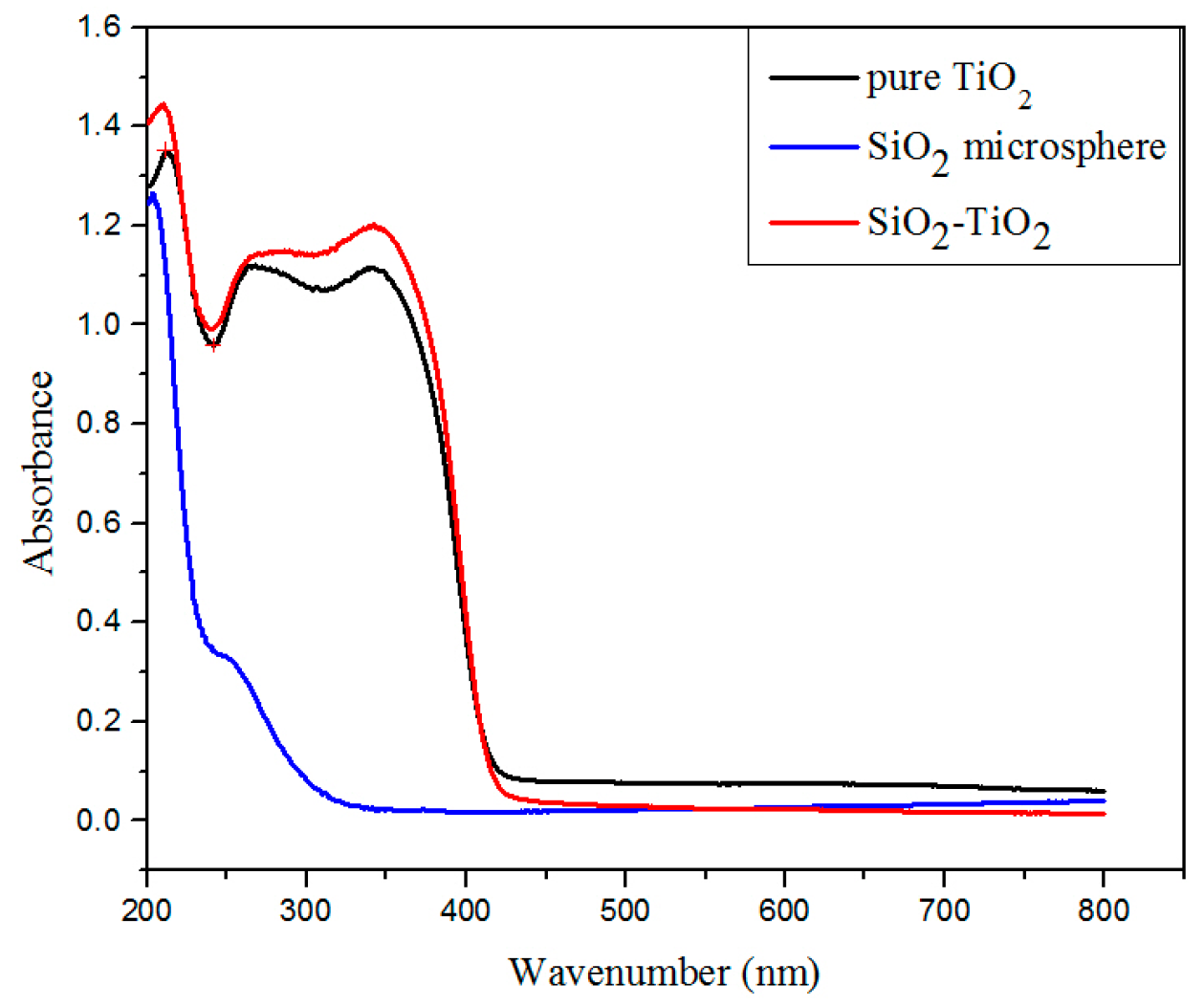
3.1.1. Photocatalytic Properties of SiO2–TiO2
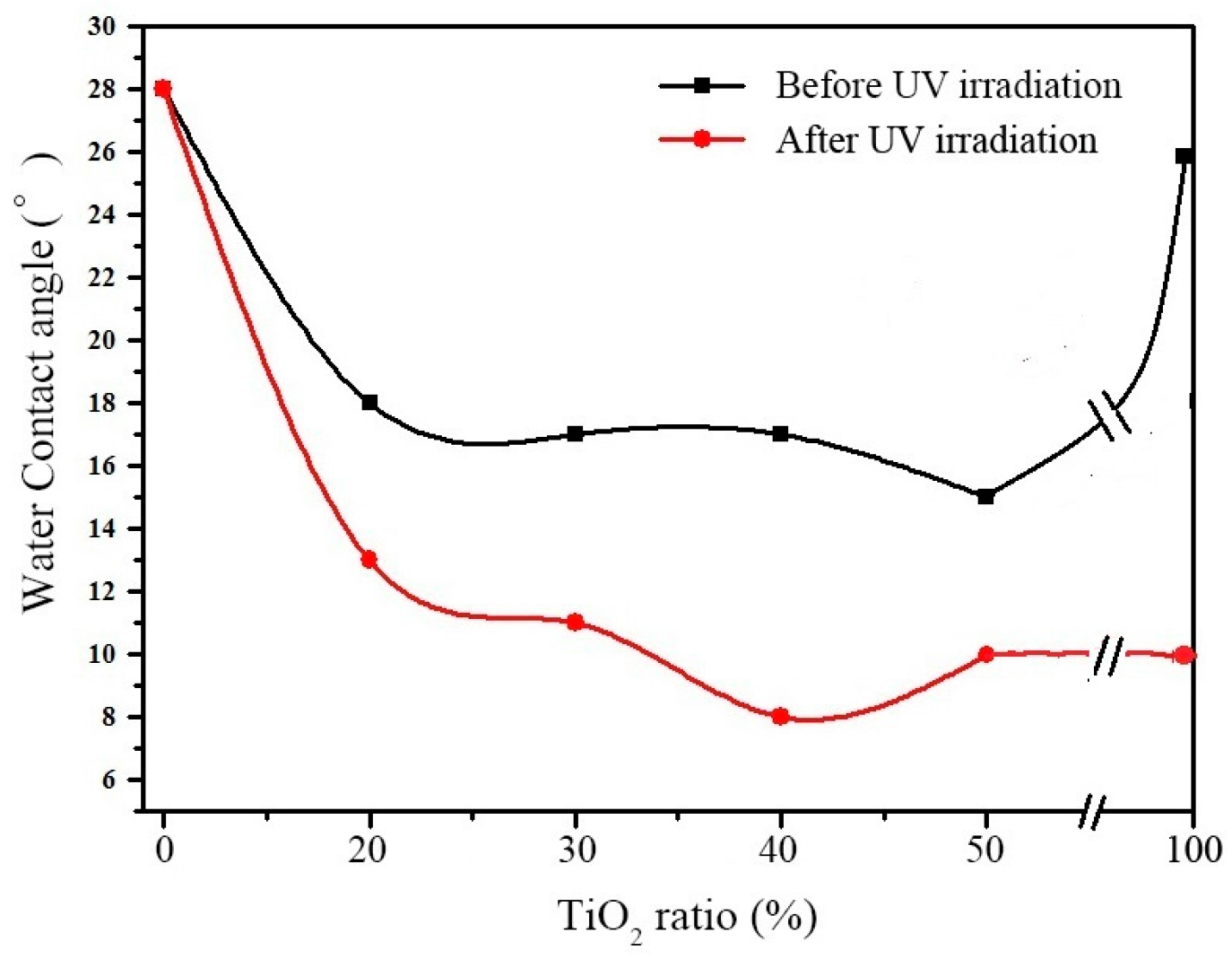
3.1.2. Hydrophilic Properties of SiO2–TiO2
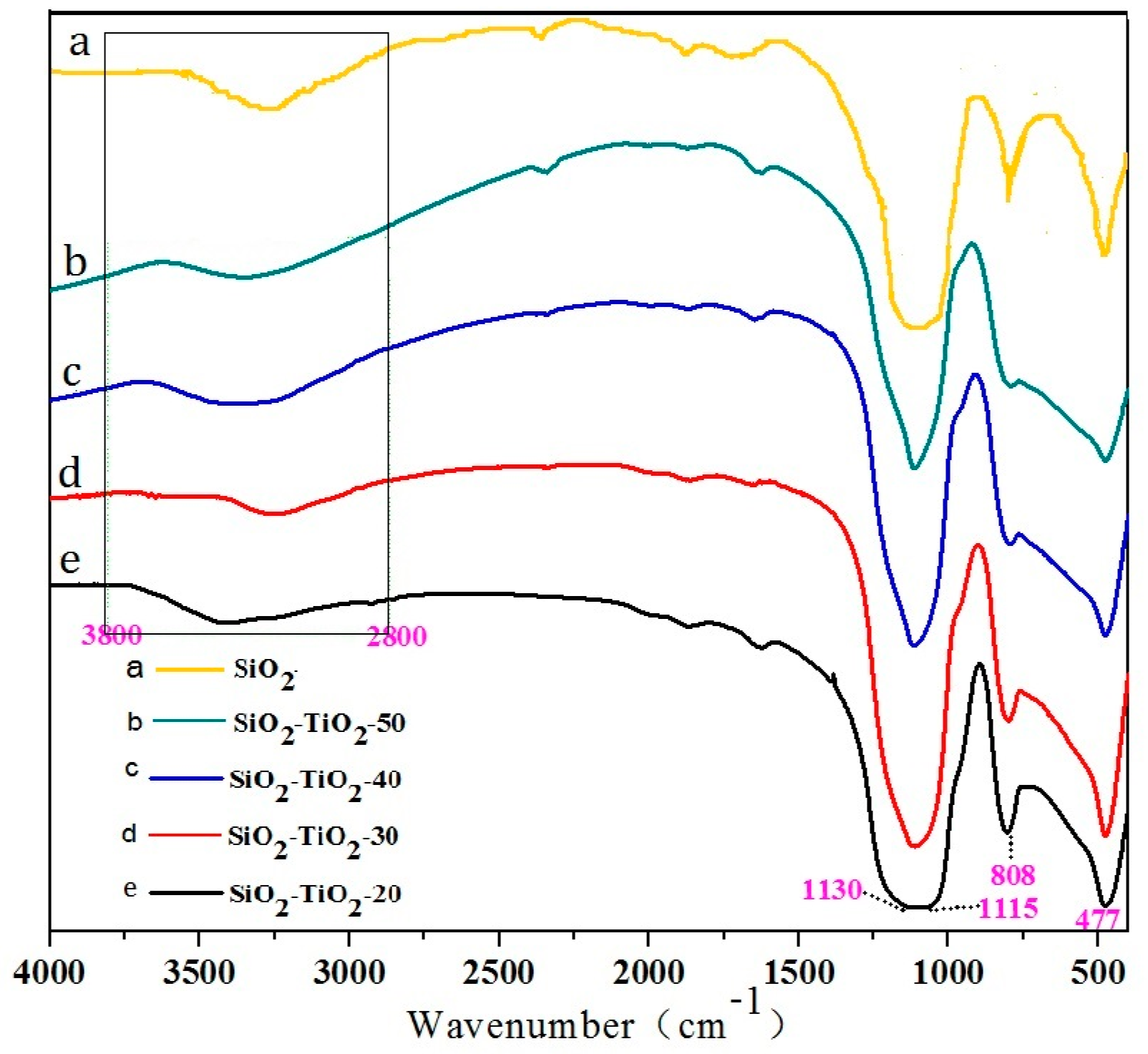
3.2. Structure and Morphology of SiO2–TiO2
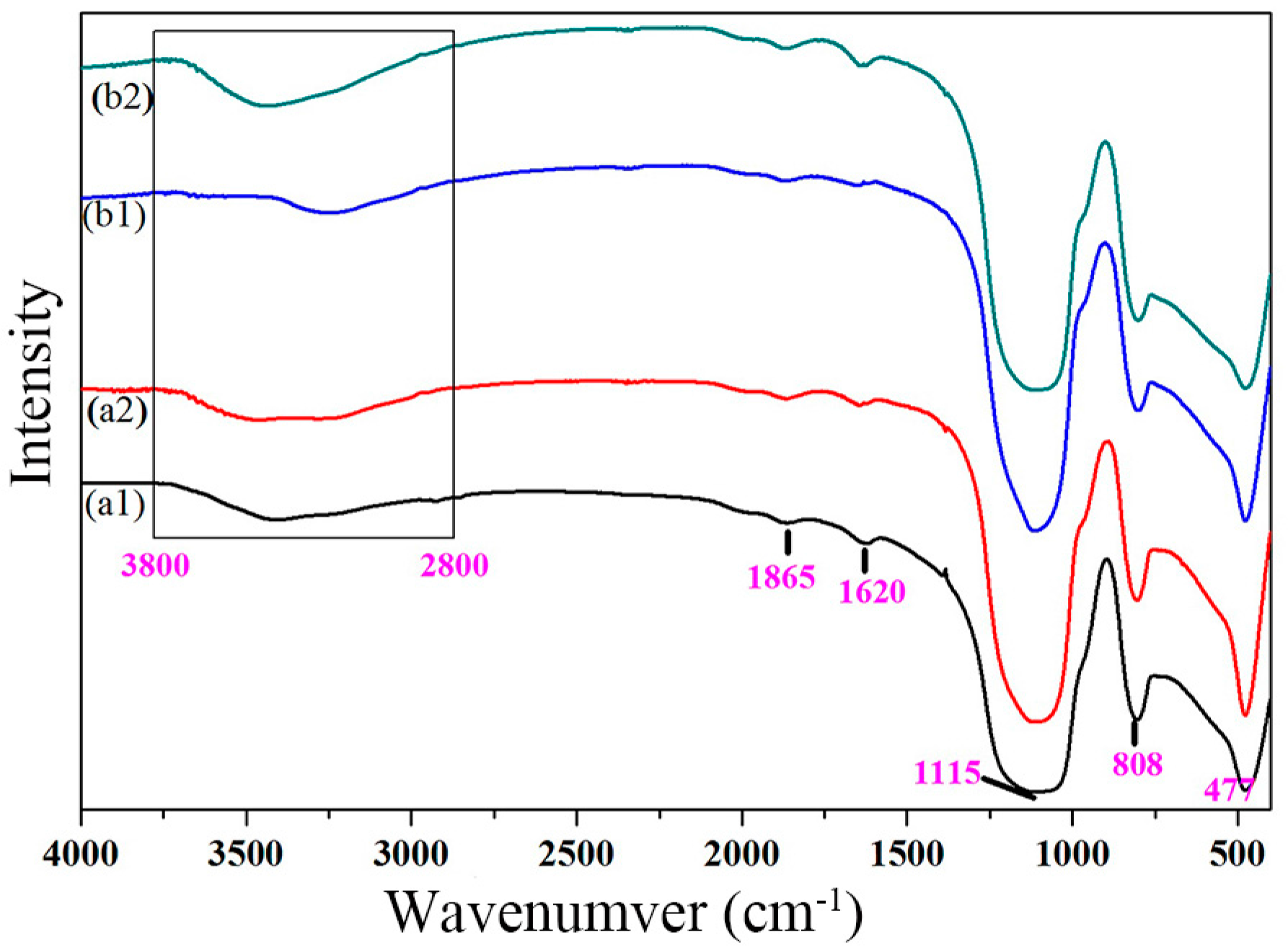
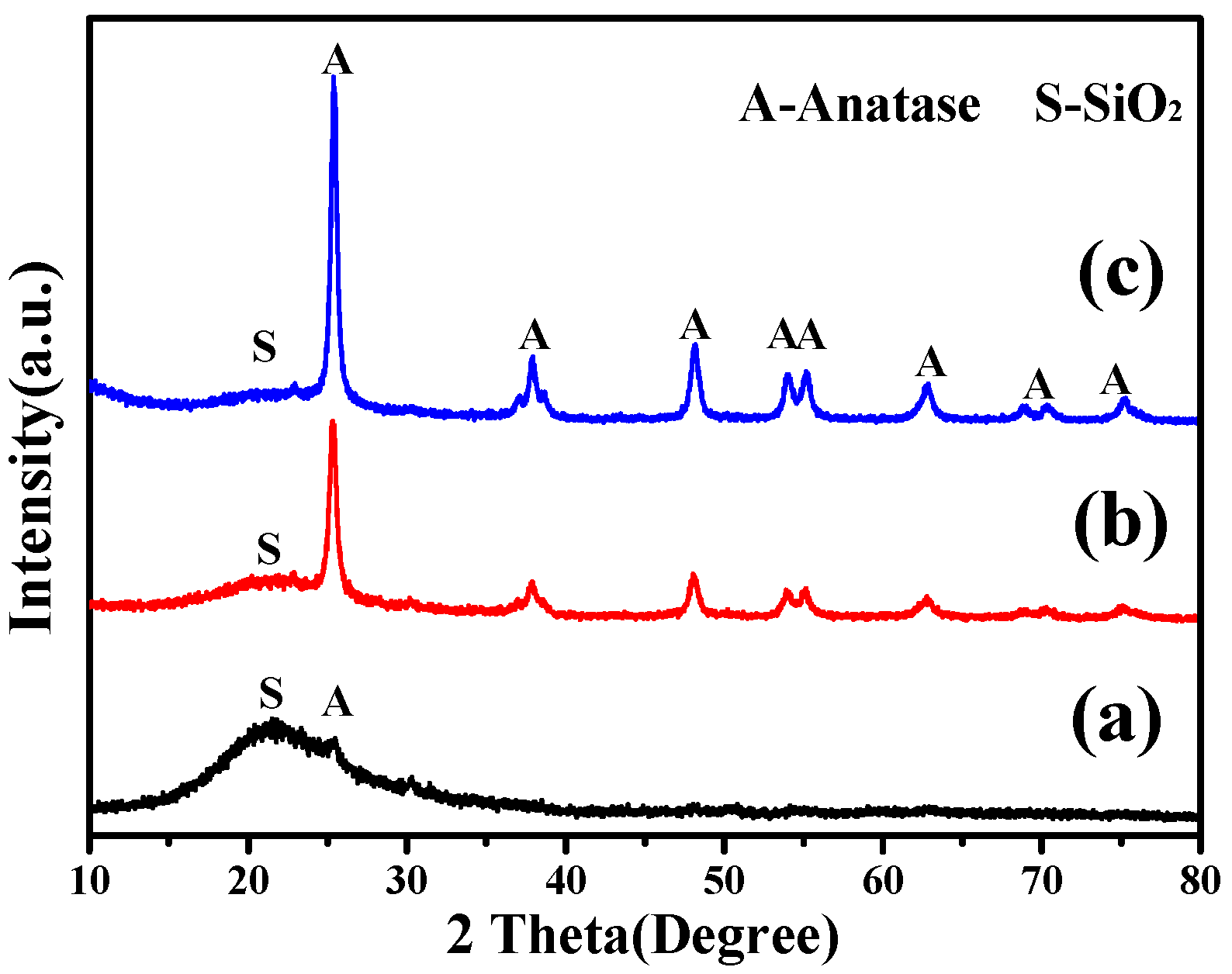
3.2.1. XRD Analysis
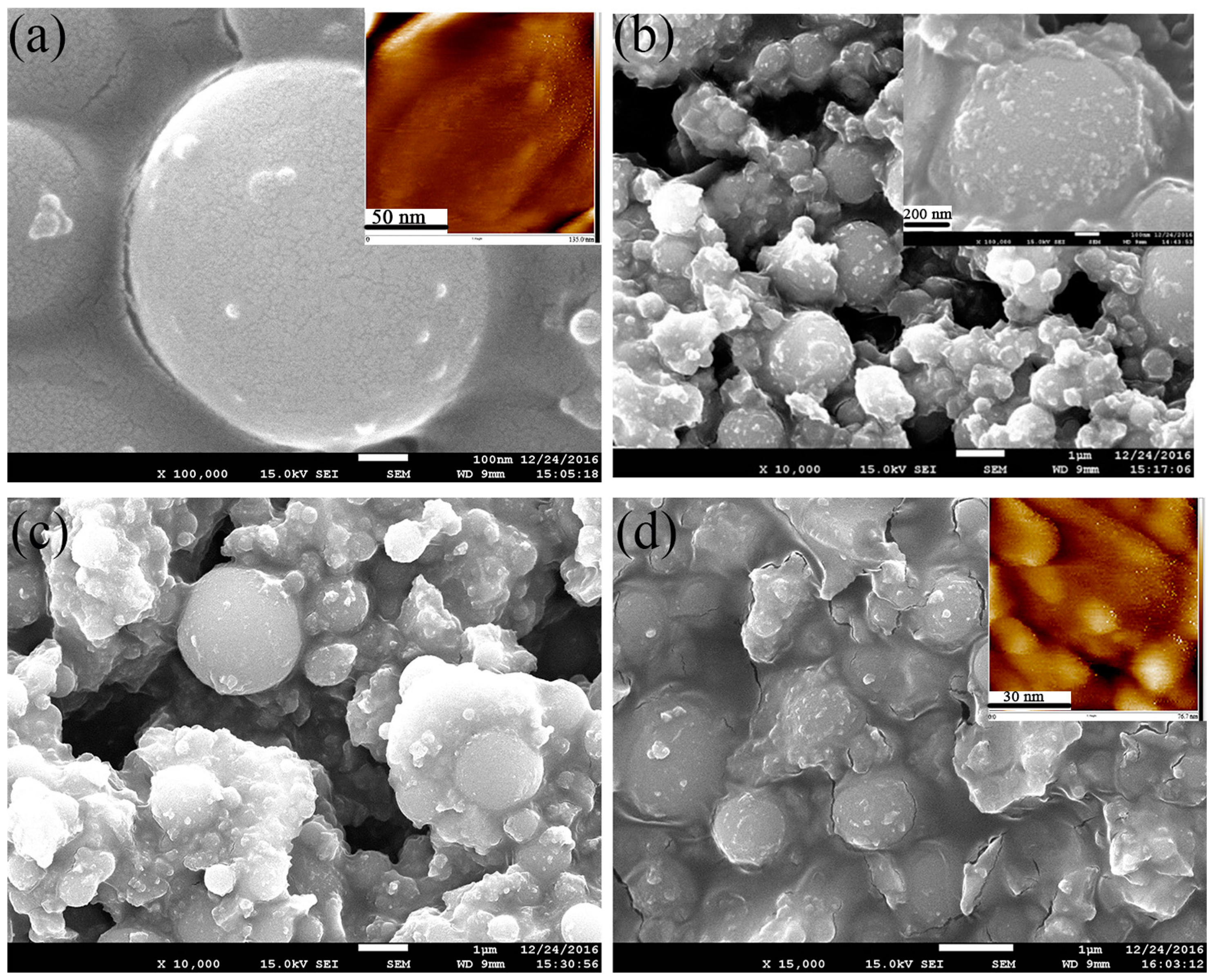
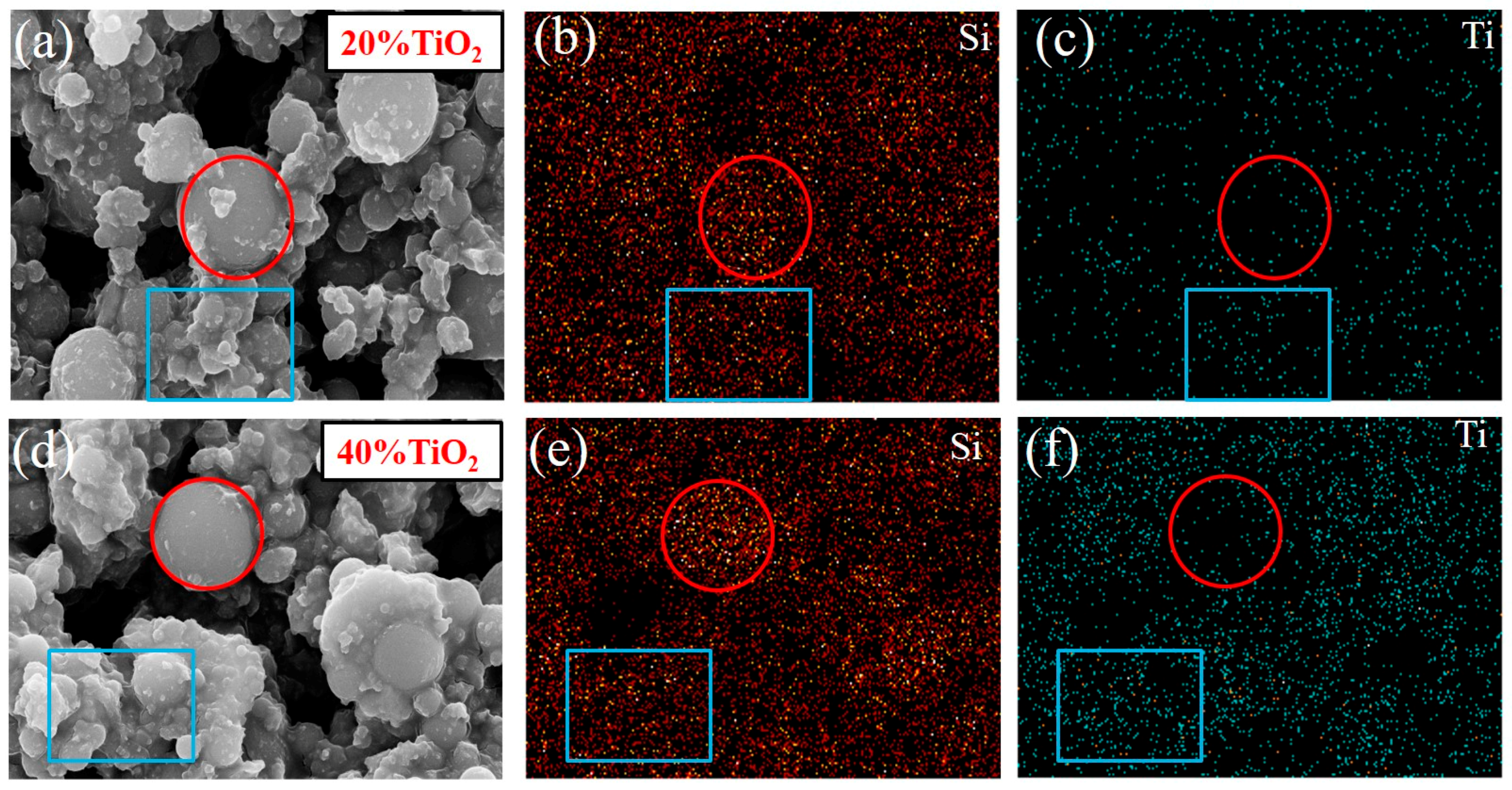
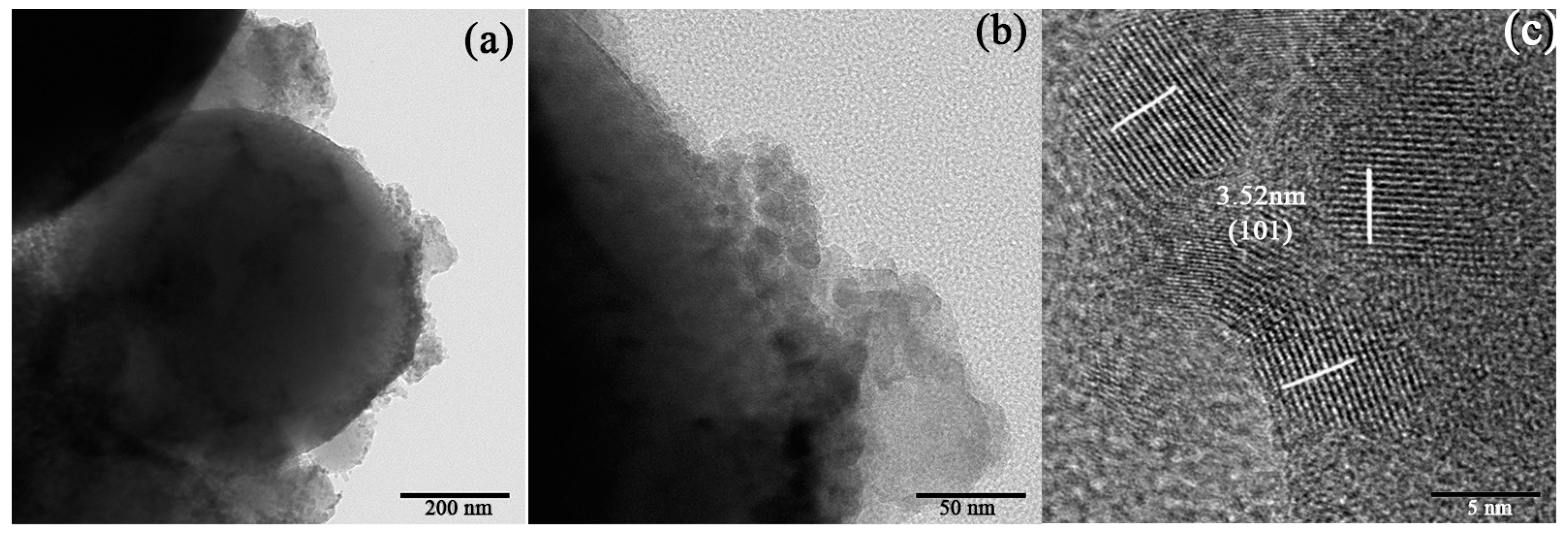
3.2.2. Morphology and Element Analysis
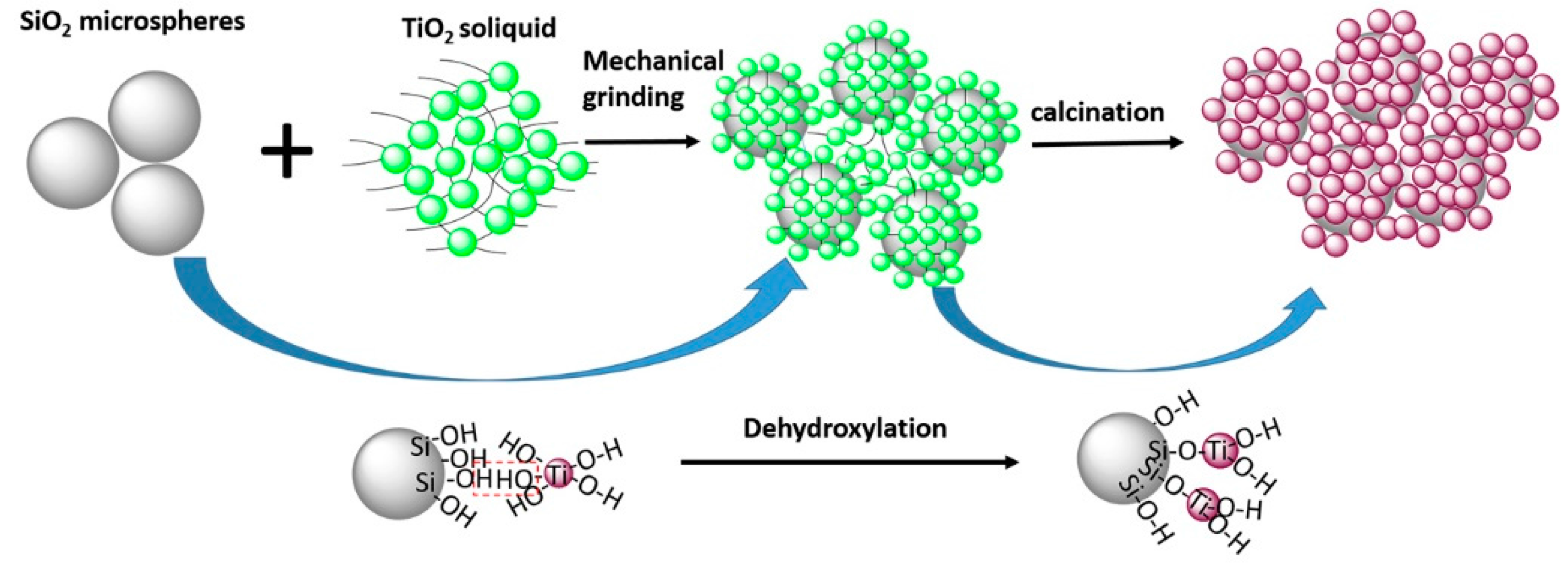
3.3. Mechanism of the Interaction between SiO2 and TiO2 Particles
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Liu, L.; Chen, X. Titanium dioxide nanomaterials: Self-structural modifications. Chem. Rev. 2014, 114, 9890–9918. [Google Scholar] [CrossRef] [PubMed]
- Kormann, C.; Bahnemann, D.W.; Hoffmann, M.R. Preparation and characterization of quantum-size titanium dioxide. J. Phys. Chem. C 1988, 92, 5196–5201. [Google Scholar] [CrossRef]
- Pu, S.; Zhu, R.; Ma, H.; Deng, D.; Pei, X.; Qi, F.; Chu, W. Facile in-situ design strategy to disperse TiO2 nanoparticles on graphene for the enhanced photocatalytic degradation of rhodamine 6G. Appl. Catal. B Environ. 2017, 218, 208–219. [Google Scholar] [CrossRef]
- Preethi, L.K.; Mathews, T.; Nand, M.; Jha, S.N.; Gopinath, C.S.; Dash, S. Band alignment and charge transfer pathway in three phase anatase-rutile-brookite TiO2, nanotubes: An efficient photocatalyst for water splitting. Appl. Catal. B Environ. 2017, 218, 9–19. [Google Scholar] [CrossRef]
- Ye, Z.; Tai, H.; Guo, R.; Yuan, Z.; Liu, C.; Su, Y.; Chen, Z.; Jiang, Y. Excellent ammonia sensing performance of gas sensor based on graphene/titanium dioxide hybrid with improved morphology. Appl. Surf. Sci. 2017, 419, 84–90. [Google Scholar] [CrossRef]
- Fu, H.; Yang, X.; An, X.; Fan, W.; Jiang, X.; Yu, A. Experimental and theoretical studies of V2O5@TiO2 core-shell hybrid composites with high gas sensing performance towards ammonia. Sens. Actuators B Chem. 2017, 252, 103–115. [Google Scholar] [CrossRef]
- Jalvo, B.; Faraldos, M.; Bahamonde, A.; Rosal, R. Antimicrobial and antibiofilm efficacy of self-cleaning surfaces functionalized by TiO2 photocatalytic nanoparticles against staphylococcus aureus and pseudomonas putida. J. Hazard. Mater. 2017, 340, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.Y.L.; Tai, M.H.; Juay, J.; Liu, Z.; Sun, D. A study on the performance of self-cleaning oil–water separation membrane formed by various TiO2, nanostructures. Sep. Purif. Technol. 2015, 156, 942–951. [Google Scholar] [CrossRef]
- Ganesh, V.A.; Raut, H.K.; Nair, A.S.; Ramakrishna, S. A review on self-cleaning coatings. J. Mater. Chem. A 2011, 21, 16304–16322. [Google Scholar] [CrossRef]
- Kim, S.M.; In, I.; Park, S.Y. Study of photo-induced hydrophilicity and self-cleaning property of glass surfaces immobilized with TiO2 nanoparticles using catechol chemistry. Surf. Coat. Technol. 2016, 294, 75–82. [Google Scholar] [CrossRef]
- Petica, A.; Gaidau, C.; Ignat, M.; Sendrea, C.; Anicai, L. Doped TiO2 nanophotocatalysts for leather surface finishing with self-cleaning properties. J. Coat. Technol. Res. 2015, 12, 1153–1163. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Paolini, R.; Rossini, M.; Aslan, A.B.; Zinzi, M.; Poli, T.; Pedeferri, M.P. Long term self-cleaning and photocatalytic performance of anatase added mortars exposed to the urban environment. Constr. Build. Mater. 2015, 96, 270–278. [Google Scholar] [CrossRef]
- Rao, X.; Liu, Y.; Fu, Y.; Liu, Y.; Yu, H. Formation and properties of polyelectrolytes/TiO2 composite coating on wood surfaces through layer-by-layer assembly method. Holzforschung 2016, 70, 361–367. [Google Scholar] [CrossRef]
- Lei, M.; Li, F.S.; Tanemura, S.; Fisher, C.A.J.; Li, L.Z.; Liang, Q.; Xu, G. Cost-effective nanoporous SiO2–TiO2, coatings on glass substrates with antireflective and self-cleaning properties. Appl. Energy 2013, 112, 1198–1205. [Google Scholar]
- Diamanti, M.V.; Gadelrab, K.R.; Pedeferri, M.P.; Stefancich, M.; Pehkonen, S.O.; Chiesa, M. Nanoscale investigation of photoinduced hydrophilicity variations in anatase and rutile nanopowders. Langmuir 2013, 29, 14512–14518. [Google Scholar] [CrossRef] [PubMed]
- Calia, A.; Lettieri, M.; Masieri, M. Durability assessment of nanostructured TiO2, coatings applied on limestones to enhance building surface with self-cleaning ability. Build. Environ. 2016, 110, 1–10. [Google Scholar] [CrossRef]
- Jo, W.K.; Tayade, R.J. Facile photocatalytic reactor development using nano-TiO2 immobilized mosquito net and energy efficient UVLED for industrial dyes effluent treatment. J. Environ. Chem. Eng. 2016, 4, 319–327. [Google Scholar] [CrossRef]
- Natarajan, K.; Natarajan, T.S.; Tayade, R.J. Photocatalytic reactor based on UV-LED/TiO2 coated quartz tube for degradation of dyes. Chem. Eng. J. 2011, 178, 40–49. [Google Scholar] [CrossRef]
- Yang, S.B.; Chun, H.H.; Tayade, R.J.; Jo, W.K. Iron-functionalized titanium dioxide on flexible glass fibers for photocatalysis of benzene, toluene, ethylbenzene, and o-xylene (BTEX) under visible- or ultraviolet-light irradiation. J. Air Waste Manag. 2015, 65, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Smitha, V.S.; Manjumol, K.A.; Baiju, K.V.; Ghosh, S.; Perumal, P.; Warrier, K.G.K. Sol–gel route to synthesize titania-silica nano precursors for photoactive particulates and coatings. J. Sol-Gel Sci. Technol. 2010, 54, 203–211. [Google Scholar] [CrossRef]
- Shi, G.; Chen, J.; Wang, L.; Wang, D.; Yang, J.; Li, Y.; Zhang, L.; Ni, C.; Chi, L. Titanium oxide/silicon moth-eye structures with antireflection, p–n heterojunctions and superhydrophilicity. Langmuir 2016, 32, 27666724. [Google Scholar] [CrossRef] [PubMed]
- Pakdel, E.; Daoud, W.A. Self-cleaning cotton functionalized with TiO2/SiO2 focus on the role of silica. J. Colloid Interface Sci. 2013, 401, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.; Cindrella, L.; Kwon, O.J.; Mohanraju, K. Superhydrophilic and self-cleaning RGO–TiO2, composite coatings for indoor and outdoor photovoltaic applications. Sol. Energy Mater. Sol. Cells 2017, 169, 304–312. [Google Scholar] [CrossRef]
- Zhou, J.; Tan, Z.; Liu, Z.; Jing, M.; Liu, W.; Fu, W. Preparation of transparent fluorocarbon/TiO2-SiO2, composite coating with improved self-cleaning performance and anti-aging property. Appl. Surf. Sci. 2017, 396, 161–178. [Google Scholar] [CrossRef]
- Zhang, H.; Fan, D.; Yu, T.; Wang, C. Characterization of anti-reflective and self-cleaning SiO2–TiO2, composite film. J. Sol-Gel Sci. Technol. 2013, 66, 274–279. [Google Scholar] [CrossRef]
- Ciprian, M.; Alexandru, E.; Anca, D. SiO2/TiO2 multi-layered thin films with self-cleaning and enhanced optical properties. Bull. Mater. Sci. 2017, 40, 1–10. [Google Scholar] [CrossRef]
- Surolia, P.K.; Tayade, R.J.; Jasra, R.V. TiO2-coated cenospheres as catalysts for photocatalytic degradation of methylene blue, p-nitroaniline, n-decane, and n-tridecane under solar irradiation. Ind. Eng. Chem. Res. 2010, 49, 8908–8919. [Google Scholar] [CrossRef]
- Mavukkandy, M.O.; Bilad, M.R.; Kujawa, J.; Al-Gharabli, S.; Arafat, H.A. On the effect of fumed silica particles on the structure, properties and application of PVDF membranes. Sep. Purif. Technol. 2017, 187, 365–373. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, J.; Liu, J.; Ke, J.; Wang, F. Properties and microstructure of reactive powder concrete having a high content of phosphorous slag powder and silica fume. Constr. Build. Mater. 2015, 101, 482–487. [Google Scholar] [CrossRef]
- Pedro, D.; Brito, J.D.; Evangelista, L. Mechanical characterization of high performance concrete prepared with recycled aggregates and silica fume from precast industry. J. Clean. Prod. 2017, 164, 939–949. [Google Scholar] [CrossRef]
- Liu, J.; Wang, D. Influence of steel slag-silica fume composite mineral admixture on the properties of concrete. Powder Technol. 2017, 320, 230–238. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, W.T.; Lian, J.S.; Jiang, Q. Photocatalytic property of Fe doped anatase and rutile TiO2 nanocrystal particles prepared by sol–gel technique. Appl. Surf. Sci. 2012, 263, 260–265. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, J.; Zhang, J.; He, P.; Ren, J.; Zhang, J.; Lu, J.; Liang, P.; Xu, K.; Shui, F. The effect of surface heterojunction between (001) and (101) facets on photocatalytic performance of anatase TiO2. Mater. Lett. 2017, 205, 173–177. [Google Scholar] [CrossRef]
- Chen, Y.; Ding, H.; Sun, S. Preparation and characterization of ZnO nanoparticles supported on amorphous SiO2. Nanomaterials 2017, 7, 217. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, S.; Deng, T.; Ding, H.; Chen, Y.; Chen, W. Preparation of Nano-TiO2-Coated SiO2 Microsphere Composite Material and Evaluation of Its Self-Cleaning Property. Nanomaterials 2017, 7, 367. https://doi.org/10.3390/nano7110367
Sun S, Deng T, Ding H, Chen Y, Chen W. Preparation of Nano-TiO2-Coated SiO2 Microsphere Composite Material and Evaluation of Its Self-Cleaning Property. Nanomaterials. 2017; 7(11):367. https://doi.org/10.3390/nano7110367
Chicago/Turabian StyleSun, Sijia, Tongrong Deng, Hao Ding, Ying Chen, and Wanting Chen. 2017. "Preparation of Nano-TiO2-Coated SiO2 Microsphere Composite Material and Evaluation of Its Self-Cleaning Property" Nanomaterials 7, no. 11: 367. https://doi.org/10.3390/nano7110367