Increased Loading, Efficacy and Sustained Release of Silibinin, a Poorly Soluble Drug Using Hydrophobically-Modified Chitosan Nanoparticles for Enhanced Delivery of Anticancer Drug Delivery Systems
Abstract
:1. Introduction
2. Results and Discussion
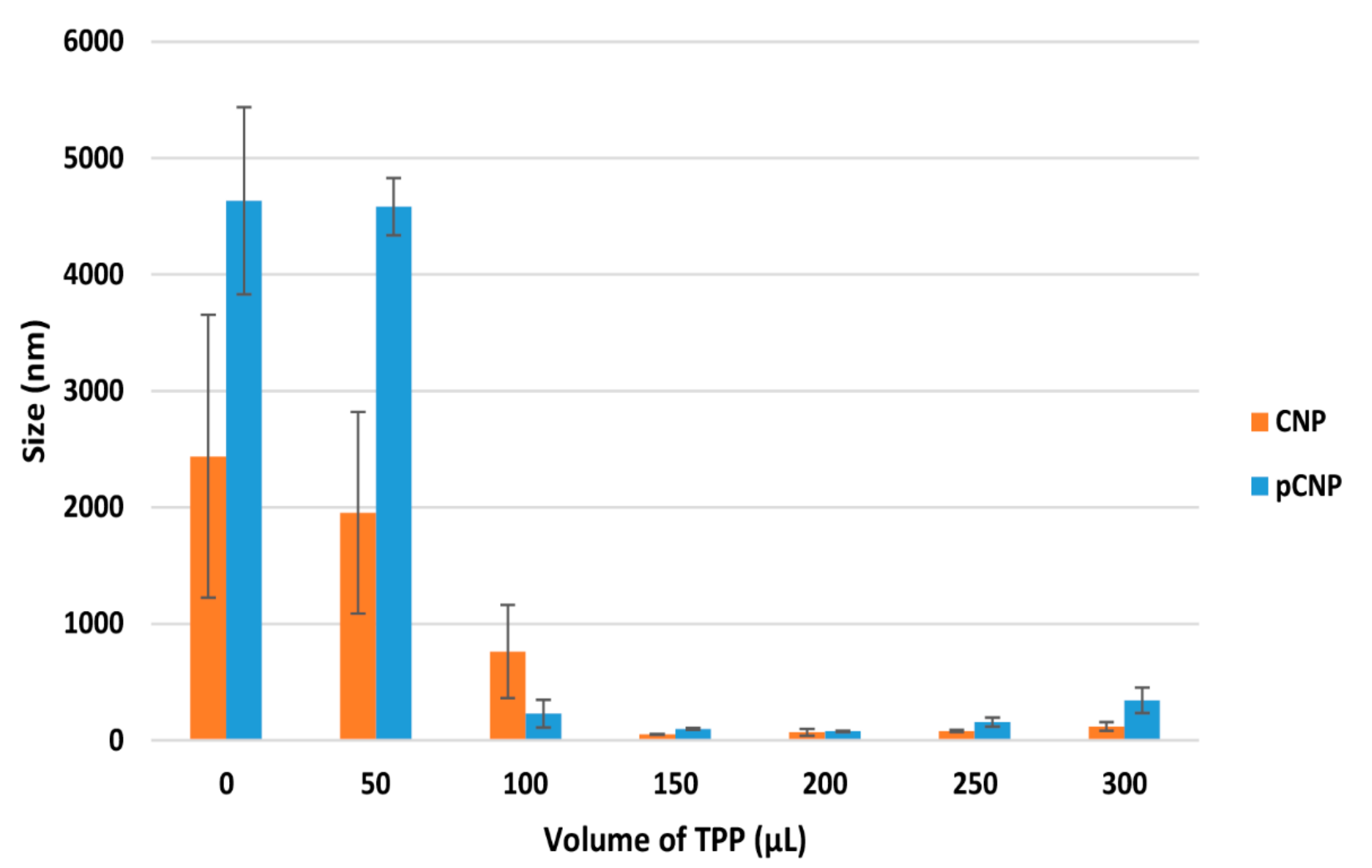
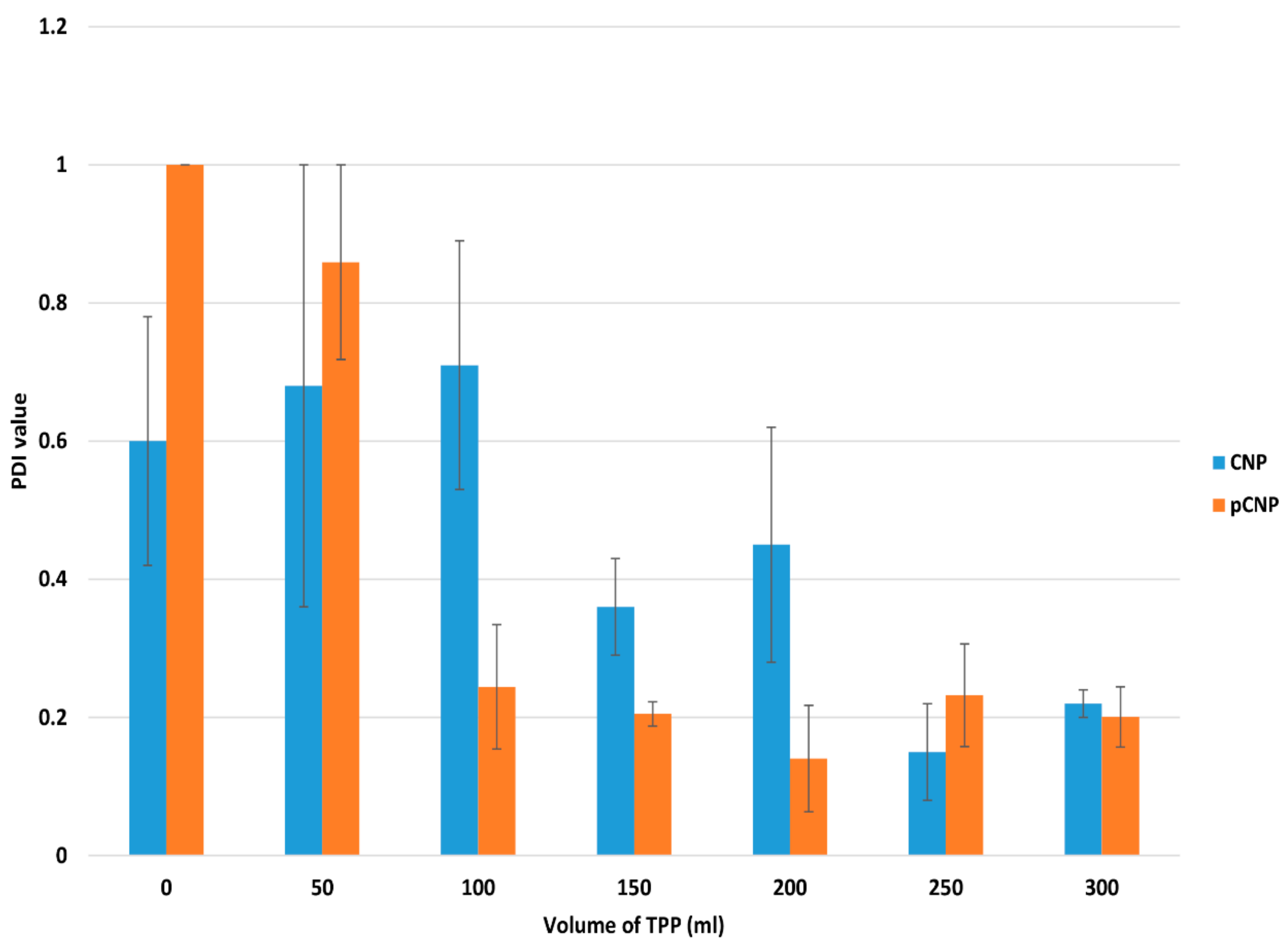
2.1. Particle Size and Polydispersity of Nanoparticle Samples
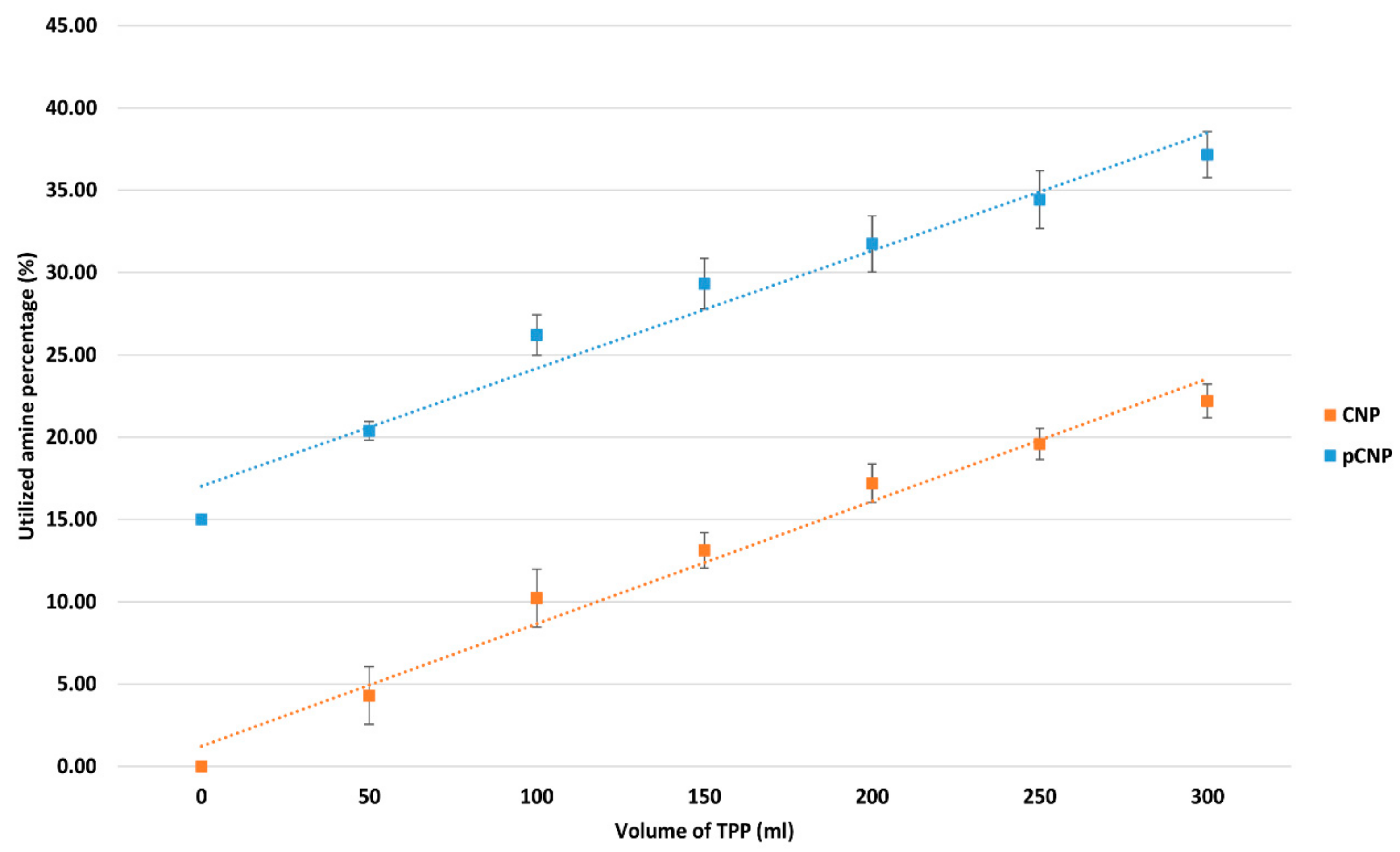
2.2. Utilization of the Free Amine Group by Nanoparticles Formation
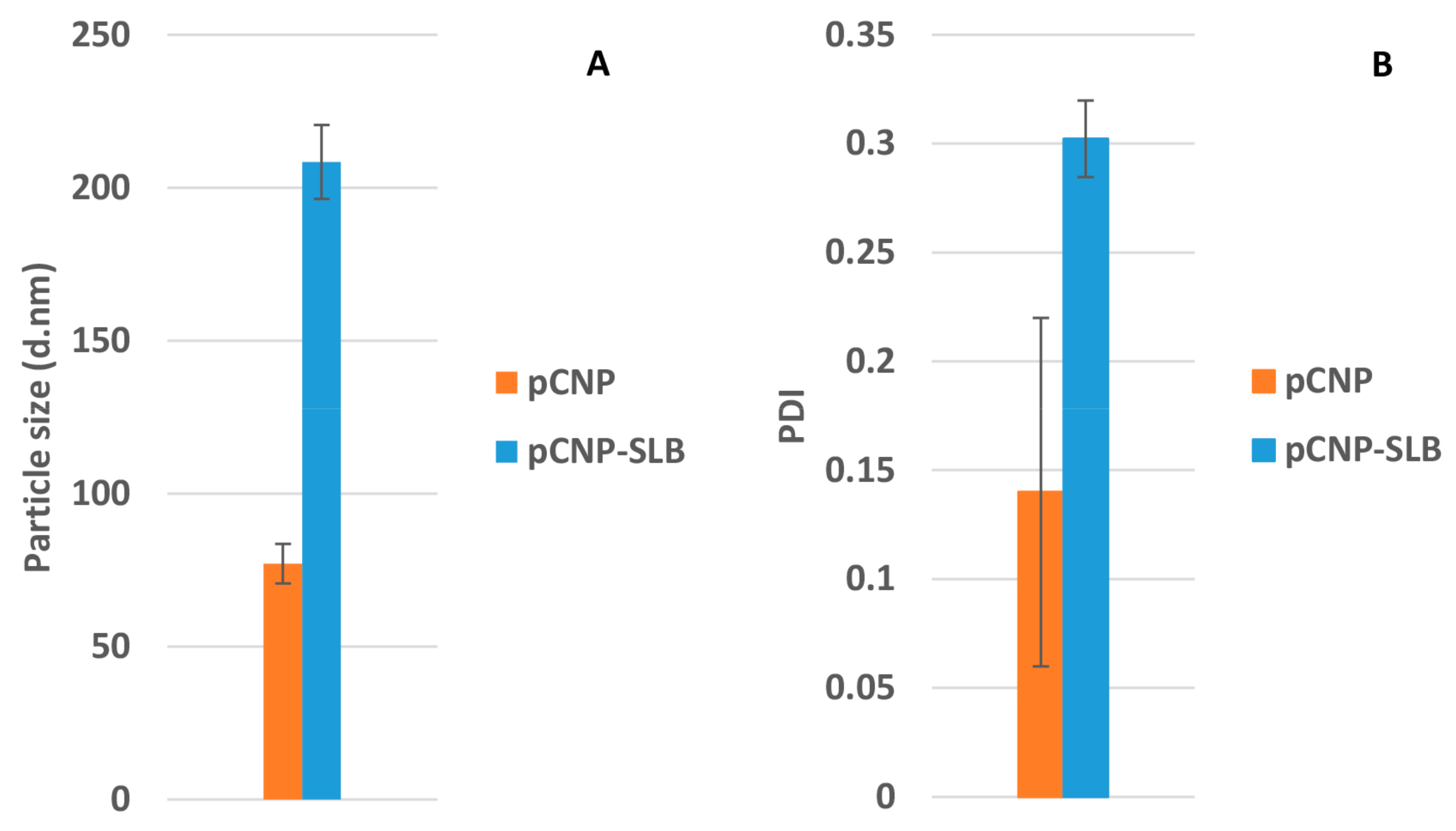
2.3. Formation of pCNP and pCNP-SLB
2.4. Morphological Analysis of Nanoparticles by FESEM
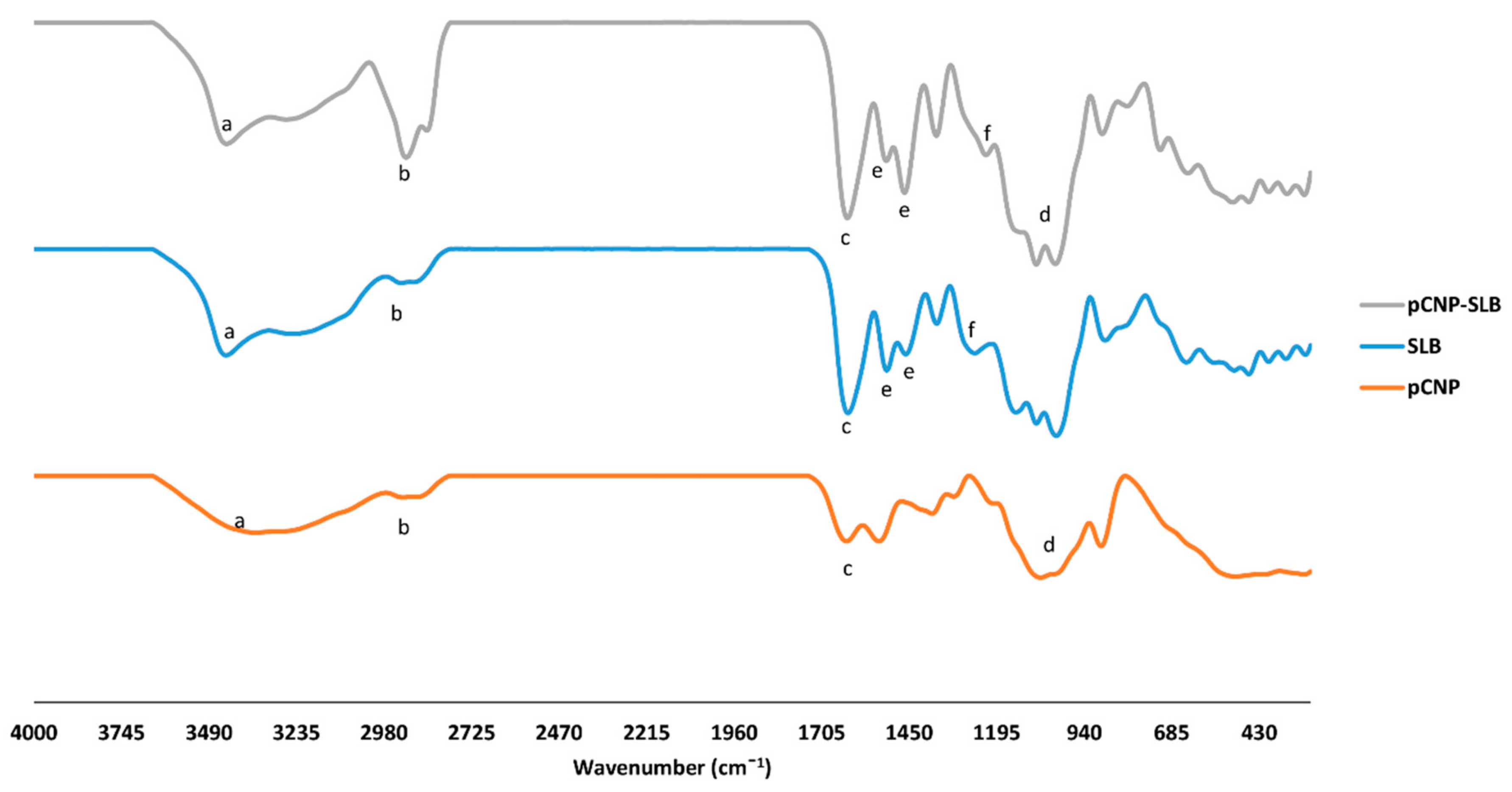
2.5. Identification of Characteristic Functional Groups in Nanoparticle Samples
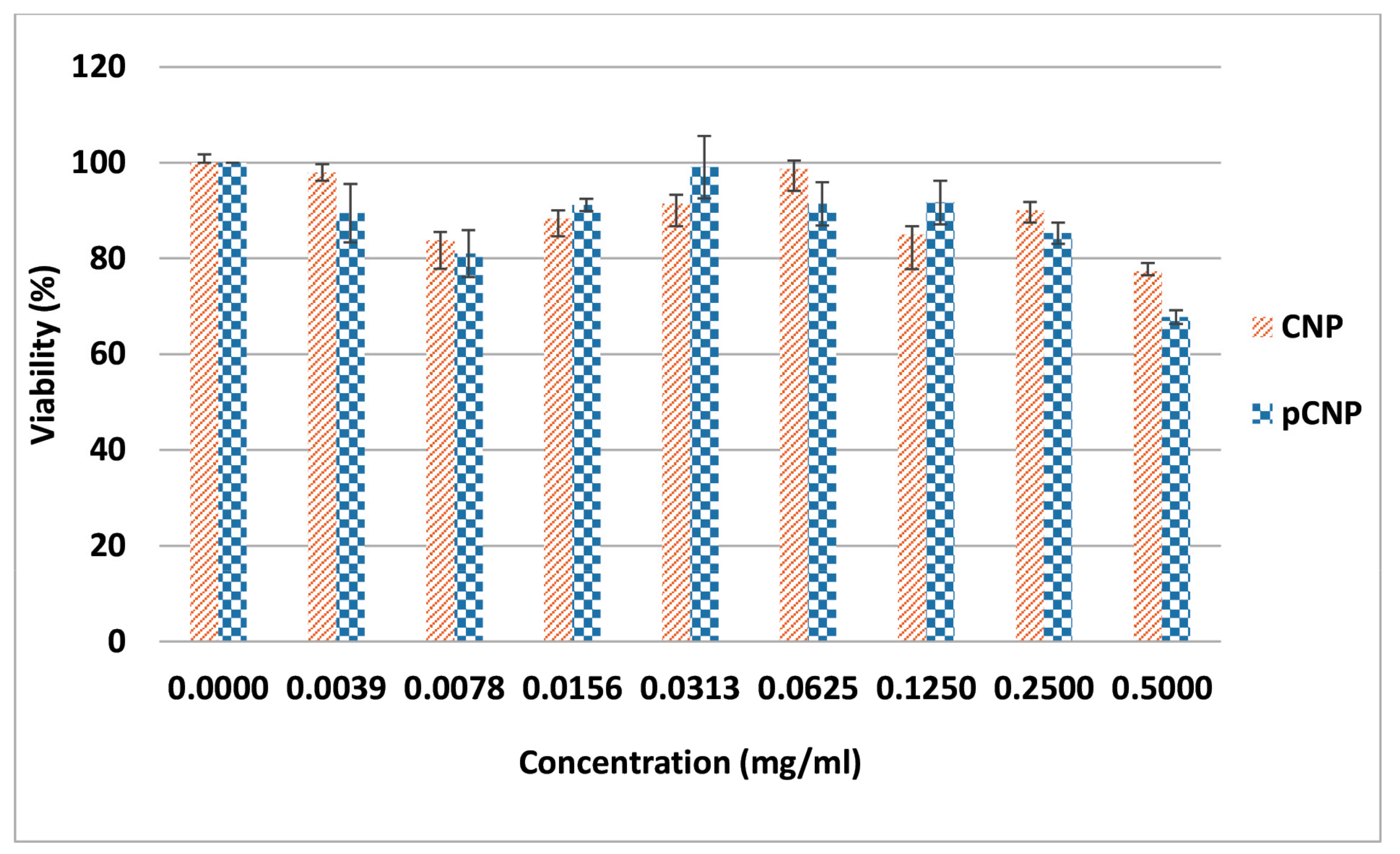
2.6. CNP and pCNP Cytotoxicity in A549 Cell Lines
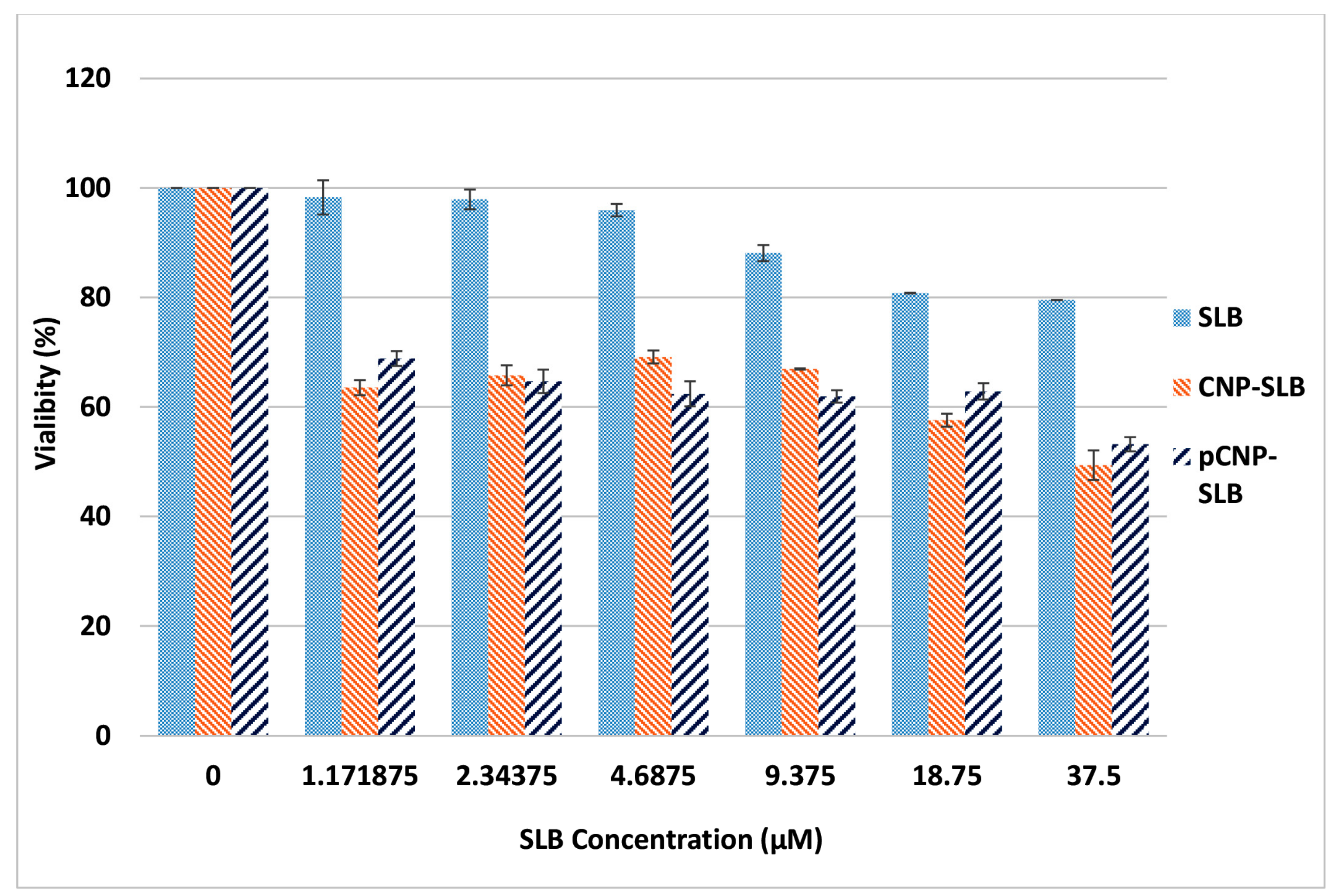
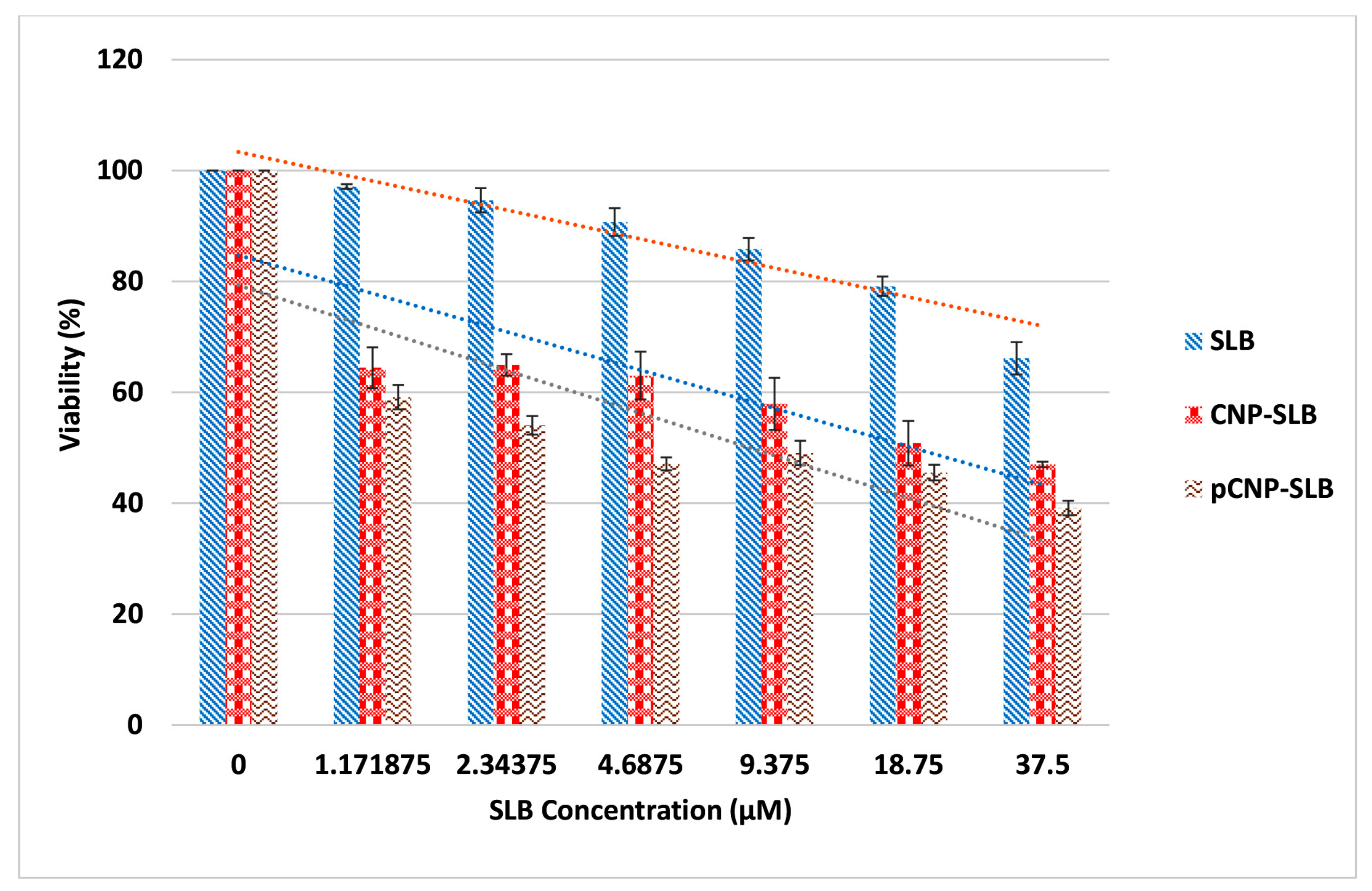
2.7. Nanoparticle-Mediated Cell Cytotoxicity of SLB CNP-SLB and pCNP-SLB in A549 Cell Lines
3. Materials and Methods
3.1. Synthesis of Chitosan Nanoparticles (CNP) and Palmitoyl Chitosan Nanoparticles (pCNP)
3.1.1. Chitosan Nanoparticles (CNP)
3.1.2. Hydrophobically-Modified Chitosan Nanoparticles (pCNP)
3.1.3. Synthesis of Silibinin-Encapsulated pCNP (pCNP-SLB)
3.2. Physicochemical Characterization of Nanoparticle Samples
3.2.1. Analysis of Particle Size Distribution and Polydispersity
3.2.2. Morphological and Surface Topological Analyses
3.2.3. Fourier Transform Infrared (FTIR) Analysis with Attenuated Total Reflectance (ATR-FTIR)
3.3. Determination of Free Amine Groups in Nanoparticle Samples Using the 2,4,6-Trinitrobenzene Sulfonic Assay
3.4. Encapsulation Efficiency of Silibinin in Nanoparticle Samples
3.5. Determination of In Vitro Cellular Efficacy Using Nanoparticle-Mediated Delivery of Silibinin
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chalasani, S.; Prakash, K.V.; Pulla, R.P.; Tekula, R.; Manasa, E.; Umasankar, B. Development and validation of doxorubicin hcl in bulk and its pharmaceutical dosage form by visible spectrophotometry. Int. J. Pharm. Sci. 2013, 3, 216–218. [Google Scholar]
- Wellington, K.; Jarvis, B. Silymarin: A review of its clinical properties in the management of hepatic disorders. BioDrugs 2001, 15, 465–489. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.C.; Graf, T.N.; Sparacino, C.M.; Wani, M.C.; Wall, M.E. Complete isolation and characterization of silybins and isosilybins from milk thistle (Silybum marianum). Org. Biomol. Chem. 2003, 1, 1684–1689. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, N.; De, R.; Begun, J.; Popat, A. Cancer therapeutics with epigallocatechin-3-gallate encapsulated in biopolymeric nanoparticles. Int. J. Pharm. 2017, 518, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, K.; Agarwal, R. Multitargeted therapy of cancer by silymarin. Cancer Lett. 2008, 269, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Mateen, S.; Raina, K.; Agarwal, R. Chemopreventive and Anti-cancer Efficacy of Silibinin against Growth and Progression of Lung Cancer. Nutr. Cancer 2013, 65, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Singh, R.P.; Chan, D.C.; Agarwal, R. Silibinin induces growth inhibition and apoptotic cell death in human lung carcinoma cells. Anticancer Res. 2003, 23, 2649–2655. [Google Scholar] [PubMed]
- Chu, S.C.; Chiou, H.L.; Chen, P.N.; Yang, S.F.; Hsieh, Y.S. Silibinin inhibits the invasion of human lung cancer cells via decreased productions of urokinase-plasminogen activator and matrix metalloproteinase-2. Mol. Carcinog. 2004, 40, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Dhanalakshmi, S.; Mallikarjuna, G.U.; Singh, R.P.; Agarwal, R. Silibinin modulates UVB-induced apoptosis via mitochondrial proteins, caspases activation, and mitogen-activated protein kinase signaling in human epidermoid carcinoma A431 cells. Biochem. Biophys. Res. Commun. 2004, 320, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Dhanalakshmi, S.; Mallikarjuna, G.U.; Singh, R.P.; Agarwal, R. Dual efficacy of silibinin in protecting or enhancing ultraviolet B radiation-caused apoptosis in HaCaT human immortalized keratinocytes. Carcinogenesis 2004, 25, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Deep, G.; Agarwal, R. Anti-metastatic Efficacy of Silibinin: Molecular Mechanisms and Therapeutic Potential against Cancer. Cancer Metastasis Rev. 2010, 29, 447–463. [Google Scholar] [CrossRef] [PubMed]
- Ting, H.; Deep, G.; Agarwal, R. Molecular Mechanisms of Silibinin-Mediated Cancer Chemoprevention with Major Emphasis on Prostate Cancer. AAPS J. 2013, 15, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Raina, K.; Agarwal, C.; Agarwal, R. Silibinin strongly inhibits the growth kinetics of colon cancer stem cell-enriched spheroids by modulating interleukin 4/6-mediated survival signals. Oncotarget 2014, 5, 4972–4989. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Ning, Z.; Zeng, J.; Fan, J.; Zhou, J.; Zhang, T.; Zhang, L.; Chen, Y.; Gao, Y.; Wang, B.; et al. Silibinin inhibits β-catenin/ZEB1 signaling and suppresses bladder cancer metastasis via dual-blocking epithelial-mesenchymal transition and stemness. Cell Signal. 2013, 25, 2625–2633. [Google Scholar] [CrossRef] [PubMed]
- Morazzoni, P.; Bombardelli, E. Silybum marianum (Carduus marianus). Fitoterapia 1995, 66, 3–42. [Google Scholar]
- Basaga, H.; Poll, G.; Tekkaya, C.; Aras, I. Free radical scavenging and antioxidative properties of “silibin” complexs on microsomal lipid peroxidation. Cell Biochem. Funct. 1997, 15, 27–33. [Google Scholar] [CrossRef]
- Skottov, N.; Kreeman, V.; Vaoa, R.; Chmela, Z.; Ulrichov, J.; Simanek, V. Effect of silymarin and silibinin-phosphatidylcholine complex on plasma and lipoprotein cholesterol, and oxidation of LDL in rats fed on high cholesterol diet supplemented with current oil. Acta Univ. Palacki. Olomuc. Fac. Med. 2000, 144, 55–58. [Google Scholar] [CrossRef]
- Leena, R.S.; Vairamani, M.; Selvamurugan, N. Alginate/Gelatin scaffolds incorporated with Silibinin-loaded Chitosan nanoparticles for bone formation in vitro. Colloids Surf. B Biointerfaces 2017, 158, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Kidd, P.; Head, K. A review of the bioavailability and clinical efficacy of milk thistle phytosome: A silybin-phosphatidylcholine complex (Siliphos). Altern. Med. Rev. 2005, 10, 193–203. [Google Scholar] [PubMed]
- Zhang, J.Q.; Liu, X.; Li, X.L.; Jasti, B.R. Preparation and Characterization of Solid Lipid Nanoparticles Containing Silibinin. Drug Deliv. 2007, 14, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhao, Y.; Feng, N.; Zhang, Y.; Liu, Y.; Dang, B. Improved dissolution and bioavailability of silymarin delivered by a solid dispersion prepared using supercritical fluids. Asian J. Pharm. Sci. 2015, 10, 194–202. [Google Scholar] [CrossRef]
- Yanyu, X.; Yunmei, S.; Zhipeng, C.; Qineng, P. The preparation of silybin—Phospholipid complex and the study on its pharmacokinetics in rats. Int. J. Pharm. 2006, 307, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Greish, K. Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. Cancer Nanotechnol. 2010, 624, 25–37. [Google Scholar]
- Hirano, S. Chitin and chitosan as novel biotechnological materials. Polym. Int. 1999, 48, 732–734. [Google Scholar] [CrossRef]
- Kang, B.S.; Lee, S.E.; Ng, C.L.; Kim, J.K.; Park, J.S. Exploring the Preparation of Albendazole-Loaded Chitosan-Tripolyphosphate Nanoparticles. Materials 2015, 8, 486–498. [Google Scholar] [CrossRef] [PubMed]
- Chai, F.; Sun, L.; He, X.; Li, J.; Liu, Y.; Xiong, F.; Ge, L.; Webster, T.J.; Zheng, C. Doxorubicin-loaded poly (lactic-co-glycolic acid) nanoparticles coated with chitosan/alginate by layer by layer technology for antitumor applications. Int. J. Nanomed. 2017, 12, 1791–1802. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.Z.; Zhu, K.J. The influence of multivalent phosphate structure on the properties of ionically cross-linked chitosan films for controlled drug release. Eur. J. Pharm. Biopharm. 2012, 54, 235–243. [Google Scholar] [CrossRef]
- Hassan, U.A.; Hussein, M.Z.; Masarudin, M.J. Synthesis of a nanoparticle system for the enhanced accumulation of fluorescently-labelled amino acids encapsulated in monodispersed chitosan nanoparticle system. Malays. Appl. Biol. 2017, 46, 171–175. [Google Scholar]
- Masarudin, M.J.; Cutts, S.M.; Evison, B.J.; Phillips, D.R.; Pigram, P.J. Factors determining the stability, size distribution, and cellular accumulation of small, monodisperse chitosan nanoparticles as candidate vectors for anticancer drug delivery: Application to the passive encapsulation of [14C]-doxorubicin. Nanotechnol. Sci. Appl. 2015, 8, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Ravikumara, N.R.; Madhusudhan, B. Chitosan nanoparticles for tamoxifen delivery and cytotoxicity to MCF-7 and Vero cells. Pure Appl. Chem. 2011, 83, 2027–2040. [Google Scholar] [CrossRef]
- Liu, H.; Gao, C. Preparation and properties of ionically crosslinked chitosan nanoparticles. Polym. Adv. Technol. 2009, 20, 613–619. [Google Scholar] [CrossRef]
- Fan, W.; Yan, W.; Xu, Z.; Ni, H. Formation mechanism of monodisperse, low molecular weight chitosan nanoparticles by ionic gelation technique. Colloids Surf. B Biointerfaces 2012, 90, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Marchiori, M.C.L.; Rigon, C.; Copetti, P.M.; Sagrillo, M.R.; Cruz, L. Nanoencapsulation Improves Scavenging Capacity and Decreases Cytotoxicity of Silibinin and Pomegranate Oil Association. AAPS PharmSciTech 2017, 18, 3236–3246. [Google Scholar] [CrossRef] [PubMed]
- Shankar, G.; Agrawal, Y.K. Formulation and evaluation of polymeric micelles for a poorly absorbed drug. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 1314–1321. [Google Scholar]
- Singh, R.; Lillard, J.W. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Ariff, S.A.Y.; Yusoff, K.; Masarudin, M.J. Encapsulation of mirna in chitosan nanoparticles as a candidate for an anti-metastatic agent in cancer therapy. Malays. Appl. Biol. 2017, 46, 165–170. [Google Scholar]
- Gomathi, T.; Prasad, P.S.; Sudha, P.N.; Anil, S. Size optimization and in vitro biocompatibility studies of chitosan nanoparticles. Int. J. Biol. Macromol. 2017, 104, 1794–1806. [Google Scholar]
- Sahu, S.K.; Maiti, S.; Maiti, T.K.; Ghosh, S.K.; Pramanik, P. Hydrophobically modified carboxymethyl chitosan nanoparticles targeted delivery of paclitaxel. J. Drug Target. 2010, 19, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpour, D.N.; Eskandari, R.; Avadi, M.R.; Zolfagharian, H.; Mir Mohammad Sadeghi, A.; Rezayat, M. Preparation and in vitro characterization of chitosan nanoparticles containing Mesobuthus eupeus scorpion venom as an antigen delivery system. J. Venom. Anim. Toxins Incl. Trop. Dis. 2012, 18, 44–52. [Google Scholar]
- Kafshgari, M.H.; Khorram, M.; Khodadoost, M.; Khavari, S. Reinforcement of Chitosan Nanoparticles Obtained by and Ionic Cross-Linking Process. Iran. Polym. J. 2011, 20, 445–456. [Google Scholar]
- Gomathi, T.; Govindarajan, C.; Rose, H.R.M.H.; Sudha, P.N.; Imran, P.K.; Venkatesan, J.; Kim, S.K. Studies on drug-polymer interaction, in vitro release and cytotoxicity from chitosan particles excipient. Int. J. Pharm. 2014, 468, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.M.; Karthivashan, G.; Gani, S.A.; Fakurazi, S.; Hussein, M.Z. In vitro drug release characteristic and cytotoxic activity of silibinin-loaded single walled carbon nanotubes functionalized with biocompatible polymers. Chem. Cent. J. 2016, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, K.; Singh, S.K.; Mishra, D.N. Chitosan Nanoparticles: A Promising System in Novel Drug Delivery. Chem. Pharm. Bull. 2010, 58, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. In Assay Guidance Manual; Eli Lilly & Company and the National Centre for Advancing Translational Sciences: Bethesda, MD, USA, 2013. [Google Scholar]
- Masarudin, M.J. Chitosan Nanoparticles as a Delivery Vehicle for [14C]-Doxorubicin and the Formaldehyde Releasing Prodrug AN-250. Ph.D. Thesis, La Trobe University, Bundoora, Australia, 2012. [Google Scholar]
- Zhang, J.; Chen, X.G.; Li, Y.Y.; Liu, C.S. Self-assembled nanoparticles based on hydrophobically modified chitosan as carriers for doxorubicin. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.L.; Ho, Y.C.; Chen, Y.M.; Peng, S.F.; Ke, C.J.; Chen, K.J.; Mi, F.L.; Sung, H.W. The characteristics, cellular uptake and intracellular trafficking of nanoparticles made of hydrophobically-modified chitosan. J. Control. Release 2010, 146, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Mateen, S.; Tyagi, A.; Agarwal, C.; Singh, R.P.; Agarwal, R. Silibinin inhibits human nonsmall cell lung cancer cell growth through cell-cycle arrest by modulating expression and function of key cell-cycle regulators. Mol. Carcinog. 2010, 49, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, P.; Truong-Dinh Tran, T.; Zhang, J.; Kong, L. Manufacturing Techniques and Surface Engineering of Polymer Based Nanoparticles for Targeted Drug Delivery to Cancer. Nanomaterials 2016, 6, 26. [Google Scholar] [CrossRef] [PubMed]

| Sample | % E.E CNP-SLB | % E.E pCNP-SLB |
|---|---|---|
| 1 | 22.46 | 50.12 |
| 2 | 28.70 | 51.35 |
| 3 | 22.58 | 46.51 |
| Average | 24.58 ± 2.06 | 49.33 ± 1.45 |
| Functional Group | Wavelength | Percentage Transmittance | Sample |
|---|---|---|---|
| Amine (a) (N–H) | 3353 | 49.62 | pCNP |
| 3446 | 49.98 | SLB | |
| 3416 | 85.97 | pCNP-SLB | |
| Alkyl group (b) (–CH, CH2) | 2888 | 80.94 | pCNP |
| 2936 | 88.91 | SLB | |
| 2851 | 24.92 | pCNP-SLB | |
| Carbonyl group (c) (C=O) | 1632 | 42.10 | pCNP |
| 1626 | 12.62 | SLB | |
| 1635 | 71.46 | pCNP-SLB | |
| Inorganic Phosphate (d) (P=O) | 1067 | 10 | pCNP |
| 1081 | 40.15 | pCNP-SLB | |
| Aromatic ring (e) (C=C) | 1507 & 1461 | 38.53 & 30.42 | SLB |
| 1536 & 1465 | 81.63 & 41.83 | pCNP-SLB | |
| Ether (f) (C–O–C) | 1268 | 10 | SLB |
| 1223 | 67.95 | pCNP-SLB |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuen, C.Y.; Fakurazi, S.; Othman, S.S.; Masarudin, M.J. Increased Loading, Efficacy and Sustained Release of Silibinin, a Poorly Soluble Drug Using Hydrophobically-Modified Chitosan Nanoparticles for Enhanced Delivery of Anticancer Drug Delivery Systems. Nanomaterials 2017, 7, 379. https://doi.org/10.3390/nano7110379
Kuen CY, Fakurazi S, Othman SS, Masarudin MJ. Increased Loading, Efficacy and Sustained Release of Silibinin, a Poorly Soluble Drug Using Hydrophobically-Modified Chitosan Nanoparticles for Enhanced Delivery of Anticancer Drug Delivery Systems. Nanomaterials. 2017; 7(11):379. https://doi.org/10.3390/nano7110379
Chicago/Turabian StyleKuen, Cha Yee, Sharida Fakurazi, Siti Sarah Othman, and Mas Jaffri Masarudin. 2017. "Increased Loading, Efficacy and Sustained Release of Silibinin, a Poorly Soluble Drug Using Hydrophobically-Modified Chitosan Nanoparticles for Enhanced Delivery of Anticancer Drug Delivery Systems" Nanomaterials 7, no. 11: 379. https://doi.org/10.3390/nano7110379





