Constructing Sheet-On-Sheet Structured Graphitic Carbon Nitride/Reduced Graphene Oxide/Layered MnO2 Ternary Nanocomposite with Outstanding Catalytic Properties on Thermal Decomposition of Ammonium Perchlorate
Abstract
:1. Introduction
2. Experimental
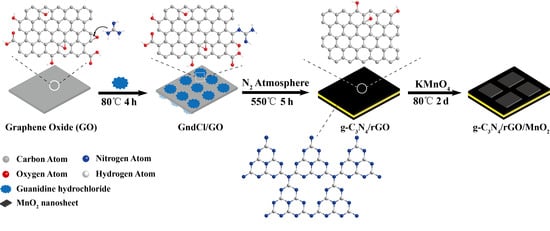
2.1. Synthesis of g-C3N4, g-C3N4/rGO and g-C3N4/rGO/MnO2 Ternary Nanocomposite
2.2. Characterization
2.3. Catalytic Activity Measurement
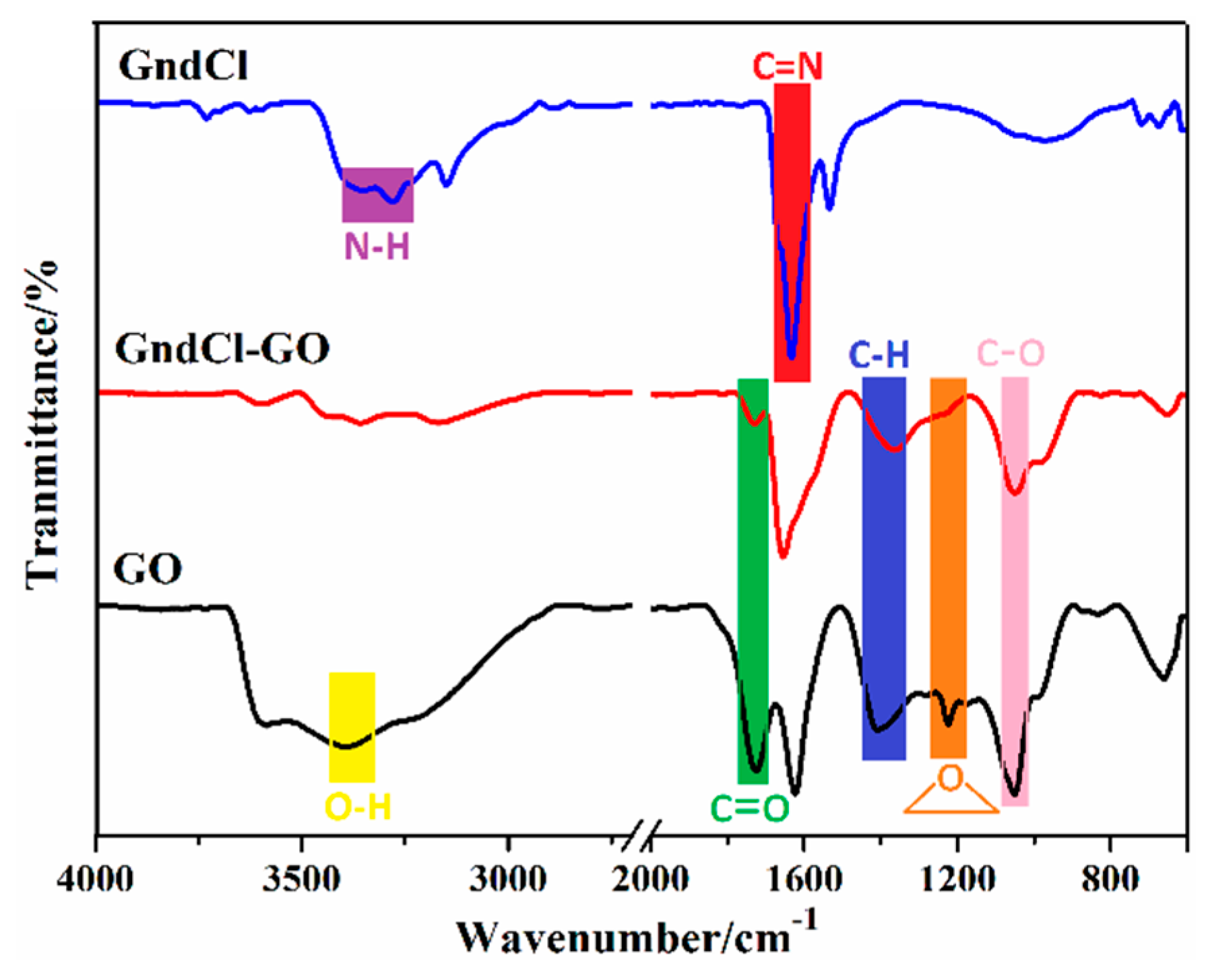
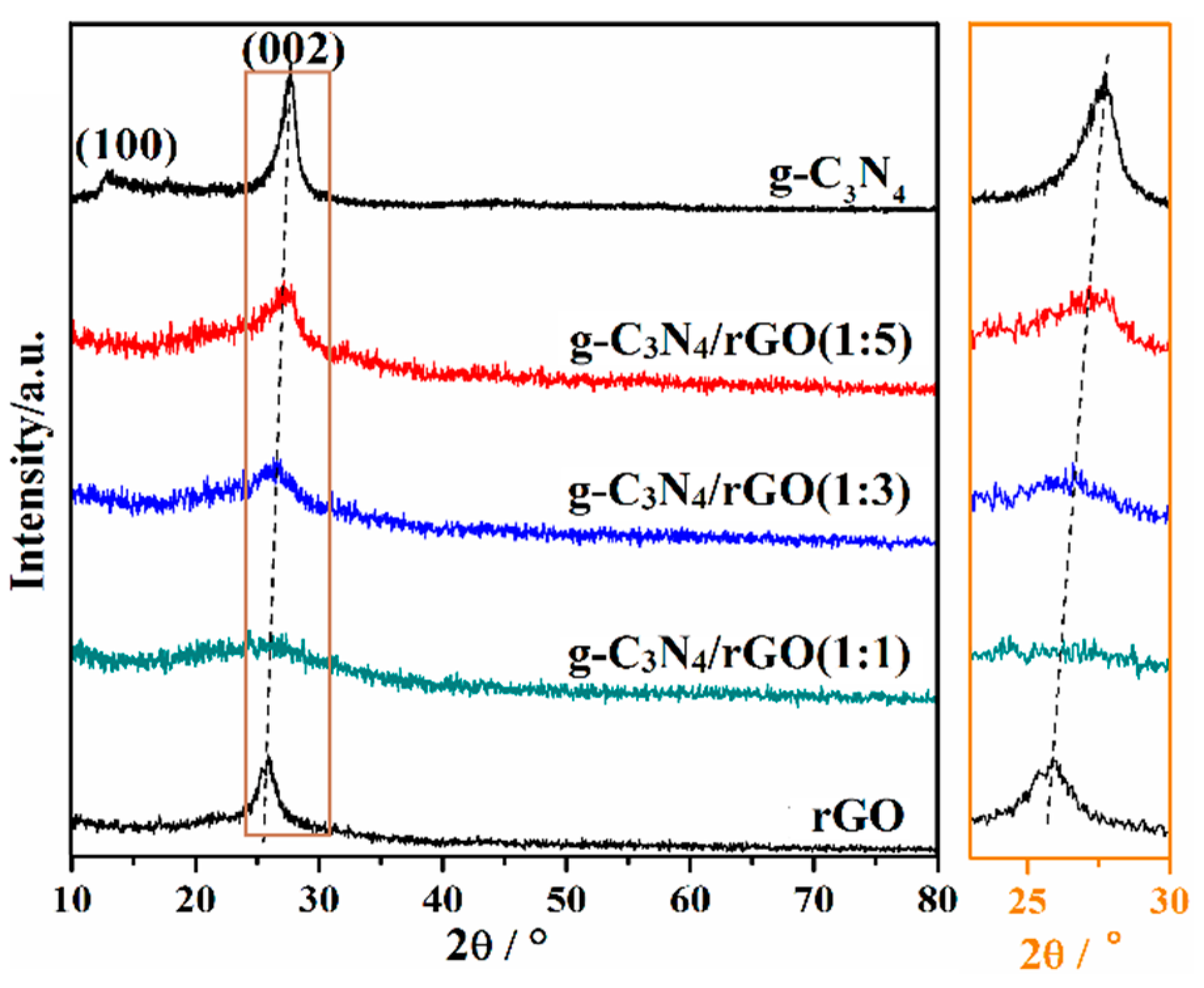
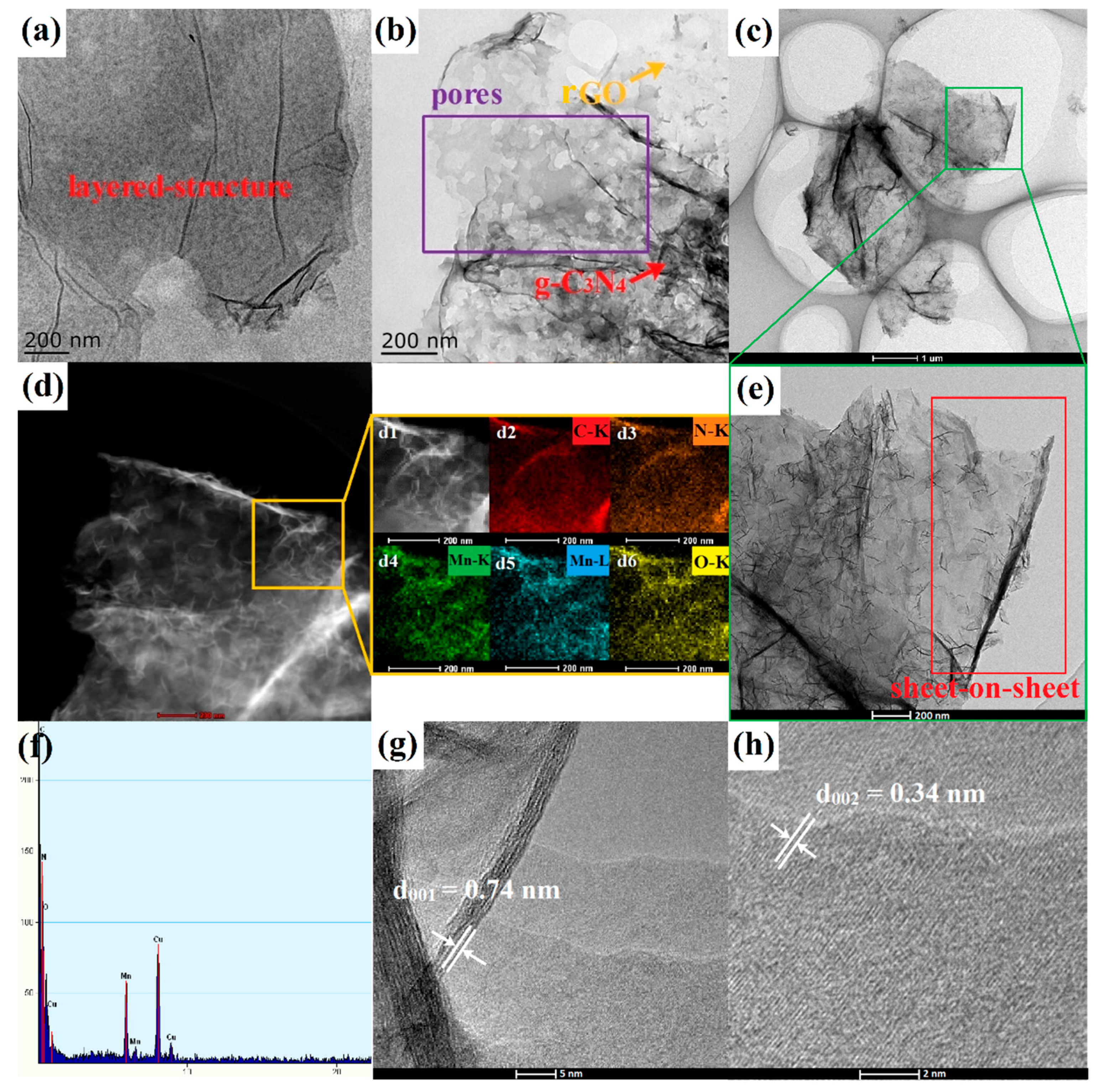
3. Result and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lu, Q.P.; Yu, Y.F.; Ma, Q.L.; Chen, B.; Zhang, H. 2D transition-metal-dichalcogenide-nanosheet-based composites for photocatalytic and electrocatalytic hydrogen evolution reactions. Adv. Mater. 2016, 28, 1917–1933. [Google Scholar] [CrossRef] [PubMed]
- Ping, J.F.; Fan, Z.X.; Sindoro, M.; Ying, Y.B.; Zhang, H. Nanosheet sensors: Recent advances in sensing applications of two-Dimensional transition metal dichalcogenide nanosheets and their composites. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Ding, J.B.; Zhou, Y.; Li, Y.G.; Guo, S.J.; Huang, X.Q. MoS2 nanosheet assembling superstructure with a three-dimensional ion accessible site: A new class of bifunctional materials for batteries and electrocatalysis. Chem. Mater. 2016, 28, 2074–2080. [Google Scholar] [CrossRef]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.H.; Kim, P.; Choi, J.Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.G.; Wei, X.D.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Qu, B.H.; Ma, C.Z.; Ji, G.; Xu, C.H.; Xu, J.; Meng, Y.S.; Wang, T.H.; Lee, J.Y. Layered SnS2-reduced graphene oxide composite—A high-capacity, high-rate and long-cycle life sodium-ion battery anode material. Adv. Mater. 2014, 26, 3854–3859. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Voiry, D.; Ahn, S.J.; Kang, D.; Kim, A.Y.; Chhowalla, M.; Shin, H.S. Two-Dimensional hybrid nanosheets of tungsten disulfide and reduced graphene oxide as catalysts for enhanced hydrogen evolution. Angew. Chem. Int. Ed. 2013, 52, 13751–13754. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.C.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.Q.; Ning, R.; Liu, Q.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X.P. Three-dimensional porous supramolecular architecture from ultrathin g-C3N4 nanosheets and reduced graphene oxide: Solution self-assembly construction and application as a highly efficient metal-free electrocatalyst for oxygen reduction reaction. ACS Appl. Mater. Interface 2014, 6, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.Z.; Chen, S.; Quan, X.; Yu, H.T.; Zhao, H.M. Graphene oxide modified g-C3N4 hybrid with enhanced photocatalytic capability under visible light irradiation. J. Mater. Chem. 2012, 22, 2721–2726. [Google Scholar] [CrossRef]
- Li, Y.B.; Zhang, H.M.; Liu, P.R.; Wang, D.; Li, Y.; Zhao, H.J. Cross-Linked g-C3N4/rGO nanocomposites with tunable band structure and enhanced visible light photocatalytic activity. Small 2013, 9, 3336–3344. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.S.; Zhu, J.W.; Hu, C.; Wu, X.D.; Wang, X. Covalently coupled hybrid of graphitic carbon nitride with reduced graphene oxide as a superior performance lithium-ion battery anode. Nanoscale 2014, 6, 12555–12564. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.J.; Tan, L.L.; Chai, S.P.; Yong, S.T. Graphene oxide as a structure-directing agent for the two-dimensional interface engineering of sandwich-like graphene-g-C3N4 hybrid nanostructures with enhanced visible-light photoreduction of CO2 to methane. Chem. Commun. 2015, 51, 858–861. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wen, Z.H.; Cui, S.M.; Guo, X.R.; Chen, J.H. Constructing 2D porous graphitic C3N4 nanosheets/nitrogen-doped graphene/layered MoS2 ternary nanojunction with enhanced photoelectrochemical activity. Adv. Mater. 2013, 25, 6291–6297. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.X.; Li, J.X.; Jiang, L.; Dong, H.L.; Wang, X.K.; Hu, W.P. Synthesizing MnO2 nanosheets from graphene oxide templates for high performance pseudosupercapacitors. Chem. Sci. 2012, 3, 433–437. [Google Scholar] [CrossRef]
- Deng, R.R.; Xie, X.J.; Vendrell, M.; Chang, Y.T.; Liu, X.G. Intracellular glutathione detection using MnO2-nanosheet-modified upconversion nanoparticles. J. Am. Chem. Soc. 2011, 133, 20168–20171. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.L.; Fan, H.H.; Zhou, G.F.; Bai, H.R.; Liang, H.; Wang, R.W.; Zhang, X.B.; Tan, W.H. Activatable fluorescence/MRI bimodal platform for tumor cell imaging via MnO2 nanosheet-aptamer nanoprobe. J. Am. Chem. Soc. 2014, 136, 11220–11223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Zheng, C.; Guo, S.S.; Li, J.; Yang, H.H.; Chen, G.N. Turn-on fluorescence sensor for intracellular imaging of glutathione using g-C3N4 nanosheet-MnO2 sandwich nanocomposite. Anal. Chem. 2014, 86, 3426–3434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.S.; Lian, J.S.; Wu, L.Y.; Duan, Z.R.; Jiang, J.; Zhao, L. Synthesis of a thin-layer MnO2 nanosheet-coated Fe3O4 nanocomposite as a magnetically separable photocatalyst. Langmuir 2014, 30, 7006–7013. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.H.; Li, S.Y.; Tan, L.H.; Kou, B. Enhanced catalytic activity of mesoporous graphitic carbon nitride on thermal decomposition of ammonium perchlorate via copper oxide modification. Mater. Res. Bull. 2017, 93, 352–360. [Google Scholar] [CrossRef]
- Xiang, Q.J.; Yu, J.G.; Jaroniec, M. Preparation and enhanced visible-light photocatalytic H2-production activity of graphene/C3N4 composites. J. Phys. Chem. C 2011, 115, 7355–7363. [Google Scholar] [CrossRef]
- Tan, L.H.; Xu, J.H.; Zhang, X.J.; Hang, Z.S.; Jia, Y.Q.; Wang, S.B. Synthesis of g-C3N4/CeO2 nanocomposites with improved catalytic activity on the thermal decomposition of ammonium perchlorate. Appl. Surf. Sci. 2015, 356, 447–453. [Google Scholar] [CrossRef]
- Jian, X.; Liu, X.; Yang, H.M.; Li, J.G.; Song, X.L.; Dai, H.Y.; Liang, Z.H. Construction of carbon quantum dots/proton-functionalized graphitic carbon nitride nanocomposite via electrostatic self-assembly strategy and its application. Appl. Surf. Sci. 2016, 370, 514–521. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, J.Y. Graphene supported Co-g-C3N4 as a novel metal-macrocyclic electrocatalyst for the oxygen reduction reaction in fuel cells. Langmuir 2013, 29, 3821–3828. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Yin, H.; Zhang, W.; Liu, Y.; Tang, Z.Y.; Zhu, M.Q. High-Performance fiber-shaped all-solid-state asymmetric supercapacitors based on ultrathin MnO2 nanosheet/carbon fiber cathodes for wearable electronics. Adv. Energy Mater. 2016, 6. [Google Scholar] [CrossRef]
- Liu, Y.C.; Miao, X.F.; Fang, J.H.; Zhang, X.X.; Chen, S.J.; Li, W.; Feng, W.D.; Chen, Y.Q.; Wang, W.; Zhang, Y.L. Layered-MnO2 nanosheet grown on nitrogen-doped graphene template as a composite cathode for flexible solid-state asymmetric supercapacitor. ACS Appl. Mater. Interface 2016, 8, 5251–5260. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Geng, Z.F.; Cao, M.H.; Ren, L.; Zhao, X.Y.; Liu, B.; Tian, Y.; Hu, C.W. Well-dispersed ultrafine Mn3O4 nanoparticles on graphene as a promising catalyst for the thermal decomposition of ammonium perchlorate. Carbon 2013, 54, 124–132. [Google Scholar] [CrossRef]
- Chen, L.J.; Zhu, D.Y. Effects of different phases of MnO2 nanorods on the catalytic thermal decomposition of ammonium perchlorate. Ceram. Int. 2015, 41, 7054–7058. [Google Scholar] [CrossRef]
- Jacobs, P.W.M.; Whitehead, H.M. Decomposition and combustion of ammonium perchlorate. Chem. Rev. 1969, 69, 551–590. [Google Scholar] [CrossRef]
- Li, Q.; He, Y.; Peng, R.F. Graphitic carbon nitride (g-C3N4) as a metal-free catalyst for thermal decomposition of ammonium perchlorate. RSC Adv. 2015, 5, 24507–24512. [Google Scholar] [CrossRef]
- Li, Q.; He, Y.; Peng, R.F. TeO2 nanoparticle loaded graphitic carbon nitride hybrids: Their preparation and catalytic activities in the thermal decomposition of ammonium perchlorate. Eur. J. Inorg. Chem. 2015, 24, 4062–4067. [Google Scholar] [CrossRef]




| Samples | C | N | O | Cl |
|---|---|---|---|---|
| GO | 67.93/61.37 | / | 32.07/38.63 | / |
| GndCl/GO | 36.04/27.05 | 21.37/18.71 | 33.06/33.08 | 9.53/21.16 |
| g-C3N4/GO | 59.54/55.14 | 33.07/35.73 | 7.39/9.13 | / |
| Samples | C=C | N–C=N | C–O–C | C–OH | –COOH |
|---|---|---|---|---|---|
| GO | 48.01 | / | 44.57 | 2.97 | 4.45 |
| GndCl/GO | 51.95 | 7.56 | 23.97 | 7.51 | 9.02 |
| g-C3N4/GO | 51.61 | 5.31 | 7.89 | 19.80 | 15.40 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Li, D.; Chen, Y.; Tan, L.; Kou, B.; Wan, F.; Jiang, W.; Li, F. Constructing Sheet-On-Sheet Structured Graphitic Carbon Nitride/Reduced Graphene Oxide/Layered MnO2 Ternary Nanocomposite with Outstanding Catalytic Properties on Thermal Decomposition of Ammonium Perchlorate. Nanomaterials 2017, 7, 450. https://doi.org/10.3390/nano7120450
Xu J, Li D, Chen Y, Tan L, Kou B, Wan F, Jiang W, Li F. Constructing Sheet-On-Sheet Structured Graphitic Carbon Nitride/Reduced Graphene Oxide/Layered MnO2 Ternary Nanocomposite with Outstanding Catalytic Properties on Thermal Decomposition of Ammonium Perchlorate. Nanomaterials. 2017; 7(12):450. https://doi.org/10.3390/nano7120450
Chicago/Turabian StyleXu, Jianhua, Dongnan Li, Yu Chen, Linghua Tan, Bo Kou, Fushun Wan, Wei Jiang, and Fengsheng Li. 2017. "Constructing Sheet-On-Sheet Structured Graphitic Carbon Nitride/Reduced Graphene Oxide/Layered MnO2 Ternary Nanocomposite with Outstanding Catalytic Properties on Thermal Decomposition of Ammonium Perchlorate" Nanomaterials 7, no. 12: 450. https://doi.org/10.3390/nano7120450