A Large-Area Nanoplasmonic Sensor Fabricated by Rapid Thermal Annealing Treatment for Label-Free and Multi-Point Immunoglobulin Sensing
Abstract
:1. Introduction
2. Experimental Methods
2.1. Au Nanostructure Fabrication
2.2. Spectroscopy and Surface Morphology Analysis
2.3. Reagent and Sample Preparation
2.4. Au Nanostructure Surface Functionalization and Immunoassay Protocol
3. Results and Discussion
3.1. Optical Images of Au Nanostructures under Various Fabrication Conditions
3.2. Optical Spectrum Measurement
3.3. Surface Morphology Analysis
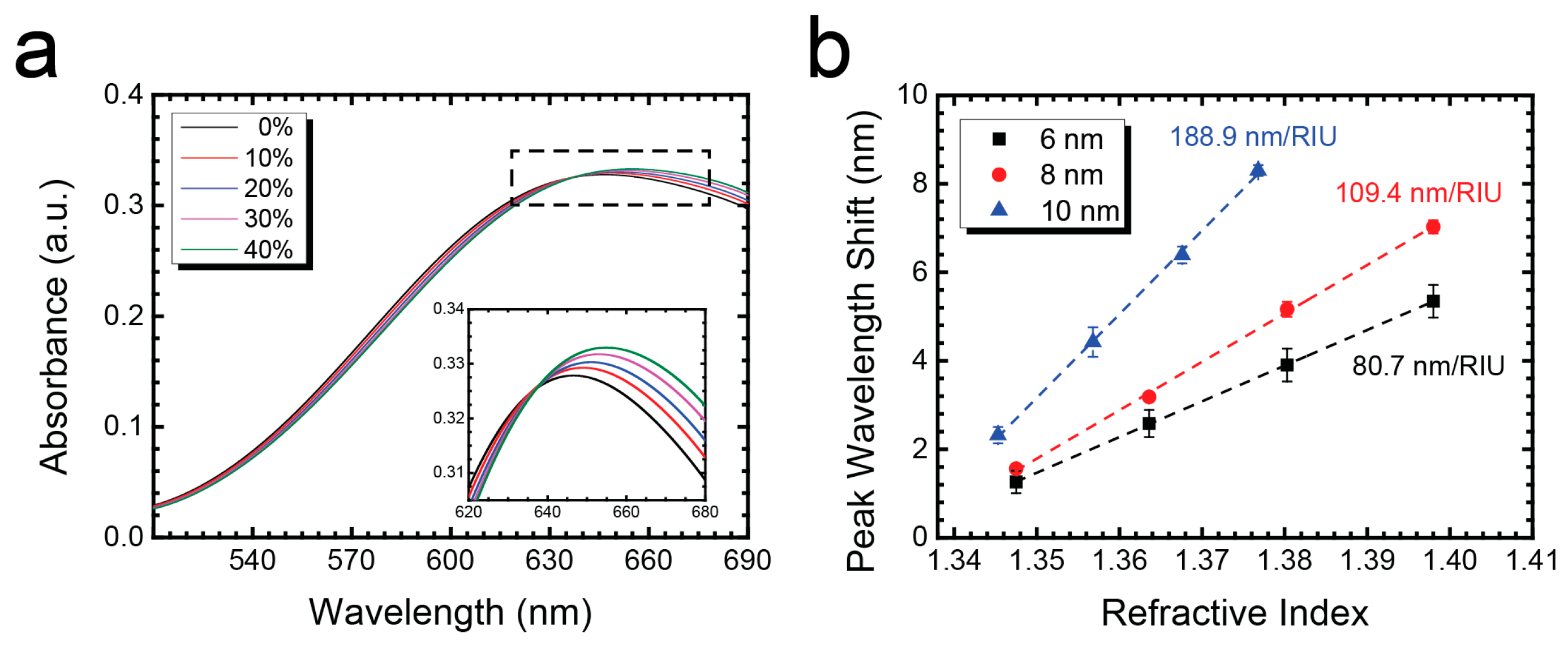
3.4. Sensitivity and Uniformity Test
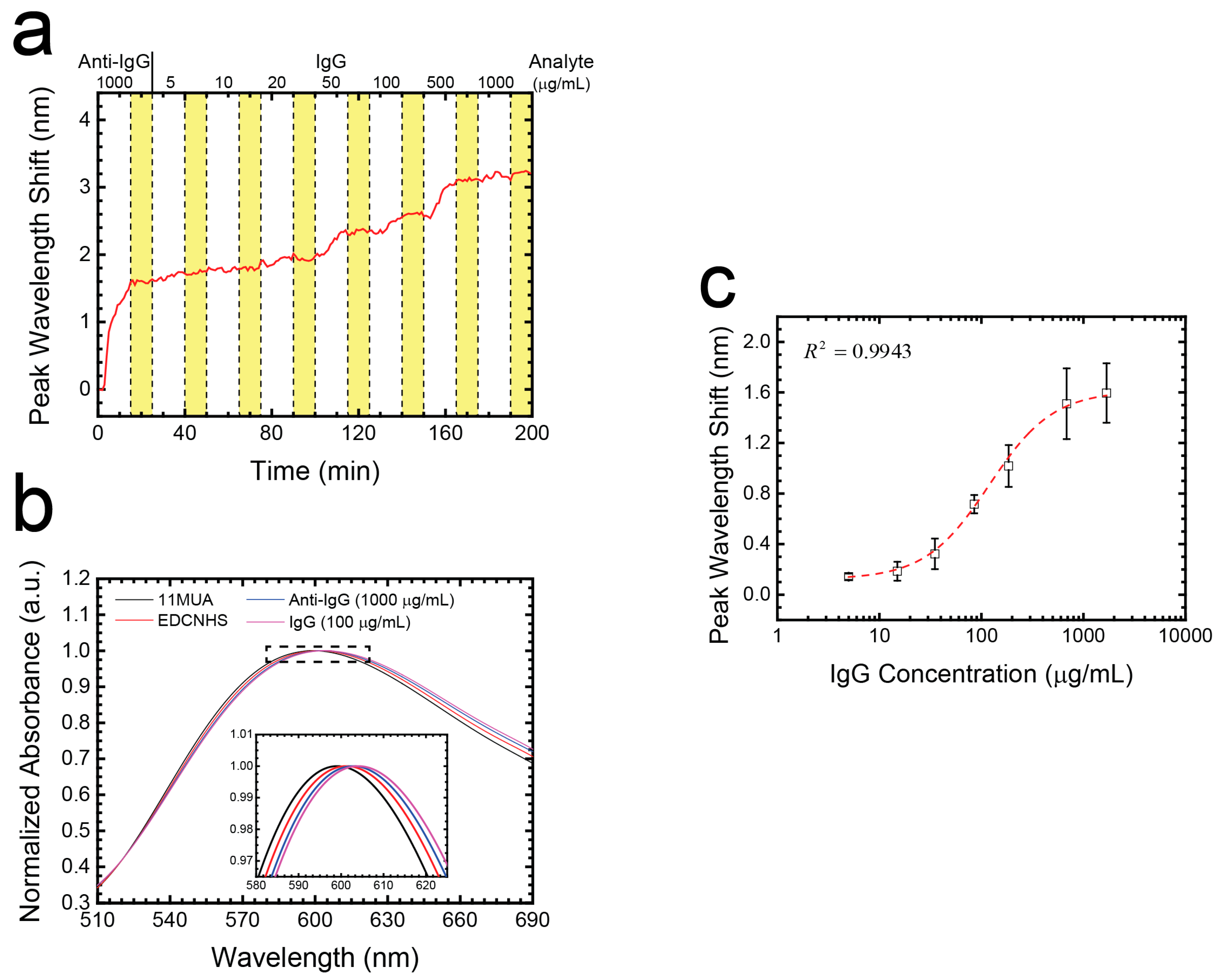
3.5. Multi-Point and Dynamic IgG Detection Using a Microchannel
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Haes, A.J.; Chang, L.; Klein, W.L.; van Duyne, R.P. Detection of a biomarker for Alzheimer’s disease from synthetic and clinical samples using a nanoscale optical biosensor. J. Am. Chem. Soc. 2005, 127, 2264–2271. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Zhao, J.; Schatz, G.C.; Sligar, S.G.; van Duyne, R.P. Screening of type I and II drug binding to human cytochrome P450–3A4 in nanodiscs by localized surface plasmon resonance spectroscopy. Anal. Chem. 2009, 81, 3754–3759. [Google Scholar] [CrossRef] [PubMed]
- Ohta, T.; Ito, M.; Kotani, T.; Hattori, T. Emission enhancement of laser-induced breakdown spectroscopy by localized surface plasmon resonance for analyzing plant nutrients. Appl. Spectrosc. 2009, 63, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-J.; Huang, K.-T.; Liu, C.-Y. Determination of organophosphorous pesticides by a novel biosensor based on localized surface plasmon resonance. Biosens. Bioelectron. 2006, 22, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Lisha, K.P.; Pradeep, T. Enhanced visual detection of pesticides using gold nanoparticles. J. Environ. Sci. Health Part B 2009, 44, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.K.; Dong, Y.; Hinds, L.; Keen, M.A.; Belisle, J.T.; Zolla-Pazner, S.; Achkar, J.M.; Nadas, A.J.; Arora, V.K.; Laal, S. Combined use of serum and urinary antibody for diagnosis of tuberculosis. J. Infect. Dis. 2003, 188, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control, New Edition ed; World Health Organization: Geneva, Switzerland, 2009.
- Fasano, A.; Catassi, C. Current approaches to diagnosis and treatment of celiac disease: An evolving spectrum. Gastroenterology 2001, 120, 636–651. [Google Scholar] [CrossRef] [PubMed]
- Lemon, S.M. Type a viral hepatitis: Epidemiology, diagnosis, and prevention. Clin. Chem. 1997, 43, 1494–1499. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Chung, M.T.; McHugh, W.; Nidetz, R.; Li, Y.; Fu, J.; Cornell, T.T.; Shanley, T.P.; Kurabayashi, K. Multiplex serum cytokine immunoassay using nanoplasmonic biosensor microarrays. ACS Nano 2015, 9, 4173–4181. [Google Scholar] [CrossRef] [PubMed]
- Oh, B.-R.; Huang, N.-T.; Chen, W.; Seo, J.; Chen, P.; Cornell, T.T.; Shanley, T.P.; Fu, J.; Kurabayashi, K. Integrated nanoplasmonic sensing for cellular functional immunoanalysis using human blood. ACS Nano 2014, 8, 2667–2676. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, K.; Watarai, H.; Itoh, H.; Nakahama, E.; Ogawa, N. Measurement of antibody binding to protein immobilized on gold nanoparticles by localized surface plasmon spectroscopy. Anal. Bioanal. Chem. 2006, 386, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Kerman, K.; Nagatani, N.; Hiepa, H.M.; Kim, D.K.; Yonezawa, Y.; Nakano, K.; Tamiya, E. Multiple label-free detection of antigen-antibody reaction using localized surface plasmon resonance-based core-shell structured nanoparticle layer nanochip. Anal. Chem. 2006, 78, 6465–6475. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Xu, S.; Zheng, X.; Wang, Y.; Xu, W. Optical fiber lspr biosensor prepared by gold nanoparticle assembly on polyelectrolyte multilayer. Sensors (Basel) 2010, 10, 3585–3596. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zou, Y.; Mo, Y.; Guo, J.; Lindquist, R.G. E-beam patterned gold nanodot arrays on optical fiber tips for localized surface plasmon resonance biochemical sensing. Sensors 2010, 10, 9397–9406. [Google Scholar] [CrossRef] [PubMed]
- Barbillon, G. Plasmonic nanostructures prepared by soft uv nanoimprint lithography and their application in biological sensing. Micromachines 2012, 3, 21–27. [Google Scholar] [CrossRef]
- Haes, A.J.; Haynes, C.L.; Duyne, R.P.V. Nanosphere lithography self-assembled photonic and magnetic materials. Mater. Res. Soc. Symp. 2001, 636. [Google Scholar] [CrossRef]
- Lucas, B.D.; Kim, J.-S.; Chin, C.; Guo, L.J. Nanoimprint lithography based approach for the fabrication of large-area, uniformly-oriented plasmonic arrays. Adv. Mater. 2008, 20, 1129–1134. [Google Scholar] [CrossRef]
- Lopatynskyi, A.M.; Lytvyn, V.K.; Nazarenko, V.I.; Guo, L.J.; Lucas, B.D.; Chegel, V.I. Au nanostructure arrays for plasmonic applications: Annealed island films versus nanoimprint lithography. Nanoscale Res. Lett. 2015, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Švorčík, V.; Kvítek, O.; Lyutakov, O.; Siegel, J.; Kolská, Z. Annealing of sputtered gold nano-structures. Appl. Phys. A 2010, 102, 747–751. [Google Scholar] [CrossRef]
- Henley, S.J.; Carey, J.D.; Silva, S.R.P. Pulsed-laser-induced nanoscale island formation in thin metal-on-oxide films. Phys. Rev. B 2005, 72, 195408. [Google Scholar] [CrossRef]
- Link, S.; Burda, C.; Mohamed, M.B.; Nikoobakht, B.; El-Sayed, M.A. Laser photothermal melting and fragmentation of gold nanorods energy and laser pulse-width dependence. J. Phys. Chem. A 1999, 103, 1165–1170. [Google Scholar] [CrossRef]
- Jia, K.; Bijeon, J.L.; Adam, P.M.; Ionescu, R.E. Sensitive localized surface plasmon resonance multiplexing protocols. Anal. Chem. 2012, 84, 8020–8027. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, N.; Lee, D.; Sathish, S.; Shen, A.Q. Dual-mode refractive index and charge sensing to investigate complex surface chemistry on nanostructures. Nanoscale 2017, 9, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hicks, E.M.; Zhao, J.; Schatz, G.C.; van Duyne, R.P. Electrochemical tuning of silver nanoparticles fabricated by nanosphere lithography. Nano Lett. 2005, 5, 1503–1507. [Google Scholar] [CrossRef] [PubMed]
- Sönnichsen, C.; Reinhard, B.M.M.; Liphardt, J.; Alivisatos, A.P. A molecular ruler based on plasmon coupling of single gold and silver nanoparticles. Nat. Biotechnol. 2005, 23, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Reinhard, B.M.M.; Siu, M.; Agarwal, H.; Alivisatos, A.P.; Liphardt, J. Calibration of dynamic molecular rulers based on plasmon coupling between gold nanoparticles. Nano Lett. 2005, 5, 2246–2252. [Google Scholar] [CrossRef] [PubMed]
- Funston, A.M.; Novo, C.; Davis, T.J.; Mulvaney, P. Plasmon coupling of gold nanorods at short distances and in different geometries. Nano Lett. 2009, 9, 1651–1658. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Huang, W.; El-Sayed, M.A. On the universal scaling behavior of the distance decay of plasmon coupling in metal nanoparticle pairs: A plasmon ruler equation. Nano Lett. 2007, 7, 2080–2088. [Google Scholar] [CrossRef]
- Petryayeva, E.; Krull, U.J. Localized surface plasmon resonance: Nanostructures, bioassays and biosensing—A review. Anal. Chim. Acta 2011, 706, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Mock, J.J.; Smith, D.R.; Schultz, S. Local refractive index dependence of plasmon resonance spectra from individual nanoparticles. Nano Lett. 2003, 3, 485–491. [Google Scholar] [CrossRef]
- Sherry, L.J.; Jin, R.; Mirkin, C.A.; Schatz, G.C.; van Duyne, R.P. Localized surface plasmon resonance spectroscopy of single silver triangular nanoprisms. Nano Lett. 2006, 6, 2060–2065. [Google Scholar] [CrossRef] [PubMed]
- Malinsky, M.D.; Kelly, K.L.; Schatz, G.C.; van Duyne, R.P. Chain length dependence and sensing capabilities of the localized surface plasmon resonance of silver nanoparticles chemically modified with alkanethiol self-assembled monolayers. J. Am. Chem. Soc. 2001, 123, 1471–1482. [Google Scholar] [CrossRef]
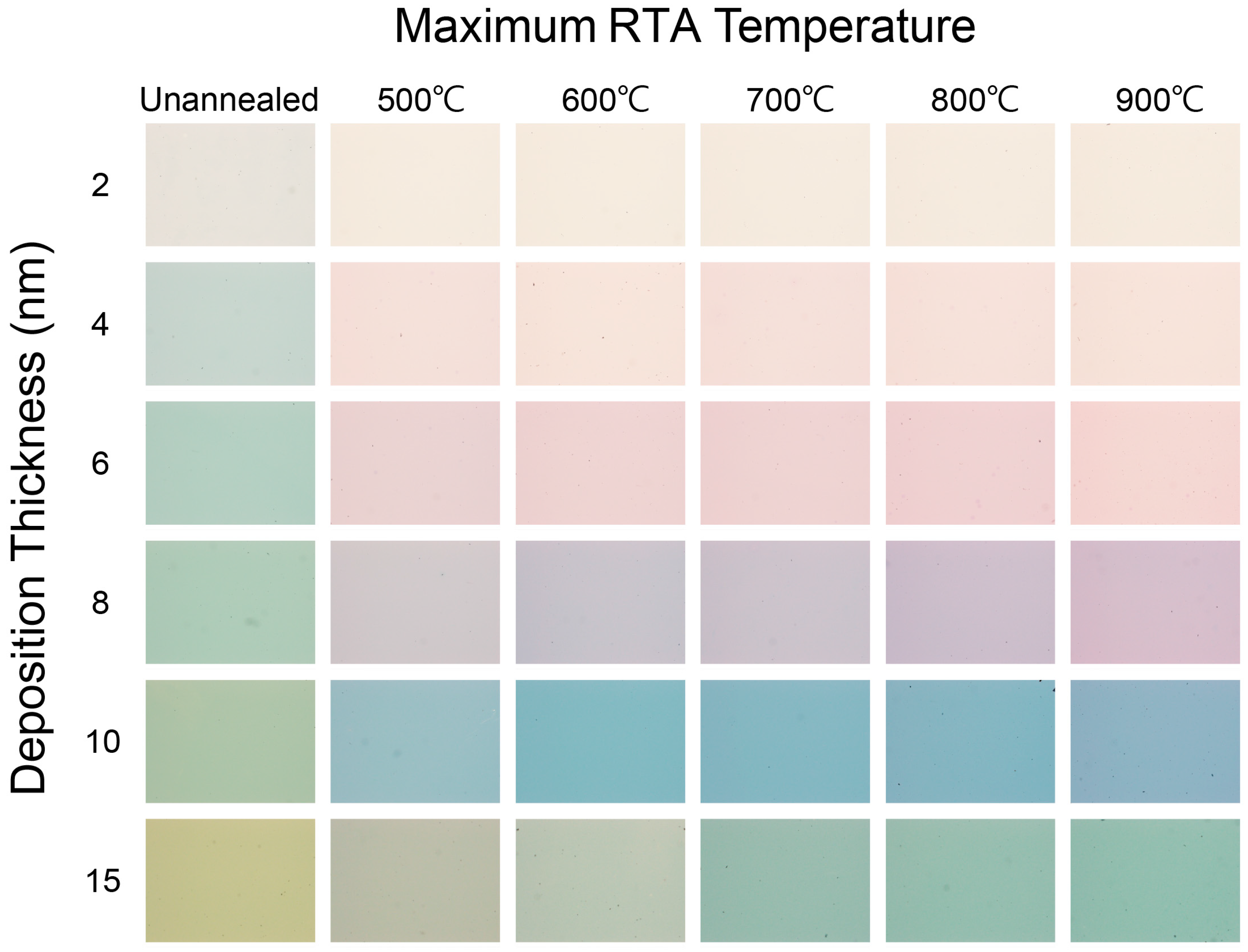

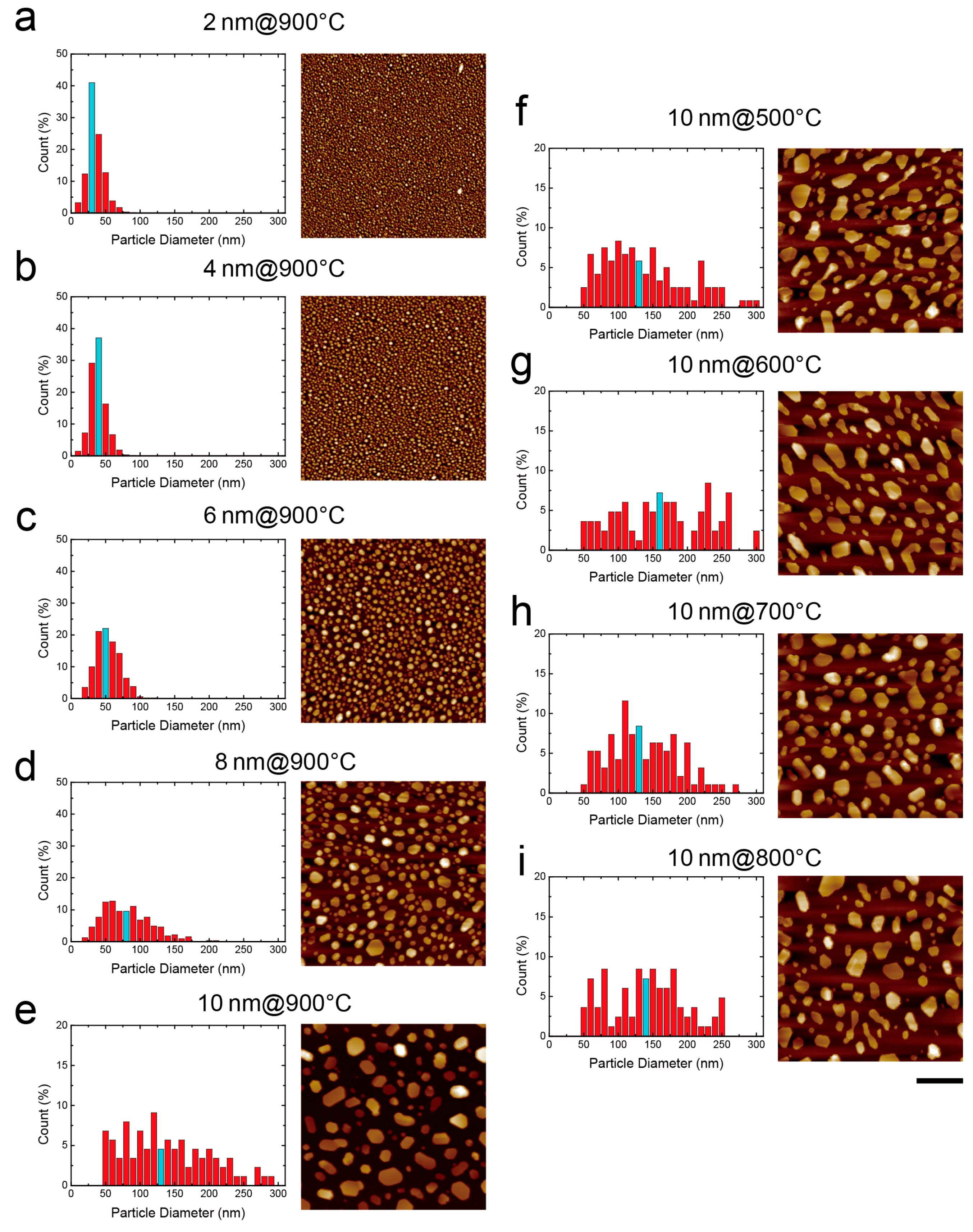
| Maximum RTA Temperature (°C ) | 900 | ||||
| Deposition thickness (nm) | 2 | 4 | 6 | 8 | 10 |
| Number of particles (Np) | 1103 ± 21 | 785 ± 22 | 281 ± 15 | 98 ± 14 | 31 ± 4 |
| Equivalent particle diameter (Da) (nm) | 20.6 ± 6.9 | 25.4 ± 7.9 | 39.9 ± 15.6 | 61.7 ± 29.4 | 113.8 ± 53.7 |
| Surface roughness (Ra) (nm) | 3.1 ± 0.0 | 5.2 ± 0.2 | 8.7 ± 0.2 | 11.9 ± 0.5 | 14.5 ± 0.6 |
| Total area of particle (Ap) (%) | 40.4 ± 0.4 | 43.4 ± 0.1 | 39.7 ± 0.5 | 33.6 ± 0.6 | 33.1 ± 1.3 |
| Deposition Thickness (nm) | 10 | ||||
| Maximum RTA temperature (°C) | 500 | 600 | 700 | 800 | 900 |
| Number of particles (Np) | 43 ± 5 | 32 ± 4 | 36 ± 3 | 31 ± 5 | 31 ± 4 |
| Equivalent particle diameter (Da) (nm) | 97.1 ± 48.1 | 114.0 ± 49.4 | 99.9 ± 47.4 | 107.1 ± 50.4 | 113.8 ± 53.7 |
| Surface roughness (Ra) (nm) | 12.9 ± 0.2 | 13.6 ± 0.2 | 14.4 ± 0.5 | 14.0 ± 0.8 | 14.5 ± 0.6 |
| Total area of particles (Ap) (%) | 34.5 ± 2.0 | 33.7 ± 1.2 | 31.1 ± 0.3 | 29.5 ± 1.3 | 33.1 ± 1.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.T.-H.; Yang, C.-K.; Lin, C.-C.; Wu, A.M.-H.; Wang, L.A.; Huang, N.-T. A Large-Area Nanoplasmonic Sensor Fabricated by Rapid Thermal Annealing Treatment for Label-Free and Multi-Point Immunoglobulin Sensing. Nanomaterials 2017, 7, 100. https://doi.org/10.3390/nano7050100
Lin HT-H, Yang C-K, Lin C-C, Wu AM-H, Wang LA, Huang N-T. A Large-Area Nanoplasmonic Sensor Fabricated by Rapid Thermal Annealing Treatment for Label-Free and Multi-Point Immunoglobulin Sensing. Nanomaterials. 2017; 7(5):100. https://doi.org/10.3390/nano7050100
Chicago/Turabian StyleLin, Hana Tzu-Han, Chuan-Kai Yang, Chi-Chen Lin, Albert Meng-Hsin Wu, Lon A. Wang, and Nien-Tsu Huang. 2017. "A Large-Area Nanoplasmonic Sensor Fabricated by Rapid Thermal Annealing Treatment for Label-Free and Multi-Point Immunoglobulin Sensing" Nanomaterials 7, no. 5: 100. https://doi.org/10.3390/nano7050100







