Deposition of Visible Light-Active C-Doped Titania Films via Magnetron Sputtering Using CO2 as a Source of Carbon
Abstract
:1. Introduction
2. Materials and Methods
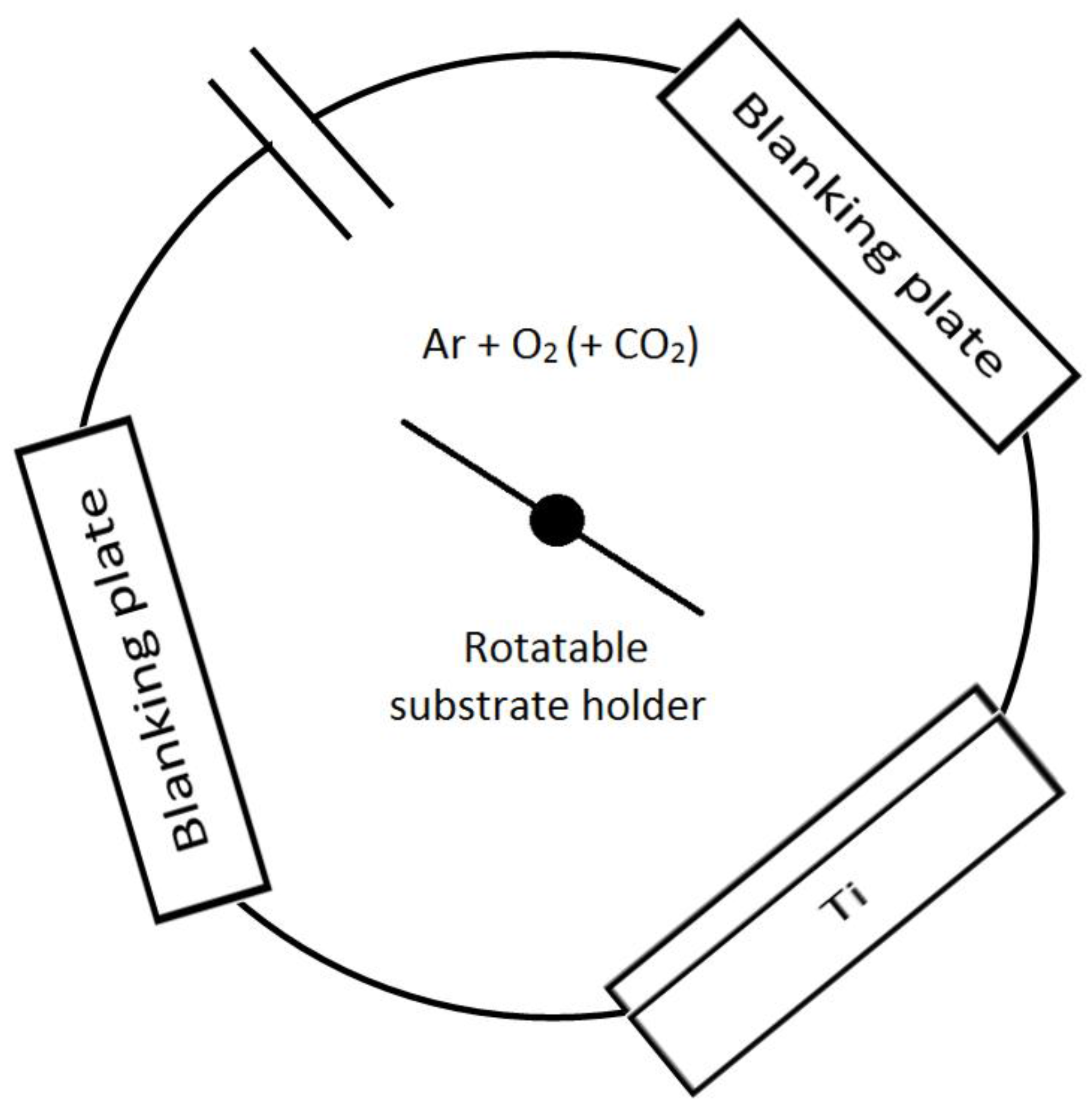
2.1. Deposition of the Coatings
2.2. Characterization of the Coatings
2.3. Photocatalytic Activity Assessment
2.3.1. Decomposition of Methylene Blue (MB)
2.3.2. Decomposition of Stearic Acid (SA)
3. Results and Discussion
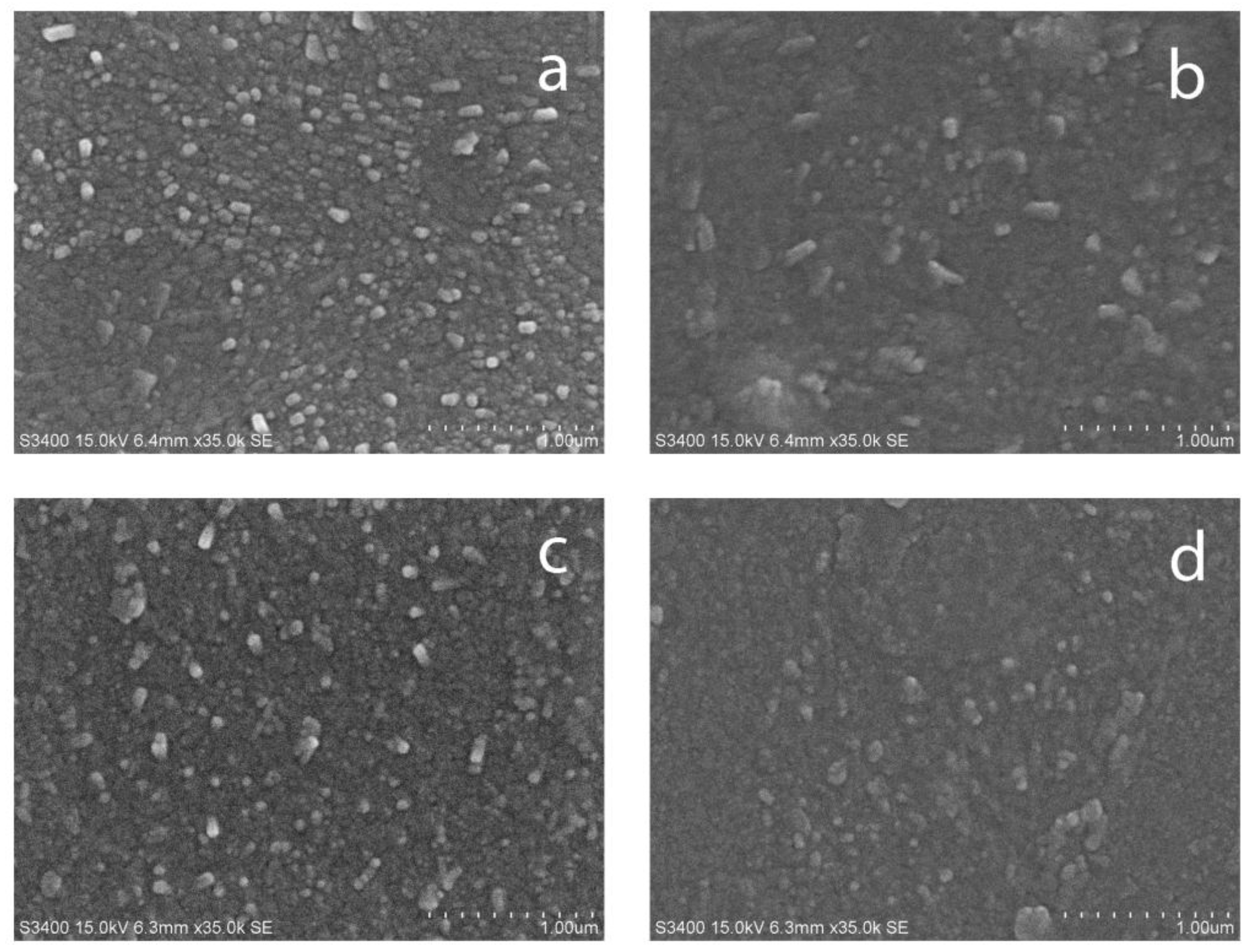
3.1. Coatings Overview
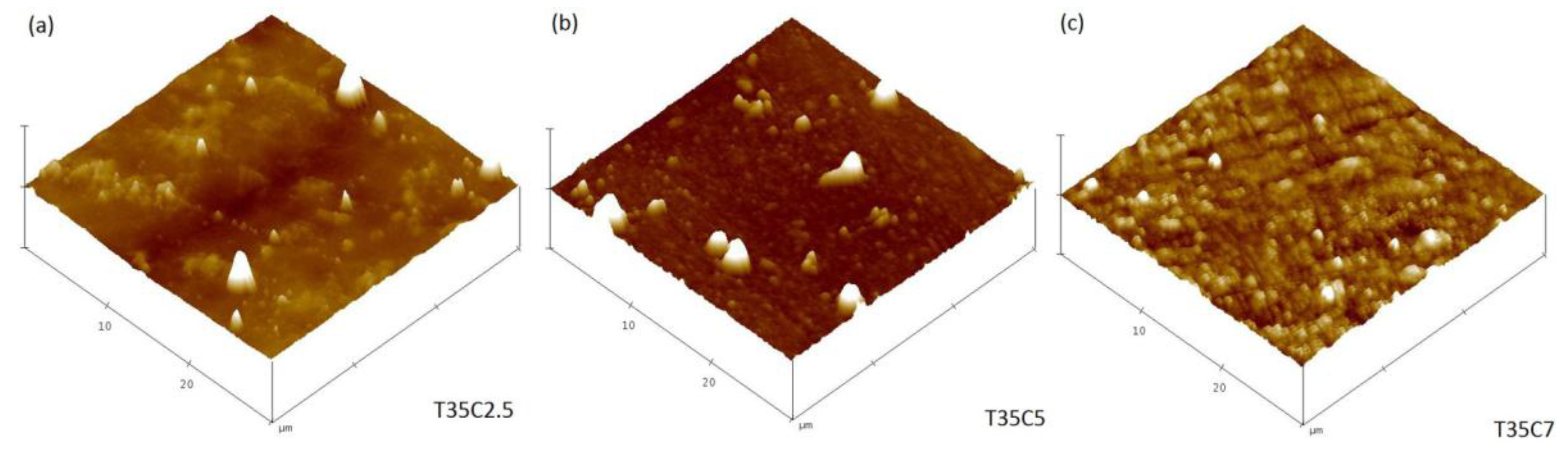
3.2. Morphology of the Films (AFM)
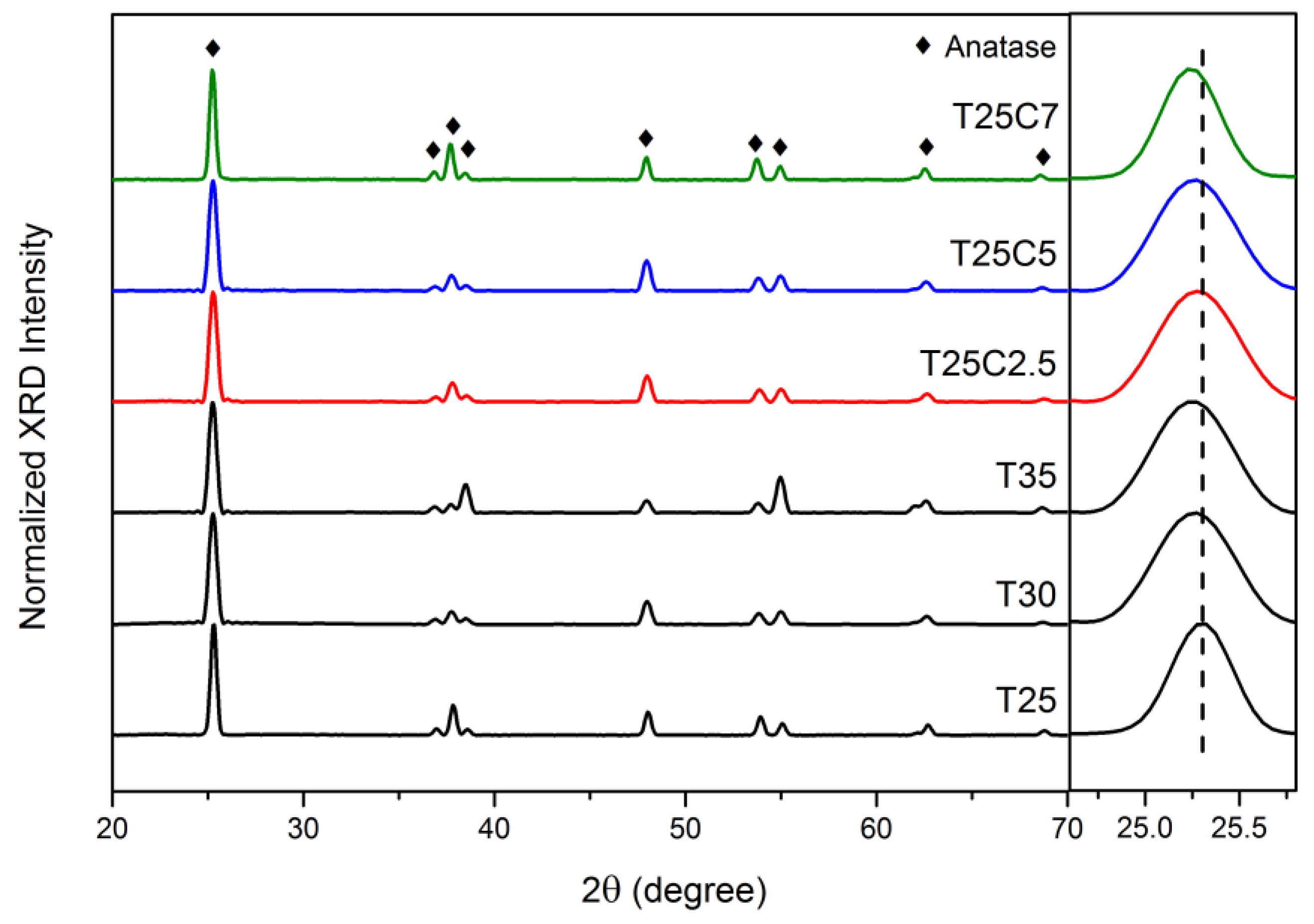
3.3. Film Crystallinity (XRD)
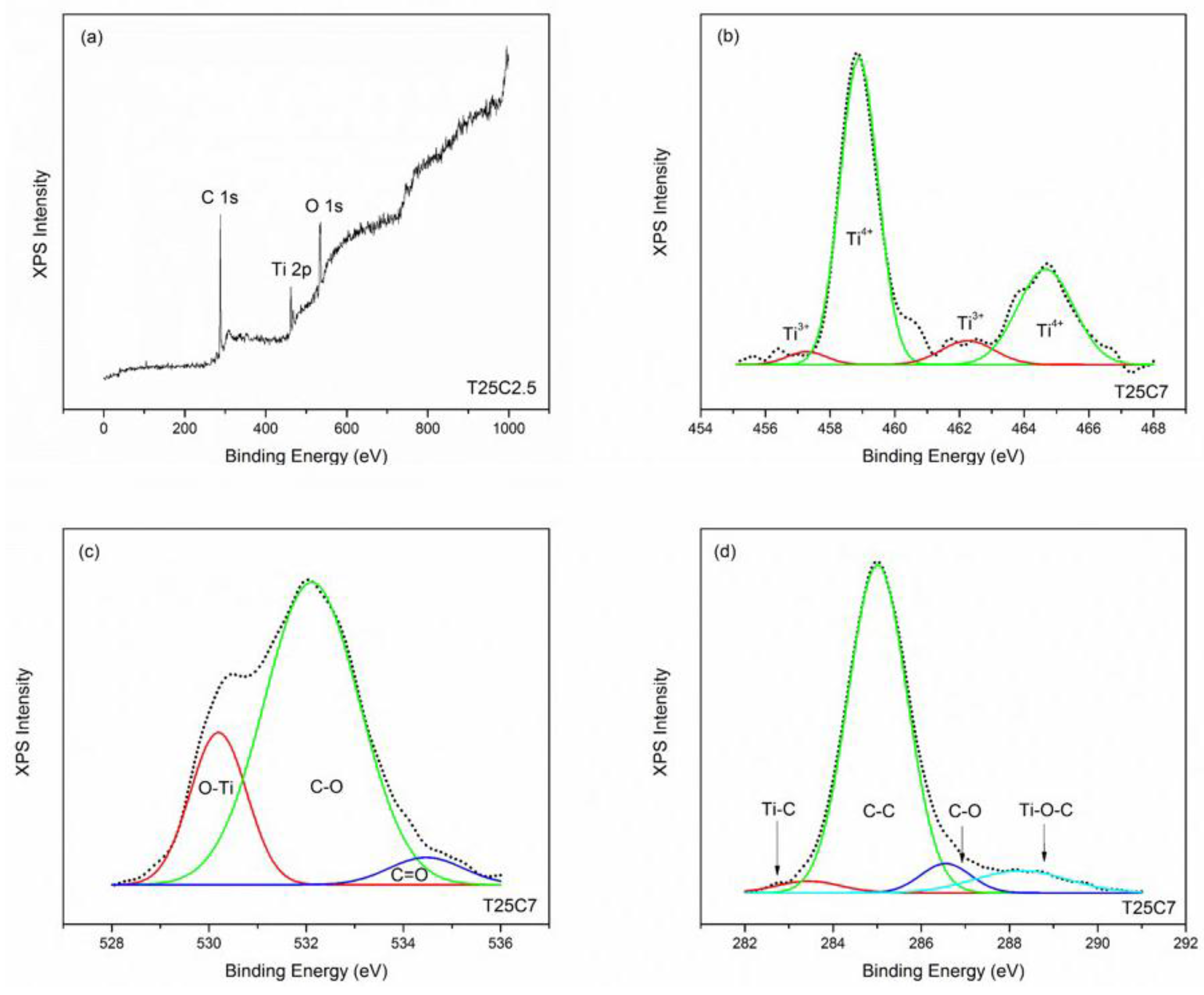
3.4. XPS Results
3.5. Optical Properties and Band Gap Calculations
3.6. Wettability Measurements
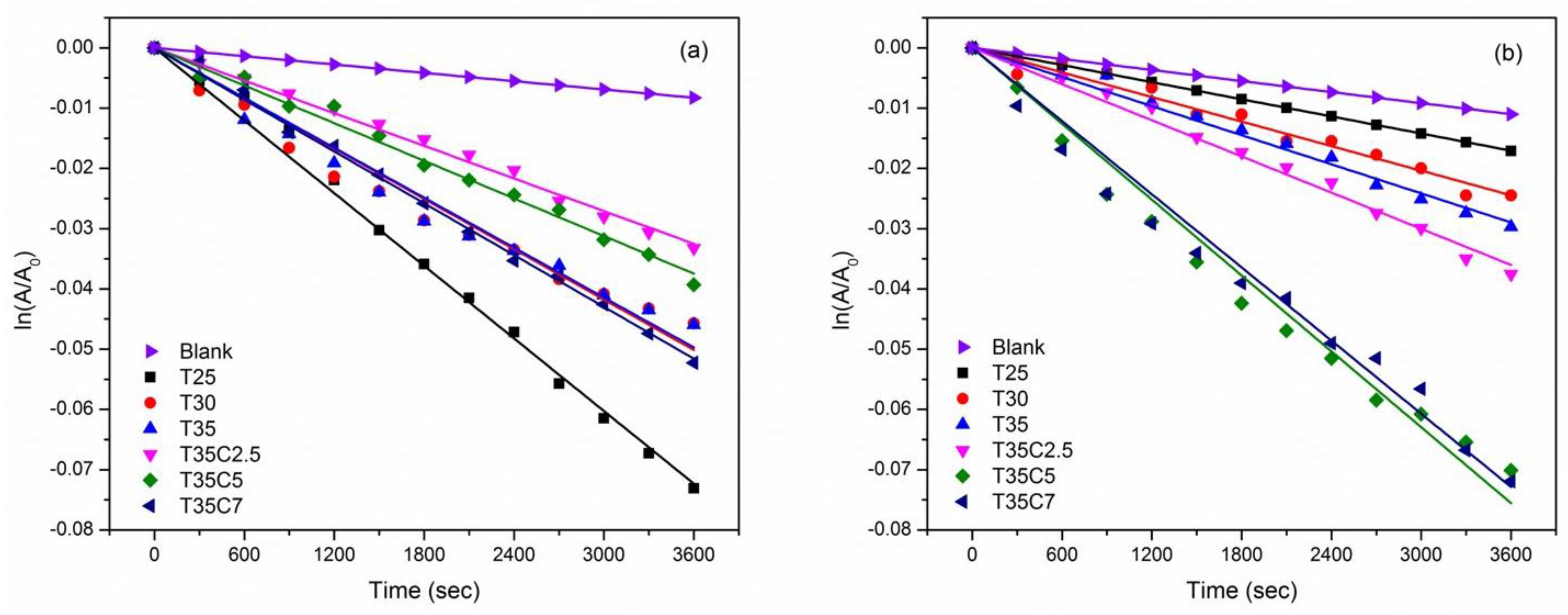
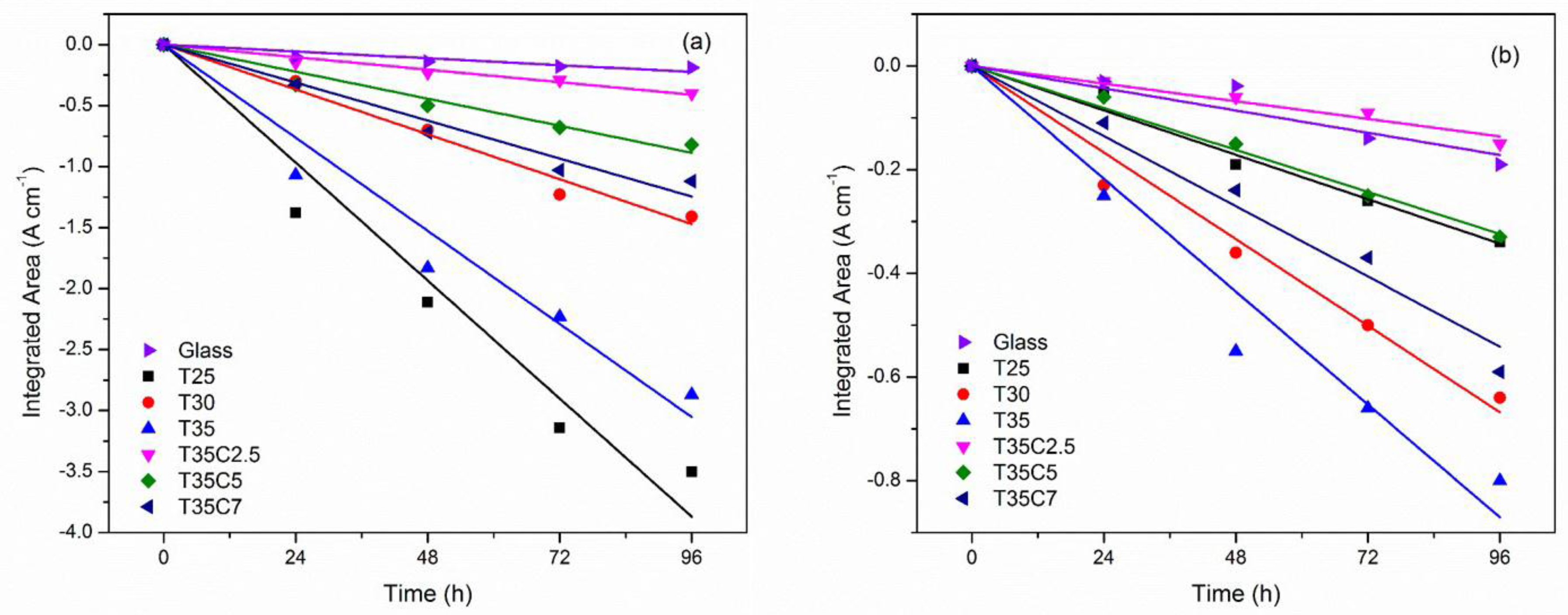
3.7. Photocatalytic Tests Results
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Taga, Y. Titanium oxide based visible light photocatalysts: Materials design and applications. Thin Solid Films 2009, 517, 3167–3172. [Google Scholar] [CrossRef]
- Paz, Y. Application of TiO2 photocatalysis for air treatment: Patents overview. Appl. Catal. B: Environ. 2010, 99, 448–460. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Ratova, M.; Kelly, P.J.; West, G.T.; Iordanova, I. Enhanced properties of magnetron sputtered photocatalytic coatings via transition metal doping. Surf. Coat. Technol. 2013, 228 (Suppl. 1), S544–S549. [Google Scholar] [CrossRef]
- Nolan, N.T.; Synnott, D.W.; Seery, M.K.; Hinder, S.J.; Van Wassenhoven, A.; Pillai, S.C. Effect of N-doping on the photocatalytic activity of sol-gel TiO2. J. Hazard. Mater. 2012, 211–212, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.S.; Chou, H.P.; Yang, T.S. Reactively sputtered N-doped titanium oxide films as visible-light photocatalyst. Thin Solid Films 2006, 494, 244–249. [Google Scholar] [CrossRef]
- Wang, M.C.; Lin, H.J.; Wang, C.H.; Wu, H.C. Effects of annealing temperature on the photocatalytic activity of N-doped TiO2 thin films. Ceram. Int. 2012, 38, 195–200. [Google Scholar] [CrossRef]
- Abadias, G.; Paumier, F.; Eyidi, D.; Guerin, P.; Girardeau, T. Structure and properties of nitrogen-doped titanium dioxide thin films produced by reactive magnetron sputtering. Surf. Interface Anal. 2010, 42, 970–973. [Google Scholar] [CrossRef]
- Bu, X.; Zhang, G.; Zhang, C. Effect of nitrogen doping on anatase-rutile phase transformation of TiO2. Appl. Surf. Sci. 2012, 258, 7997–8001. [Google Scholar] [CrossRef]
- Peng, F.; Cai, L.; Yu, H.; Wang, H.; Yang, J. Synthesis and characterization of substitutional and interstitial nitrogen-doped titanium dioxides with visible light photocatalytic activity. J. Solid State Chem. 2008, 181, 130–136. [Google Scholar] [CrossRef]
- Xie, Y.; Zhao, X.; Chen, Y.; Zhao, Q.; Yuan, Q. Preparation and characterization of porous C-modified anatase titania films with visible light catalytic activity. J. Solid State Chem. 2007, 180, 3576–3582. [Google Scholar] [CrossRef]
- Ohno, T.; Akiyoshi, M.; Umebayashi, T.; Asai, K.; Mitsui, T.; Matsumura, M. Preparation of S-doped TiO2 photocatalysts and their photocatalytic activities under visible light. Appl. Catal. A Gen. 2004, 265, 115–121. [Google Scholar] [CrossRef]
- Rockafellow, E.M.; Stewart, L.K.; Jenks, W.S. Is sulfur-doped TiO2 an effective visible light photocatalyst for remediation? Appl. Catal. B Environ. 2009, 91, 554–562. [Google Scholar] [CrossRef]
- Gombac, V.; De Rogatis, L.; Gasparotto, A.; Vicario, G.; Montini, T.; Barreca, D.; Balducci, G.; Fornasiero, P.; Tondello, E.; Graziani, M. TiO2 nanopowders doped with boron and nitrogen for photocatalytic applications. Chem. Phys. 2007, 339, 111–123. [Google Scholar] [CrossRef]
- Dozzi, M.V.; D’Andrea, C.; Ohtani, B.; Valentini, G.; Selli, E. Fluorine-doped TiO2 materials: Photocatalytic activity vs time-resolved photoluminescence. J. Phys. Chem. C 2013, 117, 25586–25595. [Google Scholar] [CrossRef]
- Ao, Y.; Xu, J.; Fu, D.; Yuan, C. Synthesis of C, N, S-tridoped mesoporous titania with enhanced visible light-induced photocatalytic activity. Microporous Mesoporous Mater. 2009, 122, 1–6. [Google Scholar] [CrossRef]
- Wu, K.R.; Hung, C.H. Characterization of N,C-codoped TiO2 films prepared by reactive dc magnetron sputtering. Appl. Surf. Sci. 2009, 256, 1595–1603. [Google Scholar] [CrossRef]
- Dozzi, M.V.; Selli, E. Doping TiO2 with p-block elements: Effects on photocatalytic activity. J. Photochem. Photobiol. C Photochem. Rev. 2013, 14, 13–28. [Google Scholar] [CrossRef]
- Serpone, N. Is the band gap of pristine TiO2 narrowed by anion- and cation-doping of titanium dioxide in second-generation photocatalysts? J. Phys. Chem. B 2006, 110, 24287–24293. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.A.; Neville, E.M.; Herron, R.; Thampi, K.R.; MacElroy, J.M.D. Routes to visible light active C-doped TiO2 photocatalysts using carbon atoms from the Ti precursors. J. Photochem. Photobiol. A Chem. 2014, 289, 60–65. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Z.; Chen, J.; Cheng, L.; Chang, J.; Sheng, W.; Hu, C.; Cao, S. C-doped hollow TiO2 spheres: In situ synthesis, controlled shell thickness, and superior visible-light photocatalytic activity. Appl. Catal. B Environ. 2015, 165, 715–722. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Li, H.; Cui, Y.; Du, Y. Visible light-driven nitrogen doped TiO2 nanoarray films: Preparation and photocatalytic activity. J. Alloy. Compd. 2010, 494, 372–377. [Google Scholar] [CrossRef]
- Kelly, P.J.; West, G.T.; Ratova, M.; Fisher, L.; Ostovarpour, S.; Verran, J. Structural formation and photocatalytic activity of magnetron sputtered titania and doped-titania coatings. Molecules 2014, 19, 16327–16348. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.J.; Arnell, R.D. Development of a novel structure zone model relating to the closed-field unbalanced magnetron sputtering system. J. Vac. Sci. Technol. A: Vac. Surf. Films 1998, 16, 2858–2869. [Google Scholar] [CrossRef]
- Irie, H.; Washizuka, S.; Hashimoto, K. Hydrophilicity on carbon-doped TiO2 thin films under visible light. Thin Solid Films 2006, 510, 21–25. [Google Scholar] [CrossRef]
- Pradhan, S.S.; Sahoo, S.; Pradhan, S.K. Influence of annealing temperature on the structural, mechanical and wetting property of TiO2 films deposited by rf magnetron sputtering. Thin Solid Films 2010, 518, 6904–6908. [Google Scholar] [CrossRef]
- Ratova, M.; West, G.T.; Kelly, P.J. Optimisation of hipims photocatalytic titania coatings for low temperature deposition. Surf. Coat. Technol. 2014, 250, 7–13. [Google Scholar] [CrossRef]
- Ratova, M.; West, G.T.; Kelly, P.J. Visible light activated photocatalytic TaON coatings deposited via pulsed-dc magnetron sputtering. Vacuum 2014, 109, 135–138. [Google Scholar] [CrossRef]
- Farahani, N.; Kelly, P.J.; West, G.; Ratova, M.; Hill, C.; Vishnyakov, V. Photocatalytic activity of reactively sputtered and directly sputtered titania coatings. Thin Solid Films 2011, 520, 1464–1469. [Google Scholar] [CrossRef]
- Ratova, M.; Kelly, P.J.; West, G.T.; Tosheva, L.; Edge, M. Reactive magnetron sputtering deposition of bismuth tungstate onto titania nanoparticles for enhancing visible light photocatalytic activity. Appl. Surf. Sci. 2017, 392, 590–597. [Google Scholar] [CrossRef]
- Schiffmann, K.I.; Fryda, M.; Goerigk, G.; Lauer, R.; Hinze, P.; Bulack, A. Sizes and distances of metal clusters in Au-, Pt-, W- and Fe-containing diamond-like carbon hard coatings: A comparative study by small angle X-ray scattering, wide angle X-ray diffraction, transmission electron microscopy and scanning tunnelling microscopy. Thin Solid Films 1999, 347, 60–71. [Google Scholar] [CrossRef]
- Chen, K.-C.; Hong, F.C.-N.; Jeng, Y.-R. Deposition of titania-containing diamond-like carbon nanocomposite films by sputtering-assisted chemical vapor deposition. Plasma Process. Polym. 2011, 8, 324–332. [Google Scholar] [CrossRef]
- Hamdy, M.S.; Amrollahi, R.; Mul, G. Surface Ti3+-containing (blue) titania: A unique photocatalyst with high activity and selectivity in visible light-stimulated selective oxidation. ACS Catal. 2012, 2, 2641–2647. [Google Scholar] [CrossRef]
- Xiong, L.-B.; Li, J.-L.; Yang, B.; Yu, Y. Ti3+ in the surface of titanium dioxide: Generation, properties and photocatalytic application. J. Nanomater. 2012, 2012, 831524. [Google Scholar] [CrossRef]
- Mai, W.; Wen, F.; Xie, D.; Leng, Y.; Mu, Z. Structure and composition study of carbon-doped titanium oxide film combined with first principles. J. Adv. Ceram. 2014, 3, 49–55. [Google Scholar] [CrossRef]
- Cheng, Y.; Sun, H.; Jin, W.; Xu, N. Photocatalytic degradation of 4-chlorophenol with combustion synthesized TiO2 under visible light irradiation. Chem. Eng. J. 2007, 128, 127–133. [Google Scholar] [CrossRef]
- Zhang, F.; Jin, S.; Mao, Y.; Zheng, Z.; Chen, Y.; Liu, X. Surface characterization of titanium oxide films synthesized by ion beam enhanced deposition. Thin Solid Films 1997, 310, 29–33. [Google Scholar] [CrossRef]
- Park, Y.; Kim, W.; Park, H.; Tachikawa, T.; Majima, T.; Choi, W. Carbon-doped TiO2 photocatalyst synthesized without using an external carbon precursor and the visible light activity. Appl. Catal. B Environ. 2009, 91, 355–361. [Google Scholar] [CrossRef]
- Xie, D.; Wen, F.; Yang, W.; Li, X.; Leng, Y.; Wan, G.; Sun, H.; Huang, N. Carbon-doped titanium oxide films by dc reactive magnetron sputtering using CO2 and O2 as reactive gas. Acta Metall. Sin. (Engl. Lett.) 2014, 27, 239–244. [Google Scholar] [CrossRef]
- Hsu, S.-W.; Yang, T.-S.; Chen, T.-K.; Wong, M.-S. Ion-assisted electron-beam evaporation of carbon-doped titanium oxide films as visible-light photocatalyst. Thin Solid Films 2007, 515, 3521–3526. [Google Scholar] [CrossRef]
- Wong, M.-S.; Hsu, S.-W.; Rao, K.K.; Kumar, C.P. Influence of crystallinity and carbon content on visible light photocatalysis of carbon doped titania thin films. J. Mol. Catal. A Chem. 2008, 279, 20–26. [Google Scholar] [CrossRef]
- Wang, S.-H.; Chen, T.-K.; Rao, K.K.; Wong, M.-S. Nanocolumnar titania thin films uniquely incorporated with carbon for visible light photocatalysis. Appl. Catal. B Environ. 2007, 76, 328–334. [Google Scholar] [CrossRef]
- Soto, G. Aes, EELS and XPS characterization of Ti (C, N, O) films prepared by PLD using a Ti target in N2, CH4, O2 and CO as reactive gases. Appl. Surf. Sci. 2004, 233, 115–122. [Google Scholar] [CrossRef]
- Taizo, K.; Satoshi, K. TiO2 patterns with wide photo-induced wettability change by a combination of reactive sputtering process and surface modification in a microfluidic channel. J. Micromech. Microeng. 2015, 25, 115014. [Google Scholar] [CrossRef]
- Sun, R.-D.; Nakajima, A.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Photoinduced surface wettability conversion of ZnO and TiO2 thin films. J. Phys. Chem. B 2001, 105, 1984–1990. [Google Scholar] [CrossRef]
- Meng, F.M.; Lu, F.; Sun, Z.Q.; Lu, J.G. A mechanism for enhanced photocatalytic activity of nano-size silver particle modified titanium dioxide thin films. Sci. China Technol. Sci. 2010, 53, 3027–3032. [Google Scholar] [CrossRef]
- Mills, A.; Hill, C.; Robertson, P.K.J. Overview of the current ISO tests for photocatalytic materials. J. Photochem. Photobiol. A Chem. 2012, 237, 7–23. [Google Scholar] [CrossRef]
- Evans, P.; Mantke, S.; Mills, A.; Robinson, A.; Sheel, D.W. A comparative study of three techniques for determining photocatalytic activity. J. Photochem. Photobiol. A Chem. 2007, 188, 387–391. [Google Scholar] [CrossRef]
- Mills, A.; Wells, N.; O’Rourke, C. Correlation between δabs, δrgb (red) and stearic acid destruction rates using commercial self-cleaning glass as the photocatalyst. Catal. Today 2014, 230, 245–249. [Google Scholar] [CrossRef]
- Xie, C.; Yang, S.; Li, B.; Wang, H.; Shi, J.-W.; Li, G.; Niu, C. C-doped mesoporous anatase TiO2 comprising 10 nm crystallites. J. Colloid Interface Sci. 2016, 476, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-T.; Weng, C.-H.; Lin, Y.-H.; Shiesh, C.-C.; Chen, F.-Y. Effect of c content and calcination temperature on the photocatalytic activity of C-doped TiO2 catalyst. Sep. Purif. Technol. 2013, 116, 114–123. [Google Scholar] [CrossRef]
- Wen, F.; Zhang, C.; Xie, D.; Sun, H.; Leng, Y.X. Research of composition and photocatalytic property of carbon-doped Ti-O films prepared by r-ms using CO2 gas resource. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2013, 307, 381–384. [Google Scholar] [CrossRef]
- Dunnill, C.W.H.; Aiken, Z.A.; Pratten, J.; Wilson, M.; Morgan, D.J.; Parkin, I.P. Enhanced photocatalytic activity under visible light in N-doped TiO2 thin films produced by apcvd preparations using t-butylamine as a nitrogen source and their potential for antibacterial films. J. Photochem. Photobiol. A Chem. 2009, 207, 244–253. [Google Scholar] [CrossRef]

| Sample ID | Optical Emission Monitoring (OEM) Setpoint, % FMS | CO2 Flow, sccm | Thickness, nm a | Ti, at. % b | O, at. % b | C, at. % b |
|---|---|---|---|---|---|---|
| T25 | - | - | 490 | 34.9 | 65.1 | - |
| T25C2.5 | 25 | 2.5 | 820 | 32.9 | 62.5 | 4.5 |
| T25C5 | 25 | 5 | 860 | 32.9 | 61.6 | 5.0 |
| T25C7 | 25 | 7 | 870 | 35.2 | 59.4 | 5.5 |
| T30 | 30 | - | 510 | 40.9 | 59.1 | - |
| T30C2.5 | 30 | 2.5 | 820 | 33.9 | 62.0 | 4.1 |
| T30C5 | 30 | 5 | 870 | 33.5 | 60.8 | 5.3 |
| T30C7 | 30 | 7 | 910 | 35.8 | 58.6 | 5.6 |
| T35 | 35 | - | 600 | 40.2 | 59.8 | - |
| T35C2.5 | 35 | 2.5 | 840 | 33.6 | 60.7 | 4.7 |
| T35C5 | 35 | 5 | 1300 | 34.1 | 59.8 | 6.1 |
| T35C7 | 35 | 7 | 1500 | 36.7 | 56.9 | 6.4 |
| Sample ID | Surface Roughness, nm a | Surface Area, µm2 a | Ti3+/Ti4+ b | Ti–C, at. % b | C–O, at. % b | Ti–C–O, at. % b | Band Gap, eV c |
|---|---|---|---|---|---|---|---|
| T25 | 5.7 | 902 | - | - | - | - | 3.22 |
| T25C2.5 | 6.0 | 901 | - | 0.06 | 0.28 | 0.28 | 3.13 |
| T25C5 | 4.2 | 901 | 0.02 | 0.09 | 0.72 | 0.39 | 3.15 |
| T25C7 | 13.0 | 902 | 0.03 | 0.18 | 0.34 | 0.48 | 3.08 |
| T30 | 6.0 | 901 | - | - | - | - | 3.20 |
| T30C2.5 | 5.8 | 901 | - | 0.08 | 0.39 | 0.16 | 3.04 |
| T30C5 | 5.8 | 901 | 0.02 | 0.13 | 0.55 | 0.42 | 3.05 |
| T30C7 | 10.0 | 902 | 0.04 | 0.27 | 0.49 | 0.34 | 2.63 |
| T35 | 6.2 | 911 | - | - | - | - | 3.18 |
| T35C2.5 | 12.8 | 902 | - | 0.11 | 0.37 | 0.51 | 3.00 |
| T35C5 | 11.0 | 902 | 0.02 | 0.18 | 0.74 | 0.32 | 2.02 |
| T35C7 | 12.4 | 902 | 0.06 | 0.31 | 0.49 | 0.46 | 2.17 |
| Sample ID | Initial CA, deg. | CA after 1 h UV, deg. | CA after 1 h vis, deg. | Kinetic Constant, k | |||
|---|---|---|---|---|---|---|---|
| MB UV, k × 105, s−1 a | MB vis, k × 105, s−1 a | SA UV, k × 102, A cm−1 h−1 b | SA vis, k × 102, A cm−1 h−1 b | ||||
| T25 | 92 | 18 | 55 | 1.8 | 0.5 | 4.0 | 0.4 |
| T25C2.5 | 60 | 10 | 15 | 1.8 | 1.5 | 0.8 | 0.6 |
| T25C5 | 11 | ~0 | ~0 | 1.3 | 1.0 | 1.1 | 0.3 |
| T25C7 | 11 | ~0 | ~0 | 1.4 | 0.8 | 1.0 | 0.2 |
| T30 | 53 | 13 | 20 | 1.4 | 0.9 | 1.6 | 0.7 |
| T30C2.5 | 58 | 13 | 16 | 0.8 | 0.9 | 1.2 | 0.1 |
| T30C5 | 11 | ~0 | ~0 | 0.7 | 1.5 | 1.3 | 0.2 |
| T30C7 | 10 | ~0 | ~0 | 0.8 | 1.4 | 0.7 | 0.2 |
| T35 | 54 | 10 | 22 | 1.4 | 0.9 | 2.3 | 0.9 |
| T35C2.5 | 63 | 12 | 14 | 0.9 | 1.0 | 0.4 | 0.1 |
| T35C5 | 10 | ~0 | ~0 | 1.0 | 2.1 | 0.9 | 0.3 |
| T35C7 | 10 | ~0 | ~0 | 1.4 | 2.0 | 1.3 | 0.6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klaysri, R.; Ratova, M.; Praserthdam, P.; Kelly, P.J. Deposition of Visible Light-Active C-Doped Titania Films via Magnetron Sputtering Using CO2 as a Source of Carbon. Nanomaterials 2017, 7, 113. https://doi.org/10.3390/nano7050113
Klaysri R, Ratova M, Praserthdam P, Kelly PJ. Deposition of Visible Light-Active C-Doped Titania Films via Magnetron Sputtering Using CO2 as a Source of Carbon. Nanomaterials. 2017; 7(5):113. https://doi.org/10.3390/nano7050113
Chicago/Turabian StyleKlaysri, Rachan, Marina Ratova, Piyasan Praserthdam, and Peter J. Kelly. 2017. "Deposition of Visible Light-Active C-Doped Titania Films via Magnetron Sputtering Using CO2 as a Source of Carbon" Nanomaterials 7, no. 5: 113. https://doi.org/10.3390/nano7050113







