Comparison of Erosion Behavior and Particle Contamination in Mass-Production CF4/O2 Plasma Chambers Using Y2O3 and YF3 Protective Coatings
Abstract
:1. Introduction
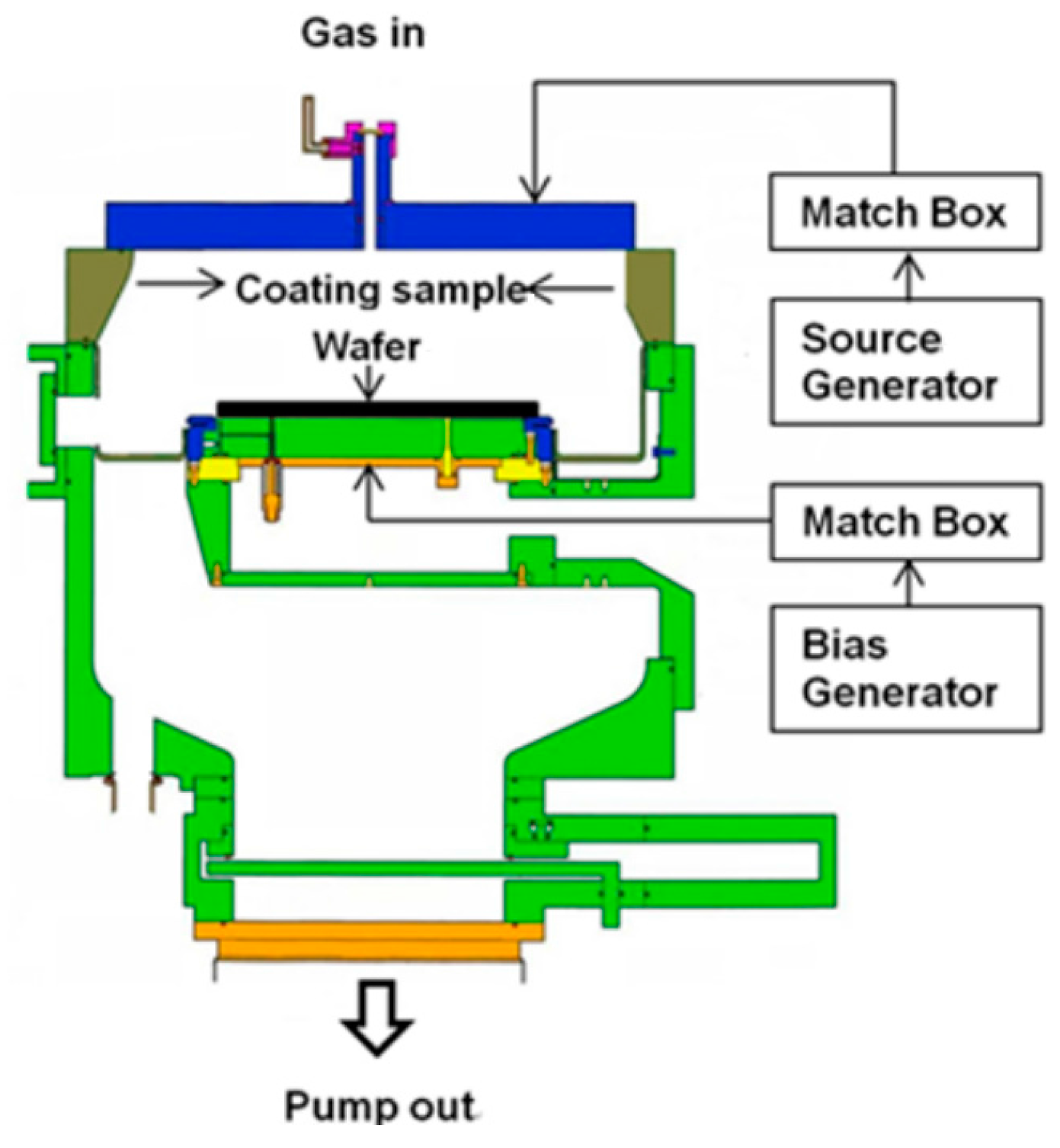
2. Materials and Methods
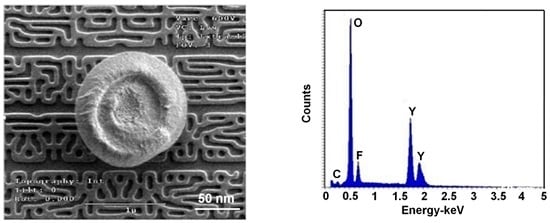
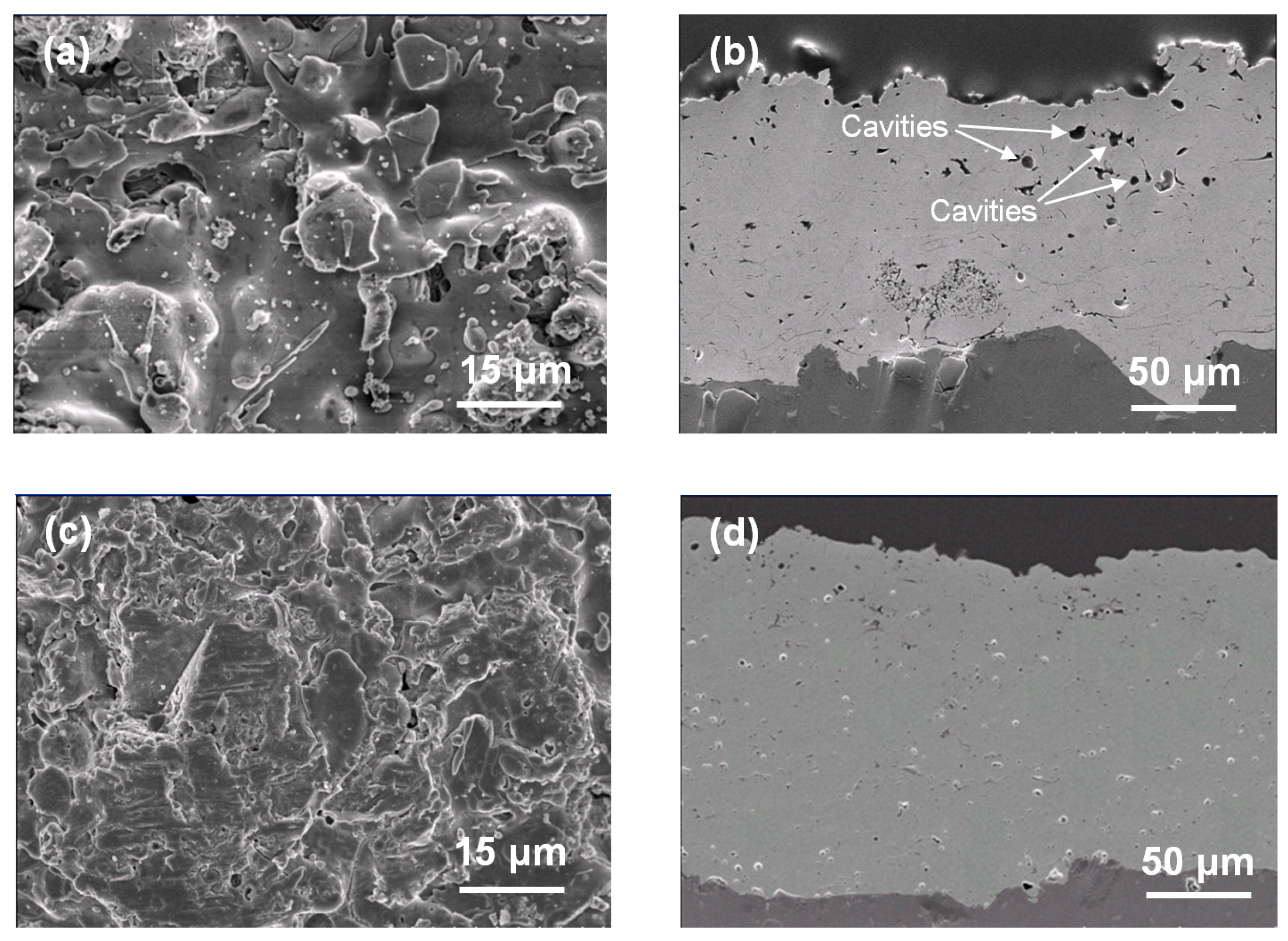
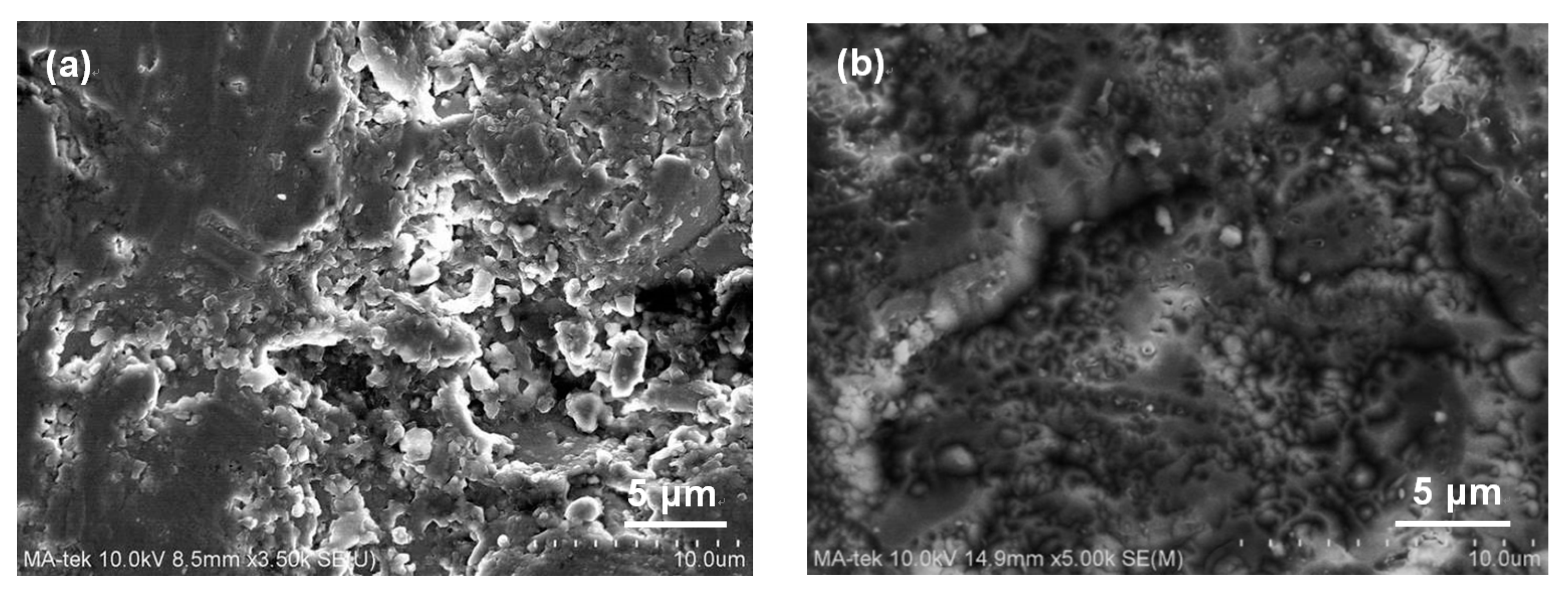
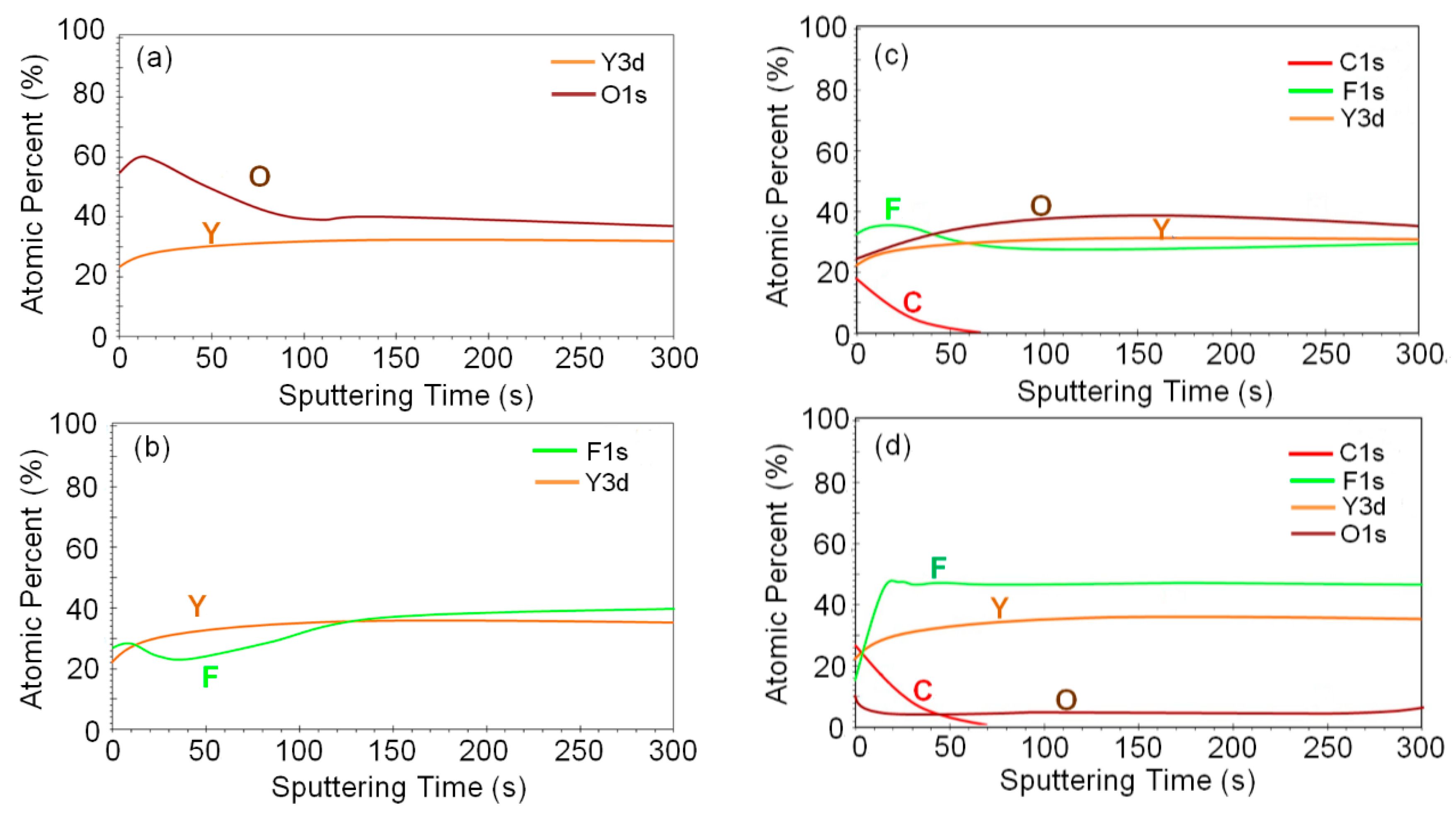
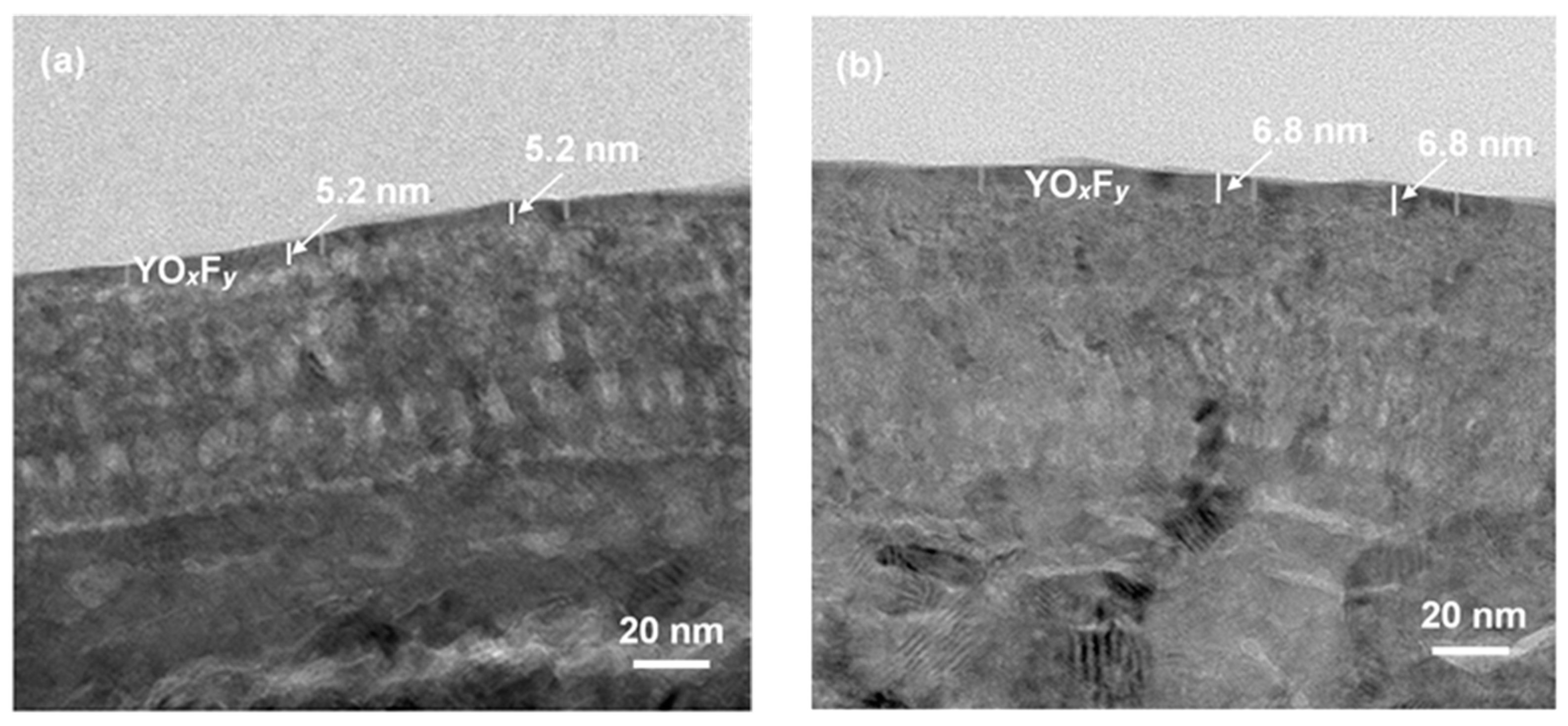
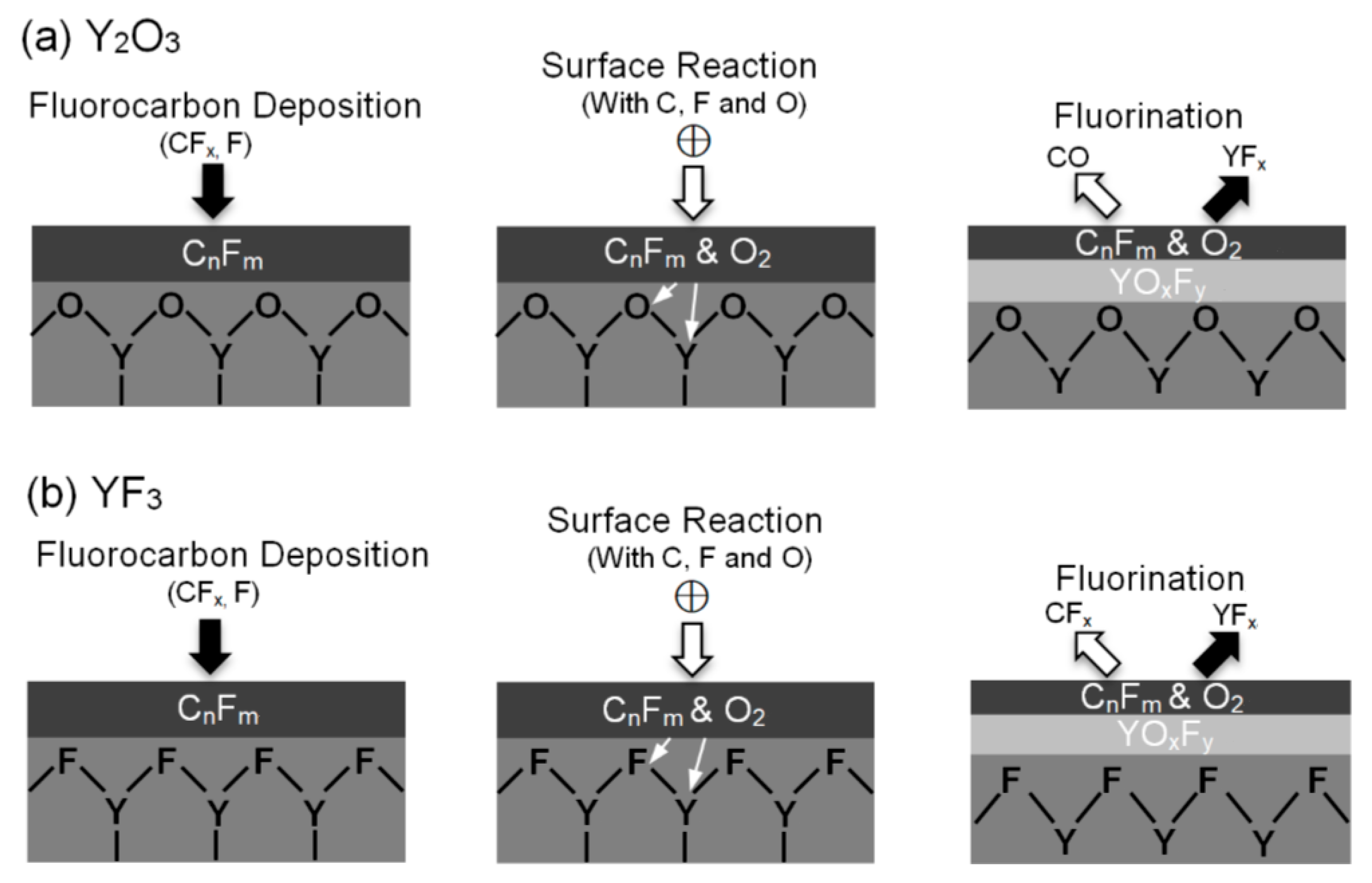
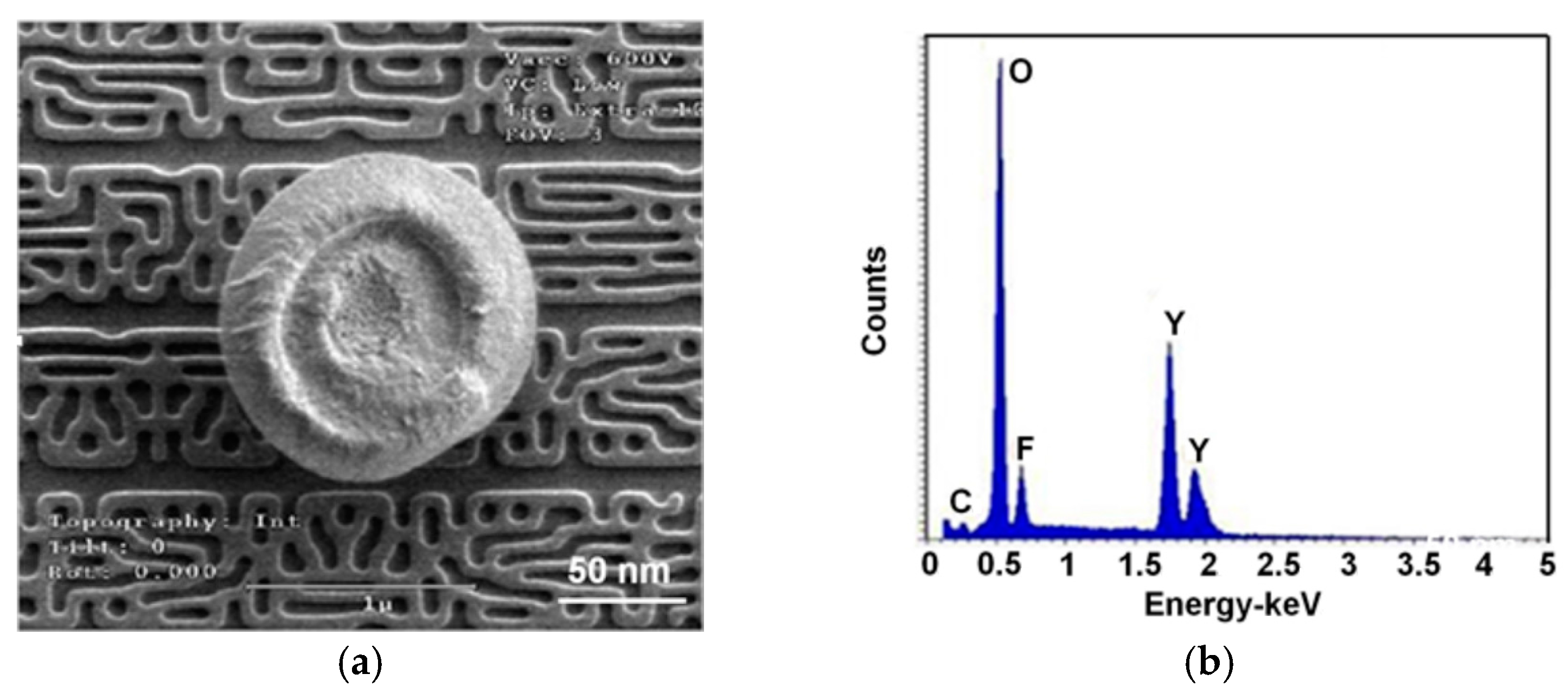
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zavareh, M.A.; Sarhen, A.A.D.M.; Razak, B.B.; Basirun, W.J. Plasma thermal spray of ceramic oxide coating on carbon steel with enhanced wear and corrosion resistance for oil and gas applications. Ceram. Int. 2014, 40, 14267–14277. [Google Scholar] [CrossRef]
- Kim, D.M.; Kim, K.B.; Yoon, S.Y.; Oh, S.Y.; Kim, H.T.; Lee, S.M. Effects of artificial pores and purity on the erosion behaviors of polycrystalline Al2O3 ceramics under fluorine plasma. J. Ceram. Soc. 2009, 117, 863–867. [Google Scholar] [CrossRef]
- Cardinaud, C.; Peignon, M.C.; Tessier, P.Y. Plasma etching: principles, mechanisms, application to micro- and nano-technologies. Appl. Surf. Sci. 2000, 164, 72–83. [Google Scholar] [CrossRef]
- Cunge, G.; Inglebert, L.; Joubert, O.; Vallier, L.; Sadeghi, N. Ion flux composition in HBr Cl2/O2 and HBr/Cl2/O2/CF4 chemistries during silicon etching in industrial high-density plasmas. J. Vac. Sci. Technol. 2002, 20, 2137–2148. [Google Scholar] [CrossRef]
- Fukumoto, H.; Fujikake, I.; Takao, Y.; Eriguchi, K.; Ono, K. Plasma chemical behavior of reactants and reaction products during inductively coupled CF4 plasma etching of SiO2. Plasma Sources Sci. Technol. 2009, 18, 045027. [Google Scholar] [CrossRef]
- Ito, N.; Moriya, T.; Uesugi, F.; Matsumoto, M.; Liu, S.; Kitayama, Y. Reduction of particle contamination in plasma-etching equipment by dehydration of chamber wall. Jpn. J. Appl. Phys. 2008, 47, 3630–3634. [Google Scholar] [CrossRef]
- Sato, N.; Uchida, G.; Kaneko, T.; Shimizu, S.; Iizuka, S. Dynamics of fine particles in magnetized plasmas. Phys. Plasmas 2001, 8, 1786–1790. [Google Scholar] [CrossRef]
- Kim, D.M.; Lee, S.H.; Alexander, W.B.; Kim, K.B.; Oh, Y.S.; Lee, S.M. X-Ray photoelectron spectroscopy study on the interaction of yttrium–aluminum oxide with fluorine-based plasma. J. Am. Ceram. Soc. 2011, 94, 3455–3459. [Google Scholar] [CrossRef]
- Qin, X.P.; Zhou, G.H.; Yang, H.; It Wong, J.; Zhang, J.; Luo, D.W.; Wang, S.W.; Ma, J.; Tang, D.Y. Fabrication and plasma resistance properties of transparent YAG ceramics. Ceram. Int. 2012, 38, 2529–2535. [Google Scholar] [CrossRef]
- Iwasawa, J.; Nishimizu, R.; Tokita, M.; Kiyohara, M. Plasma-resistant dense yttrium oxide film prepared by aerosol deposition process. J. Am. Ceram. Soc. 2007, 90, 2327–2332. [Google Scholar] [CrossRef]
- Mun, S.Y.; Shin, K.C.; Lee, S.S.; Kwak, J.S.; Jeong, J.Y.; Jeong, Y.H. Etch defect reduction using SF6/O2 plasma cleaning and optimizing etching recipe in photo resist masked gate poly silicon etch process. Jpn. J. Appl. Phys. 2005, 44, 4891–4894. [Google Scholar] [CrossRef]
- Ramos, R.; Gunge, G.; Joubert, O.; Sadeghi, N.; Mori, M.; Vallier, L. Plasma/react walls interactions in advanced gate etching process. Thin Solid Films. 2007, 515, 4846–4852. [Google Scholar] [CrossRef]
- Rueger, N.R.; Beulens, J.J.; Schaepkens, M.; Doemling, M.F.; Mirza, J.M.; Standaert, T.E.F.M.; Oehrlein, G.S. Role of steady state fluorocarbon films in the etching of silicon dioxide using CHF3 in an inductively coupled plasma reactor. J. Vac. Sci. Technol. 1997, 15, 1881–1889. [Google Scholar] [CrossRef]
- Standaert, T.E.F.M.; Hedlund, C.; Joseph, E.A.; Oehrlein, G.S.; Dalton, T.J. Role of fluorocarbon film formation in the etching of silicon, silicon dioxide, silicon nitride, and amorphous hydrogenated silicon carbide. J. Vac. Sci. Technol. 2004, 22, 53–60. [Google Scholar] [CrossRef]
- Schaepkens, M.; Standaert, T.E.F.M.; Rueger, N.R.; Sebel, P.G.M.; Cook, J.M. Study of the SiO2-to-Si3N4 etch selectivity mechanism in inductively coupled fluorocarbon plasmas and a comparison with the SiO2-to-Si mechanism. J. Vac. Sci. Technol. 1999, 17, 26–37. [Google Scholar] [CrossRef]
- Lin, T.K.; Wuu, D.S.; Huang, S.Y.; Wang, W.K. Characteristics of yttrium fluoride and yttrium oxide coatings for plasma process equipment prepared by atmospheric plasma spraying. Jpn. J. Appl. 2016, 55, 162601. [Google Scholar] [CrossRef]
- Cao, Y.C.; Zhao, L.; Luo, J.; Wang, K.; Zhang, B.P.; Yotoka, H.; Ito, Y.; Li, J.F. Plasma etching behavior of Y2O3 ceramics: Comparative study with Al2O3. App. Surf. Sci. 2016, 336, 304–309. [Google Scholar] [CrossRef]
- Kim, D.M.; Oh, Y.S.; Kim, S.; Kim, H.T.; Lim, D.S.; Lee, S.M. The erosion behaviors of Y2O3 and YF3 coatings under fluorocarbon plasma. Thin Solid Films 2011, 519, 6698–6702. [Google Scholar] [CrossRef]
- Standaert, T.E.F.M.; Schaepkens, M.; Rueger, N.R.; Sebel, P.G.M.; Oehrlein, G.S.; Cook, J.M. High density fluorocarbon etching of silicon in an inductively coupled plasma: Mechanism of etching through a thick steady state fluorocarbon layer. J. Vac. Sci. Technol. 1998, 16, 239–249. [Google Scholar] [CrossRef]
- Tatsumi, T.; Matsui, M.; Okigawa, M.; Sekine, M. Control of surface reactions in high-performance SiO2 etching. J. Vac. Sci. Technol. 2000, 18, 1897–1902. [Google Scholar] [CrossRef]
- Matsui, M.; Tatsumi, T.; Sekine, M. Observation of surface reaction layers formed in highly selective SiO2 etching. J. Vac. Sci. Technol. 2001, 19, 1282–1888. [Google Scholar] [CrossRef]
- Korzenski, M.B.; Lecoeur, P.; Mercey, B.; Chippaux, D.; Raveau, B. PLD-grown Y2O3 thin films from Y metal: An advantageous alternative to films deposited from yttria. Chem. Mater. 2000, 12, 3139–3150. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Physical Electronics: Eden Prairie, MN, USA, 1995; p. 10. [Google Scholar]
- Pei, L.; Jiapi, Z.; Yuankun, Z.; Jiecai, H. Preparation and optical properties of sputtered-deposition yttrium fluoride film. Nucl. Instrum. Methods Phys. Res. 2013, 307, 429–433. [Google Scholar] [CrossRef]
- Vickerman, J.C.; Gilmore, I.S. Surface Analysis, 2nd ed.; Wiley: New York, NY, USA, 2009; p. 53. [Google Scholar]
- Cunge, G.; Pelissier, B.; Joubert, O.; Ramos, R.; Maurice, C. New chamber walls conditioning and cleaning strategies to improve the stability of plasma processes. Plasma Sources Sci. Technol. 2005, 14, 599–609. [Google Scholar] [CrossRef]
- Zhong, H.X.; Hong, J.M.; Cao, X.F.; Chen, X.T.; Xue, Z.L. Ionic-liquid-assisted synthesis of YF3 with different crystalline phases and morphologies. Mater. Res. Bull. 2009, 44, 623–628. [Google Scholar] [CrossRef]
- Barve, S.A.; Jagannath; Mithal, N.; Deo, M.N.; Chand, N.; Bhanage, B.M.; Gantayet, L.M.; Patil, D.S. Microwave ECR plasma CVD of cubic Y2O3 coatings and their characterization. Surf. Coat. Technol. 2010, 204, 3167–3172. [Google Scholar] [CrossRef]
- Kim, D.M.; Jang, M.R.; Oh, Y.S.; Kim, S.; Lee, S.M.; Lee, S.H. Relative sputtering rates of oxides and fluorides of aluminum and yttrium. Surf. Coat. Technol. 2017, 309, 694–697. [Google Scholar] [CrossRef]
- Levin, I.; Huang, Q.Z.; Lawrence, P.C.; Winnie, W.N. Non-quenchable chemical order-disorder phase transition in yttrium oxyfluoride YOF. Eur. J. Inorg. Chem. 2005, 1, 87–91. [Google Scholar] [CrossRef]
- Miwa, K.; Takada, N.; Sasaki, K. Fluorination mechanisms of Al2O3 and Y2O3 surfaces irradiated by high-density CF4/O2 and SF6/O2 plasmas. J. Vac. Sci. Technol. 2009, 27, 831–835. [Google Scholar] [CrossRef]
- Tsunoura, T.; Yoshida, K.; Yano, T.; Kishi, Y. Fabrication, characterization, and fluorine-plasma exposure behavior of dense yttrium oxyfluoride ceramics. Jpn. J. Appl. 2017, 56, 06HC02. [Google Scholar]

| Condition | Y2O3 | YF3 |
|---|---|---|
| Primary gas flow rate (L/min) | 45 | 45 |
| Secondary current (L/min) | 6 | 6 |
| Gun moving rate (cm/s) | 10 | 10 |
| System voltage (V) | 50 | 50 |
| Gun power (kW) | 15 | 15 |
| Stand-off (cm) | 10 | 10 |
| Condition | Y2O3 | YF3 |
|---|---|---|
| RF source power (W) | 1300 | 1300 |
| RF bias power (W) | 500 | 500 |
| Chamber pressure (Pa) | 1.06 | 1.06 |
| CF4:O2 (sccm) | 30:5 | 30:5 |
| Etching time (min) | 60 | 60 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, T.-K.; Wang, W.-K.; Huang, S.-Y.; Tasi, C.-T.; Wuu, D.-S. Comparison of Erosion Behavior and Particle Contamination in Mass-Production CF4/O2 Plasma Chambers Using Y2O3 and YF3 Protective Coatings. Nanomaterials 2017, 7, 183. https://doi.org/10.3390/nano7070183
Lin T-K, Wang W-K, Huang S-Y, Tasi C-T, Wuu D-S. Comparison of Erosion Behavior and Particle Contamination in Mass-Production CF4/O2 Plasma Chambers Using Y2O3 and YF3 Protective Coatings. Nanomaterials. 2017; 7(7):183. https://doi.org/10.3390/nano7070183
Chicago/Turabian StyleLin, Tzu-Ken, Wei-Kai Wang, Shih-Yung Huang, Chi-Tsung Tasi, and Dong-Sing Wuu. 2017. "Comparison of Erosion Behavior and Particle Contamination in Mass-Production CF4/O2 Plasma Chambers Using Y2O3 and YF3 Protective Coatings" Nanomaterials 7, no. 7: 183. https://doi.org/10.3390/nano7070183