The Bioactivity and Photocatalytic Properties of Titania Nanotube Coatings Produced with the Use of the Low-Potential Anodization of Ti6Al4V Alloy Surface
Abstract
:1. Introduction
2. Results
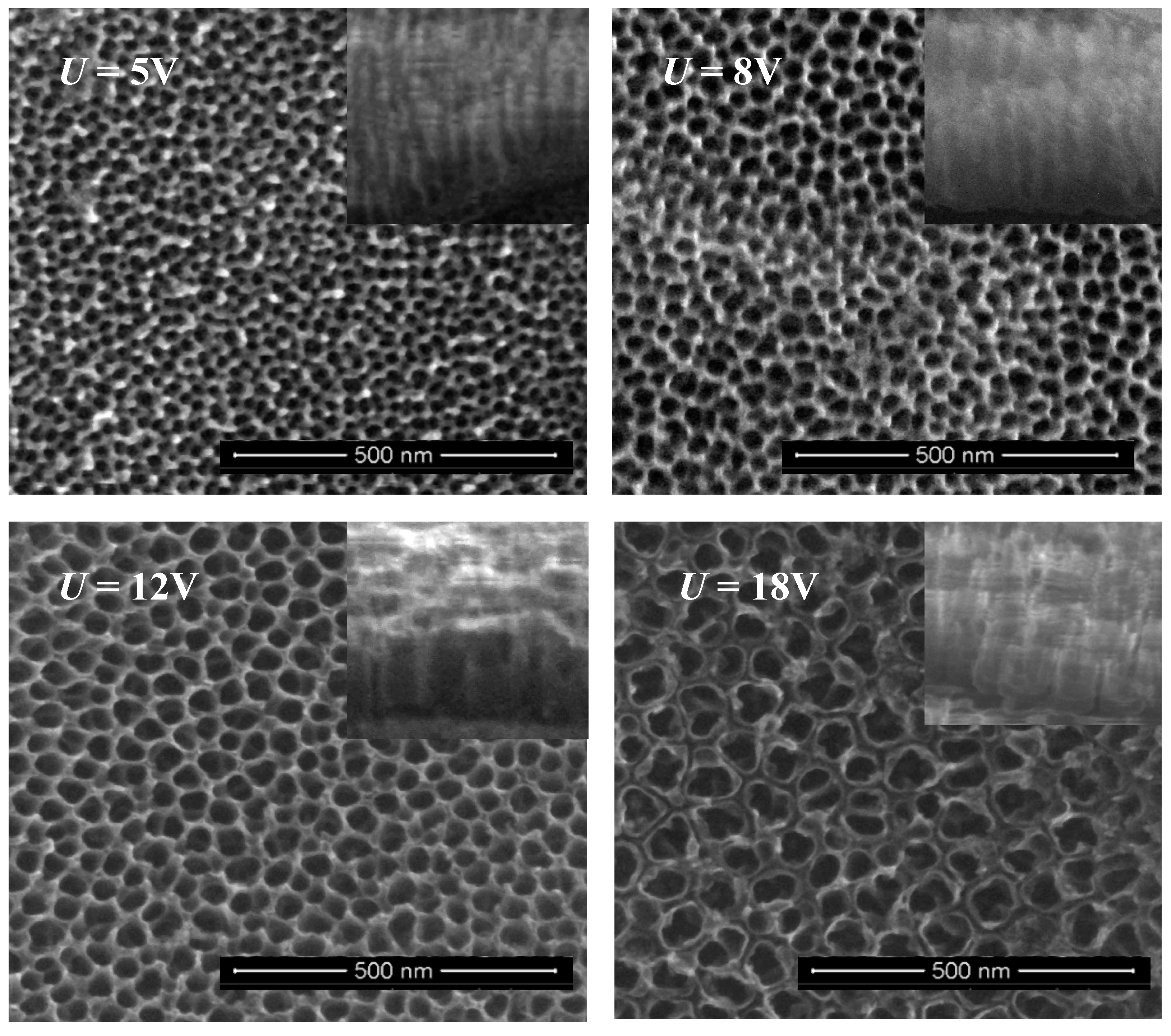
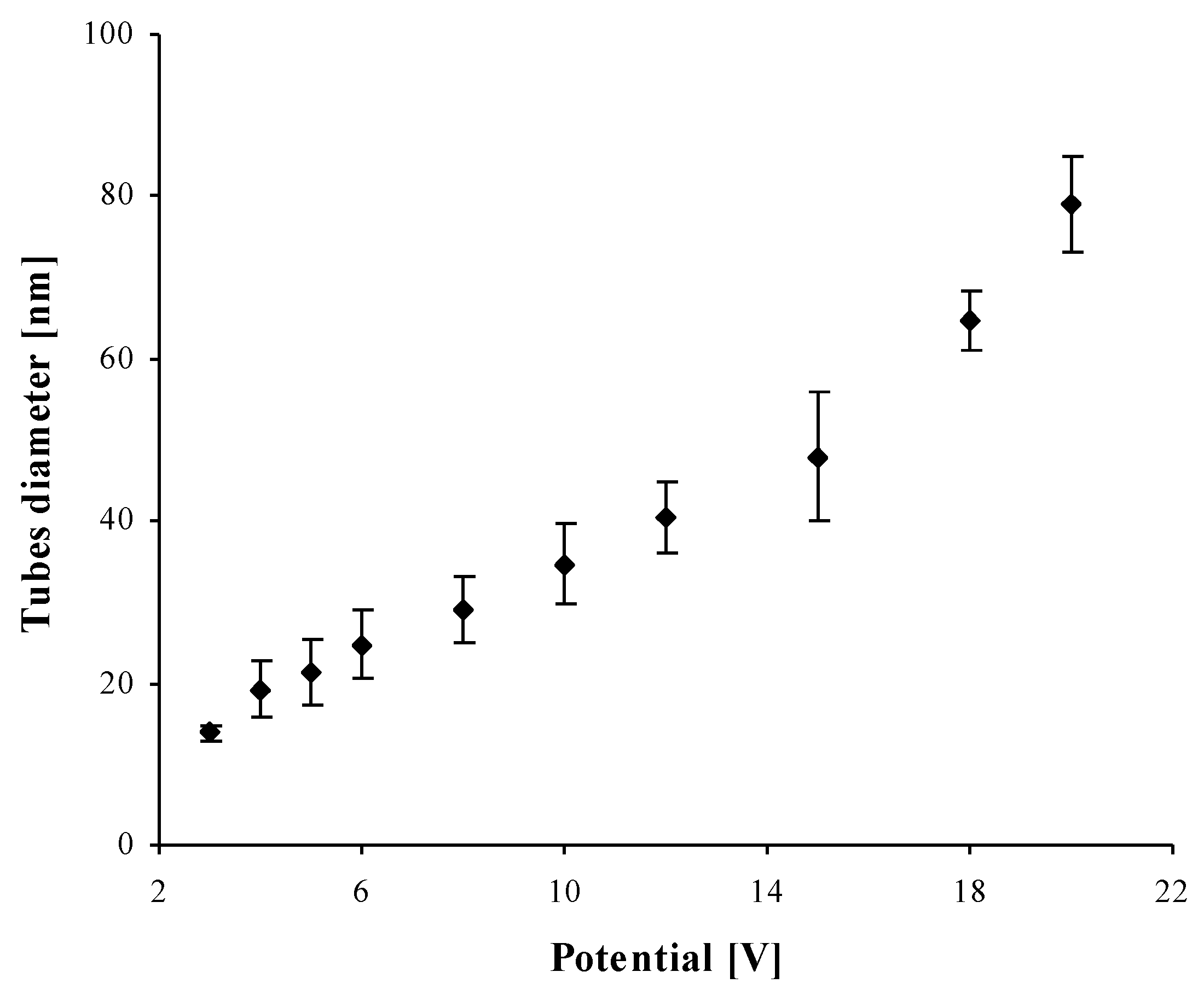
2.1. Structural Characterization of TNT Coatings
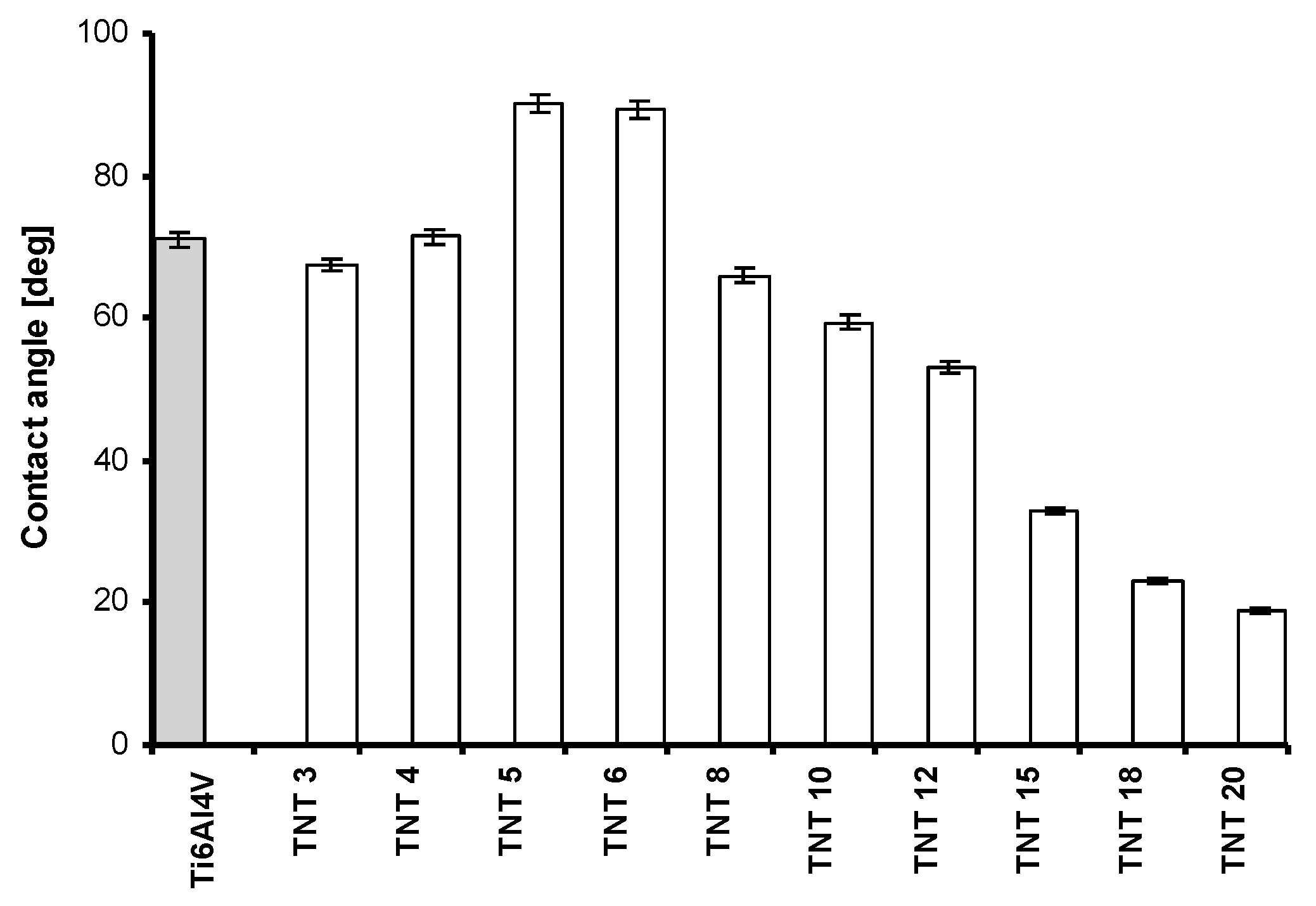
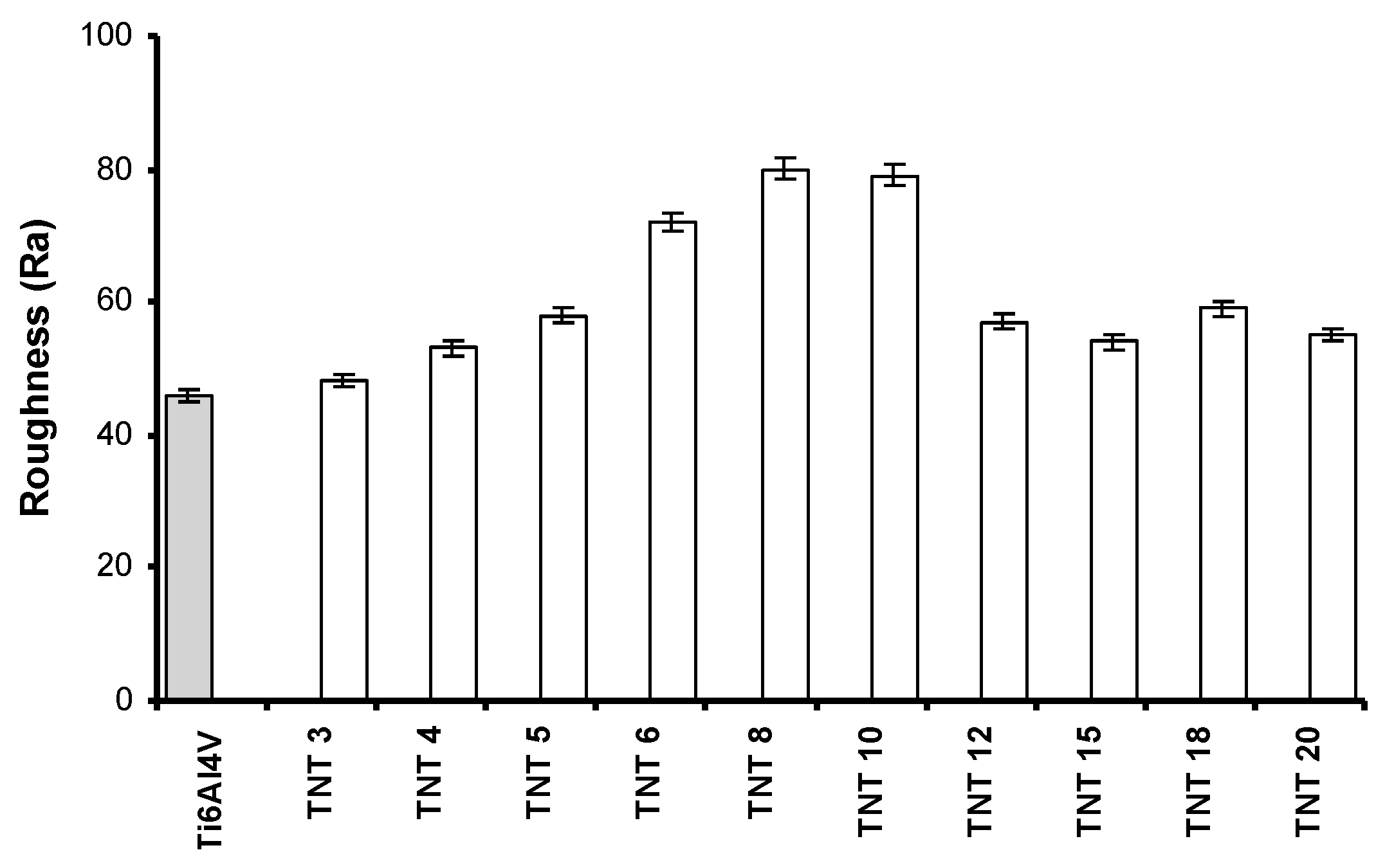
2.2. The Wettability and the Roughness of TNT Coatings
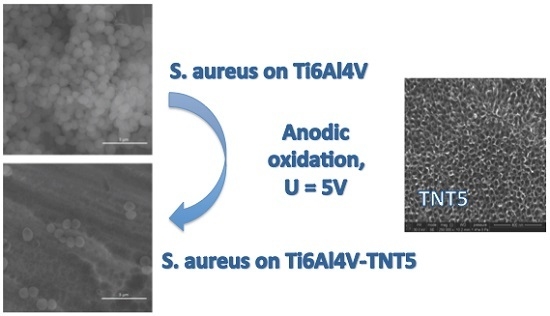
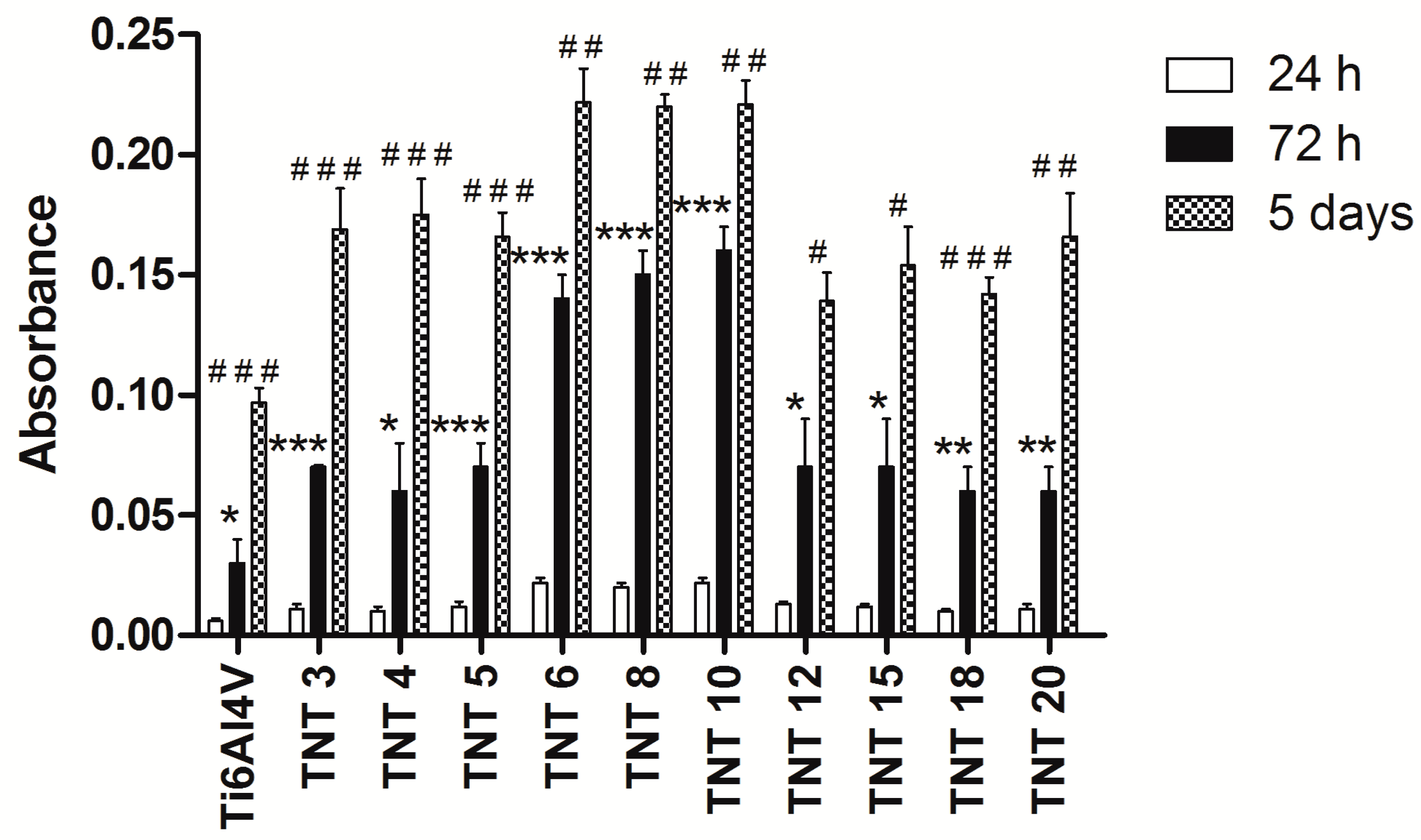
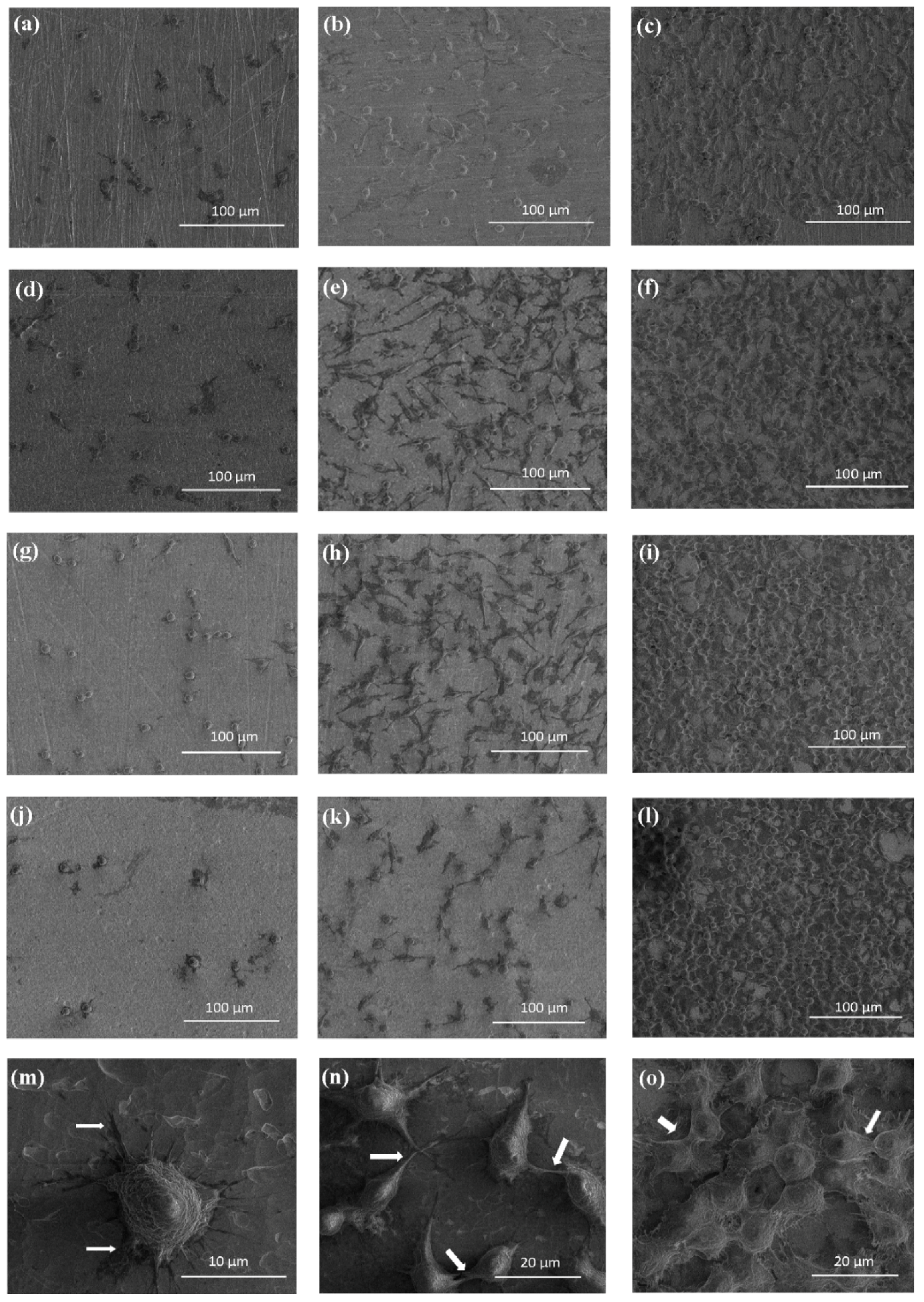
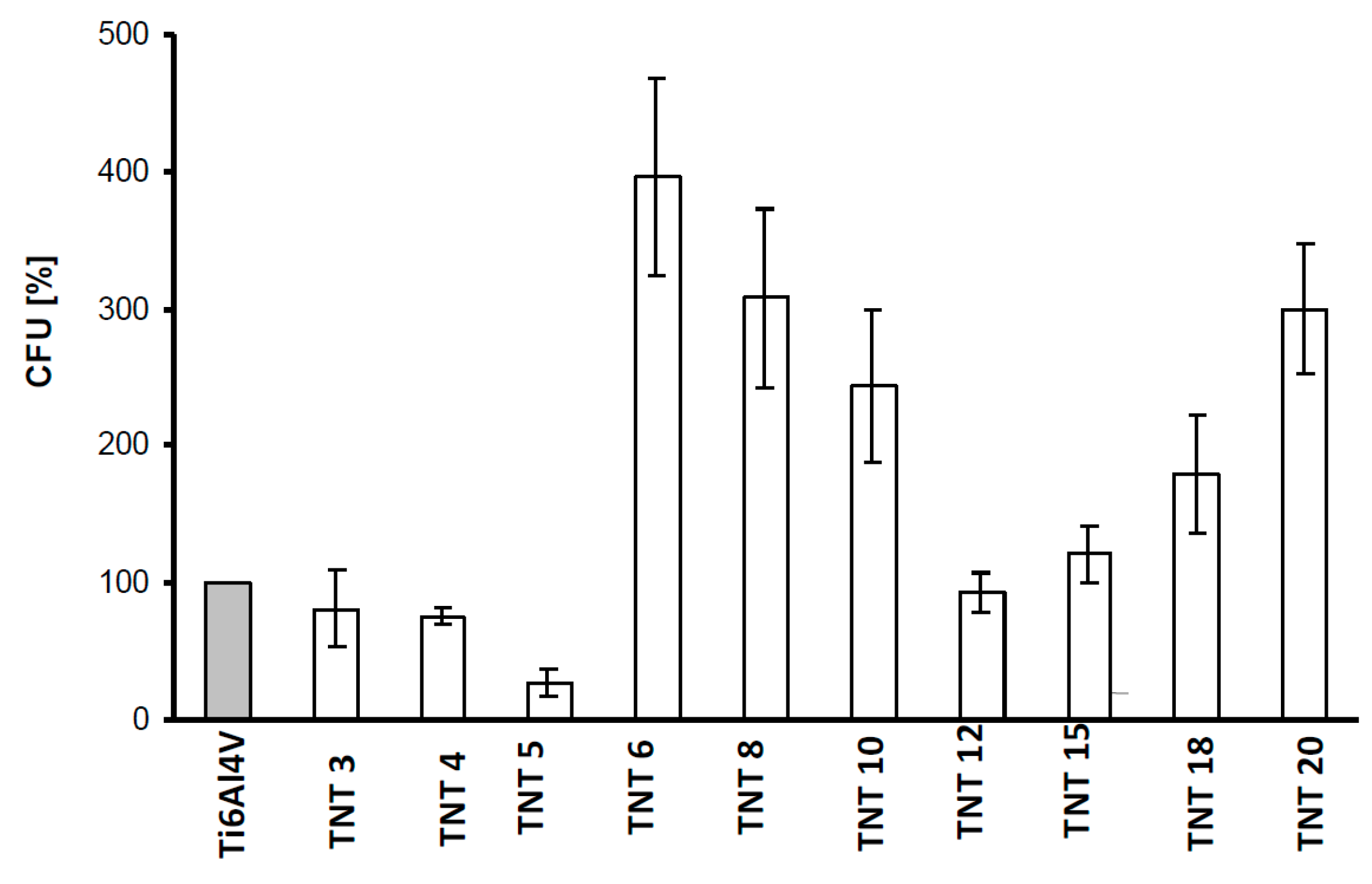
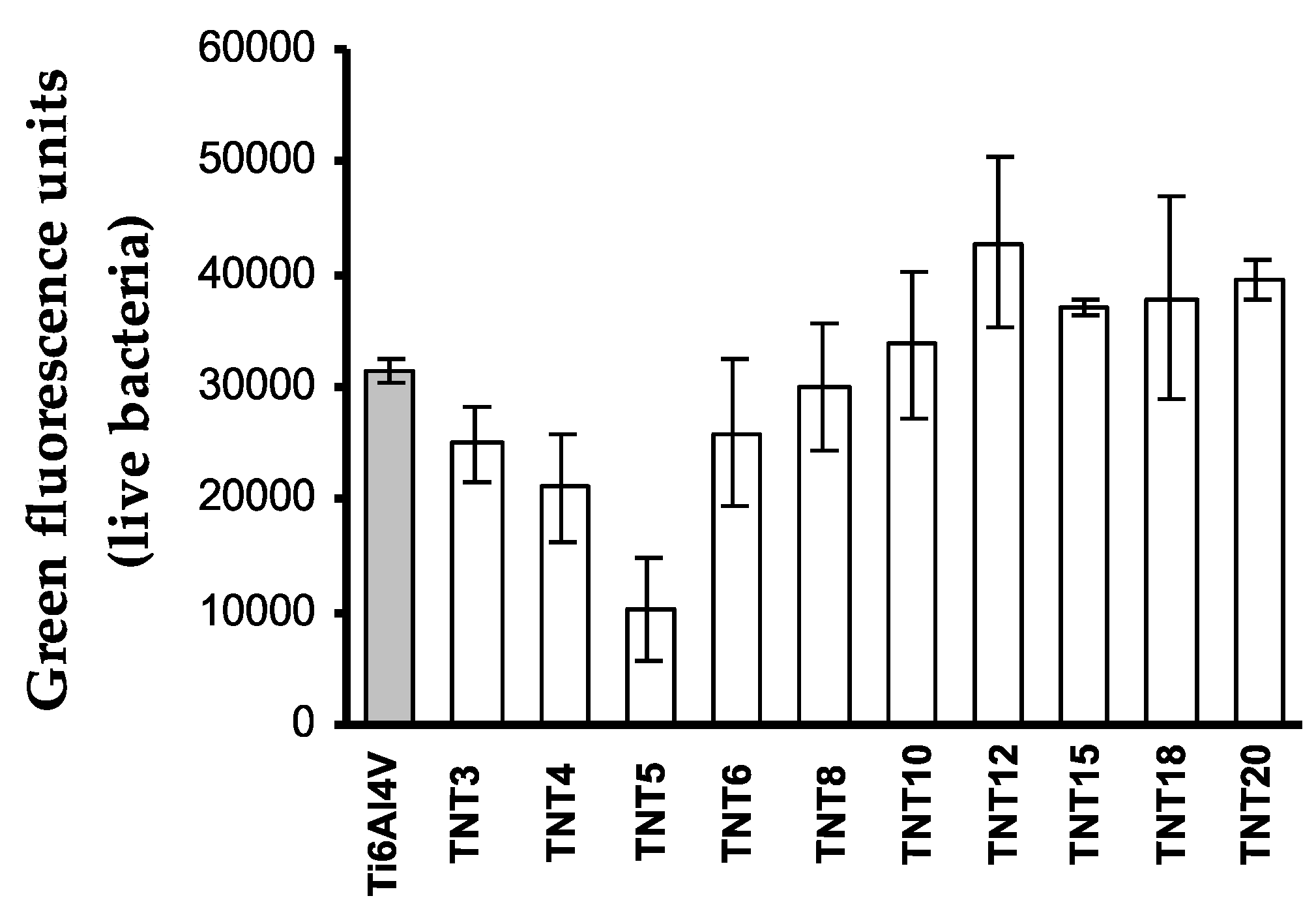
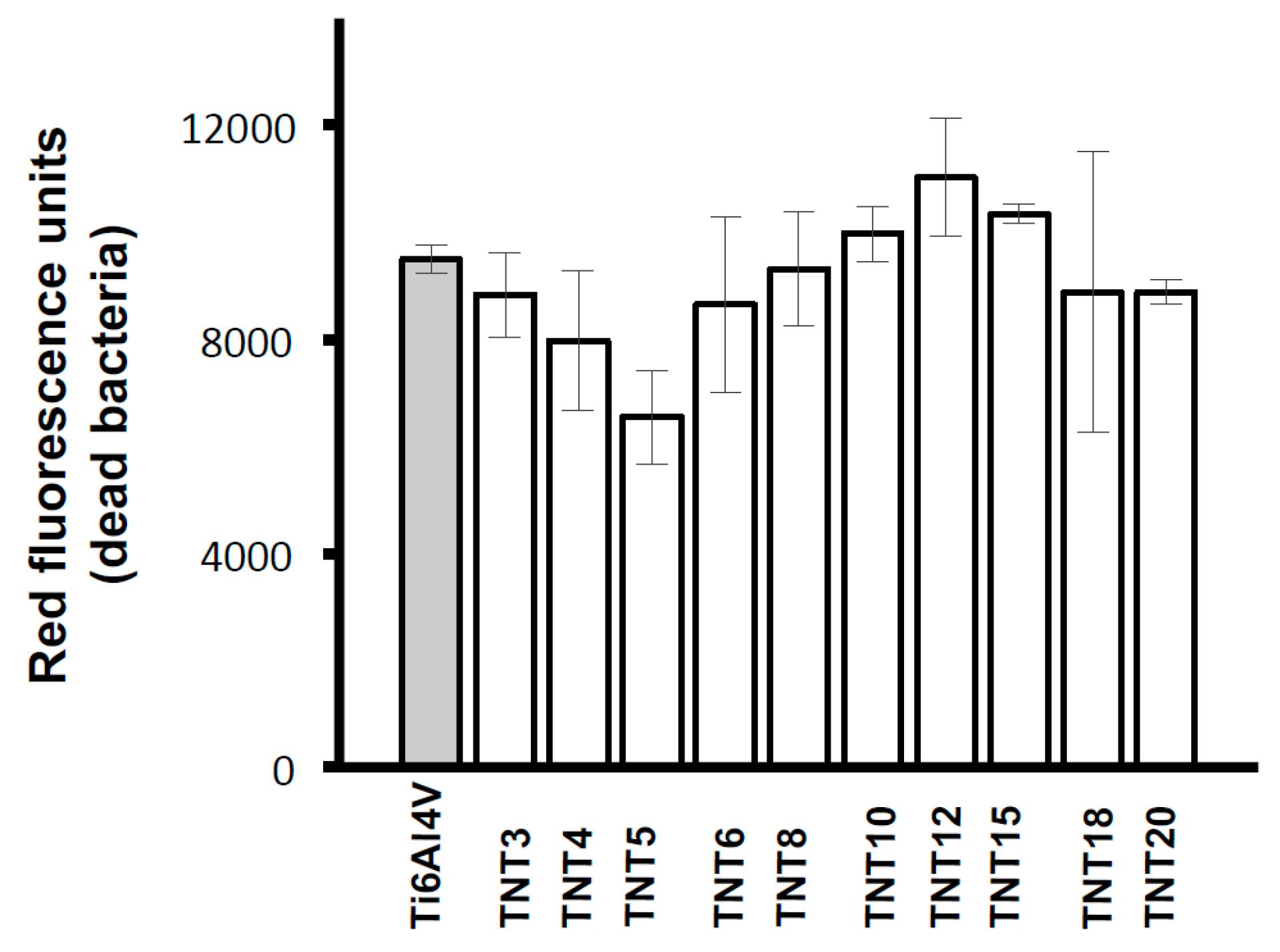
2.3. Biological Activity of TNT Coatings Produced on the Surface of Ti6Al4V Foil
2.4. Photocatalytic Properties of TNT Coatings Produced on the Surface of Ti6Al4V Foil
3. Discussion
4. Materials and Methods
4.1. Synthesis of TiO2 Nanotubes Coatings (TNT)
4.2. Morphological and Structural Characterization of TNT Coatings
4.3. Wettability Measurements
4.4. Cell Adhesion and Proliferation Assay on TNT Coatings
4.5. Microbial Aggregates/Biofilm Formation on TNT Coatings
4.6. Photocatalytic Degradation of Methylene Blue (MB)
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Williams, D.F. The Williams Dictionary of Biomaterials; Liverpool University Press: Liverpool, UK, 2008; Volume 42. [Google Scholar]
- Sáenz, A.; Rivera-Muñoz, E.; Brostow, W.; Castaño, V.M. Ceramic biomaterials: An introductory overview. J. Mater. Educ. 1999, 21, 297–306. [Google Scholar]
- Kokubo, T.; Kim, H.M.; Kawashita, M. Novel bioactive materials with different mechanical properties. Biomaterials 2003, 13, 2161–2175. [Google Scholar] [CrossRef]
- Kokubo, T.; Kim, H.M.; Kawashita, M.; Nakamura, T. Bioactive metals: Preparation and properties. J. Mater. Sci. Mater. Med. 2004, 15, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Park, J.P.; Bronzino, J.D. Biomaterials: Principles and Applications. In Metallic Biomaterials; Kim, Y.K., Park, J.B., Eds.; CRC Press LLC: Boca Raton, FL, USA, 2003; pp. 1–20. [Google Scholar]
- Teo, A.J.T.; Mishra, A.; Park, I.; Kim, Y.-J.; Park, W.-T.; Yoon, Y.-J. Polymeric biomaterials for medical implants and devices. ACS Biomater. Sci. Eng. 2016, 2, 454–472. [Google Scholar] [CrossRef]
- Ananth, H.; Kundapur, V.; Mohammed, H.S.; Anand, M.; Amarnath, G.S.; Mankar, S. Review on biomaterials in dental implantology. Int. J. Biomed. Sci. 2015, 11, 113–120. [Google Scholar] [PubMed]
- Hench, L.L.; Polak, J.M. Third-Generation Biomedical Materials. Science 2002, 295, 1014–1017. [Google Scholar] [CrossRef] [PubMed]
- Manivasagam, G.; Dhinasekaran, D.; Rajamanickam, A. Biomedical Implants: Corrosion and its Prevention—A Review. Recent Pat. Corros. Sci. 2010, 2, 40–54. [Google Scholar] [CrossRef]
- Özcan, M.; Hämmerle, C.H. Titanium as a Reconstruction and Implant Material in Dentistry: Advantages and Pitfalls. Materials 2012, 5, 1528–1545. [Google Scholar] [Green Version]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Ratner, B. Titanium in Medicine: Material Science, Surface Science, Engineering, Biological Responses and Medical Applications; Brunette, D.M., Tengvall, P., Textor, M., Thompson, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 1–12. [Google Scholar]
- Cui, C.; Liu, H.; Li, Y.; Sun, J.; Wang, R.; Liu, S.; Greer, A.L. Fabrication and biocompatibility of nano-TiO2/titanium alloys biomaterials. Mater. Lett. 2005, 59, 3144–3148. [Google Scholar] [CrossRef]
- Al-Mobarak, N.A.; Al-Swayih, A.A. Development of Titanium Surgery Implants for Improving Osseointegration Through Formation of a Titanium Nanotube Layer. Int. J. Electrochem. Sci. 2014, 9, 32–45. [Google Scholar]
- López-Huerta, F.; Cervantes, B.; González, O.; Hernández-Torres, J.; García-González, L.; Vega, R.; Herrera-May, A.L.; Soto, E. Biocompatibility and Surface Properties of TiO2 Thin Films Deposited by DC Magnetron Sputtering. Materials 2014, 7, 4105–4117. [Google Scholar] [CrossRef]
- Majeed, A.; He, J.; Jiao, L.; Zhong, X.; Sheng, Z. Surface properties and biocompatibility of nanostructured TiO2 film deposited by RF magnetron sputtering. Nanoscale Res. Lett. 2015, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M. Multiscale Fabrication of Functional Materials for Regenerative Medicine; Springer: Berlin/Heidelberg, Germany, 2011; p. 45. [Google Scholar]
- Heimann, R.B. Thermal spraying of biomaterials. Surf. Coat. Technol. 2006, 201, 2012–2019. [Google Scholar] [CrossRef]
- Oshida, Y.; Tuna, E.B.; Aktören, O.; Gençay, K. Dental Implant Systems. Int. J. Mol. Sci. 2010, 11, 1580–1678. [Google Scholar] [CrossRef] [PubMed]
- Sobieszczyk, S.; Klotzke, R. Nanotubular titanium oxide layers for enhancement of bone-implant bonding and bioactivity. Adv. Mater. Sci. 2011, 11, 17–26. [Google Scholar] [CrossRef]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 Nanotubes: Synthesis and Applications. Angew. Chem. Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef] [PubMed]
- Minagar, S.; Berndt, C.C.; Wang, J.; Ivanova, E.; Wen, C. A review of the application of anodization for the fabrication of nanotubes on metal implant surfaces. Acta Biomater. 2012, 8, 2875–2888. [Google Scholar] [CrossRef] [PubMed]
- Macak, J.M.; Tsuchiya, H.; Ghicov, A.; Yasuda, K.; Hahn, R.; Bauer, S.; Schmuki, P. TiO2 nanotubes: Self-organized electrochemical formation, properties and applications. Curr. Opin. Solid State Mater. Sci. 2007, 11, 3–18. [Google Scholar] [CrossRef]
- Macak, J.M.; Hildebrand, H.; Marten-Jahns, U.; Schmuki, P. Mechanistic aspects and growth of large diameter self organized TiO2 nanotubes. J. Electroanal. Chem. 2008, 621, 254–266. [Google Scholar] [CrossRef]
- Tan, A.W.; Pingguan-Murphy, B.; Ahmad, R.; Akbar, S.A. Review of titania nanotubes: Fabrication and cellular response. Ceram. Int. 2012, 38, 4421–4435. [Google Scholar] [CrossRef]
- Brammer, K.S.; Frandsen, C.J.; Jin, S. TiO2 nanotubes for bone regeneration. Trends Biotechnol. 2012, 30, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Montaro, L.; Costerton, J.W. New trends in diagnosis and control strategies for implant infections. Int. J. Artif. Organs 2011, 34, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Montanaro, L.; Speziale, P.; Campoccia, D.; Ravaioli, S.; Cangini, I.; Pietrocola, G.; Giannini, S.; Arciola, C.R. Scenery of Staphylococcus implant infections in orthopedics. Future Microbiol. 2011, 6, 1329–1349. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Yang, B. Bioactive titanium surfaces with the effect of inhibiting biofilm formation. J. Bionic Eng. 2014, 11, 589–599. [Google Scholar] [CrossRef]
- Hanke, M.L.; Kielian, T. Deciphering mechanisms of staphylococcal biofilm evasion of host immunity. Front. Cell. Infect. Microbiol. 2012, 2, 62. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Zhong, H.; Zhang, M.; Hong, Y. Effects of antimicrobial peptides on Staphylococcus aureus growth and biofilm formation in vitro following isolation from implant-associated infections. Int. J. Clin. Exp. Med. 2015, 8, 1546–1551. [Google Scholar] [PubMed]
- Lewandowska, Ż.; Piszczek, P.; Radtke, A.; Jędrzejewski, T.; Kozak, W.; Sadowska, B. The evaluation of the impact of titania nanotube covers morphology and crystal phase on their biological properties. J. Mater. Sci. Mater. Med. 2015, 26, 163. [Google Scholar] [CrossRef] [PubMed]
- Radtke, A.; Piszczek, P.; Topolski, A.; Hald Andersen, I.; Pleth Nielsen, L.; Heikkilä, M.; Leskelä, M. The evaluation of the impact of titania nanotube covers morphology and crystal chase on their biological properties. Appl. Surf. Sci. 2016, 368, 165–172. [Google Scholar] [CrossRef]
- Lai, Y.; Sun, L.; Chen, Y.; Zhuang, H.; Lin, C.; Chin, J.W. Effects of the structure of TiO2 nanotube array on Ti substrate on its photocatalytic activity. J. Electrochem. Soc. 2006, 153, 123–127. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Capping antibacterial Ag nanorods aligned on Ti interlayer by mesoporous TiO2 layer. Surf. Interface Anal. 2009, 203, 3123–3128. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Self-accumulated Ag nanoparticles on mesoporous TiO2 thin film with high bactericidal activities. Surf. Coat. Technol. 2010, 204, 3676–3683. [Google Scholar] [CrossRef]
- McCafferty, E.; Wightman, J.P. Determination of the concentration of surface hydroxyl groups on metal oxide films by a quantitative XPS method. Surf. Interface Anal. 1998, 26, 549–564. [Google Scholar] [CrossRef]
- Tan, X.; Fan, Q.; Wang, X.; Grambow, B. Eu(III) sorption to TiO2 (anatase and rutile): Batch, XPS, and EXAFS studies. Environ. Sci. Technol. 2009, 43, 3115–3121. [Google Scholar] [CrossRef] [PubMed]
- Haija, M.A.; Guimond, S.; Uhl, A.; Kuhlenbeck, H.; Freund, H.-J. Adsorption of water on thin V2O3(0001) films. Surf. Sci. 2006, 600, 1040–1047. [Google Scholar] [CrossRef]
- Henderson, M.A. Water adsorption on TiO2 surfaces probed by soft X-ray spectroscopies: Bulk materials vs. isolated nanoparticles. Surf. Sci. Rep. 2002, 46, 15088. [Google Scholar]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.-M. Photochemical Characterization and Photocatalytic Properties of a Nanostructure Composite TiO2 Film. Appl. Catal. B Environ. 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Ninham, B.W.; Kurihara, K.; Vinogradova, O. Hydrophobicity, specific ion adsorption and reactivity. Colloid Surf. A 1997, 123–124, 7–12. [Google Scholar] [CrossRef]
- Park, J.; Bauer, S.; Schleger, K.A.; Neukam, F.W.; Von Mark, K.D.; Schmuki, P. TiO2 nanotube surfaces: 15 nm–an optimal length scale of surface topography for cell adhesion and differentiation. Small 2009, 5, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Brammer, K.S.; Oh, S.; Gallagher, J.O.; Jin, S. Enhanced cellular mobility guided by TiO2 nanotube surfaces. Nano Lett. 2008, 8, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Wang, M. A comparative study on titania layers formed on Ti, Ti-6Al-4V and NiTi shape memory alloy through a low temperature oxidation process. Surf. Coat. Technol. 2010, 205, 92–101. [Google Scholar] [CrossRef]
- Clem, W.C.; Konovalov, V.; Chowdhury, S.; Vohra, Y.K.; Catledge, S.A.; Bellis, S.L. Mesenchymal stem cell adhesion and spreading on microwave plasma-nitrided titanium alloy. J. Biomed. Mater. Res. A 2006, 76, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.H.; Shokuhfar, T.; Choi, C.K.; Lee, S.-H.; Friedrich, C. Wettability changes of TiO2 nanotube surfaces. Nanotechnology 2011, 22, 315704. [Google Scholar] [CrossRef] [PubMed]
- Giordano, C.; Saino, E.; Rimondini, L.; Pedeferri, M.P.; Visai, L.; Cigada, A.; Chiesa, R. Electrochemically induced anatase inhibits bacterial colonization on Titanium Grade 2 and Ti6Al4V alloy for dental and orthopedic devices. Colloids Surf. B Biointerfaces 2011, 88, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Cevc, G.; Vierl, U. Nanotechnology and the transdermal route: A state of the art review and critical appraisal. J. Control. Release 2010, 141, 277–299. [Google Scholar] [CrossRef] [PubMed]
- Puckett, S.D.; Taylor, E.; Raimondo, T.; Webster, T.J. The relationship between the nanostructure of titanium surfaces and bacterial attachment. Biomaterials 2010, 31, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Yoshinari, M.; Oda, Y.; Kato, T.; Okuda, K. Influence of surface modifications to titanium on antibacterial activity in vitro. Biomaterials 2001, 22, 2043–2048. [Google Scholar] [CrossRef]
- Raulio, M.; Jarn, M.; Ahola, J.; Peltonen, J.; Rosenholm, J.B.; Tervakangas, S. Microbe repelling coated stainless steel analysed by field emission scanning electron microscopy and physicochemical methods. J. Ind. Microbiol. Biotechnol. 2008, 35, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Hyde, F.W.; Alberg, M.; Smith, K. Comparison of fluorinated polymers against stainless steel, glass and polypropylene in microbial biofilm adherence and removal. J. Ind. Microbiol. Biotechnol. 1997, 19, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Palma, M.; Haggar, A.; Flock, J.I. Adherence of Staphylococcus aureus is enhanced by an endogenous secreted protein with broad binding activity. J. Bacteriol. 1999, 181, 2840–2845. [Google Scholar] [PubMed]
| Sample | Ti4+ | O2− | OH− | H2O | H2O | |
|---|---|---|---|---|---|---|
| Ti(2p3/2) | Ti(2p1/2) | O(1s) | O(1s) | O(1s) | O(1s) | |
| TNT4 | 459.0 | 464.8 | 530.3 (53%) | 531.6 (19%) | 532.7 (17%) | 533.7 (11%) |
| TNT5 | 459.2 | 465.0 | 530.4 (67%) | 531.8 (23%) | 533.1 (10%) | |
| TNT6 | 459.0 | 464.8 | 530.3 (66%) | 531.7 (24%) | 533.0 (10%) | |
| TNT8 | 458.7 | 464.7 | 530.4 (64%) | 531.9 (25%) | 533.2 (11%) | |
| TNT10 | 458.8 | 464.6 | 530.2 (53%) | 532.0 (32%) | 533.4 (15%) | |
| TNT15 | 459.0 | 464.8 | 530.4 (69%) | 531.8 (21%) | 532.8 (10%) | |
| TNT18 | 458.7 | 464.5 | 530.3 (66%) | 532.0 (23%) | 533.4 (11%) | |
| Sample | TNT3 | TNT4 | TNT5 | TNT6 | TNT8 | TNT10 | TNT12 | TNT15 | TNT18 | TNT20 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rate Constant | |||||||||||
| 103 × kobs (min−1) | 1.70 ± 0.14 | 1.63 ± 0.15 | 1.91 ± 0.13 | 1.62 ± 0.14 | 1.77 ± 0.16 | 1.89 ± 0.20 | 1.70 ± 0.16 | 1.80 ± 0.14 | 1.90 ± 0.16 | 1.69 ± 0.15 | |
| Sample | TNT4 | TNT5 | TNT6 | TNT8 | TNT10 | TNT15 | TNT18 |
|---|---|---|---|---|---|---|---|
| F % | 4.2 | 6.3 | 4.5 | 1.5 | 2.9 | 2.4 | 1.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radtke, A.; Topolski, A.; Jędrzejewski, T.; Kozak, W.; Sadowska, B.; Więckowska-Szakiel, M.; Szubka, M.; Talik, E.; Pleth Nielsen, L.; Piszczek, P. The Bioactivity and Photocatalytic Properties of Titania Nanotube Coatings Produced with the Use of the Low-Potential Anodization of Ti6Al4V Alloy Surface. Nanomaterials 2017, 7, 197. https://doi.org/10.3390/nano7080197
Radtke A, Topolski A, Jędrzejewski T, Kozak W, Sadowska B, Więckowska-Szakiel M, Szubka M, Talik E, Pleth Nielsen L, Piszczek P. The Bioactivity and Photocatalytic Properties of Titania Nanotube Coatings Produced with the Use of the Low-Potential Anodization of Ti6Al4V Alloy Surface. Nanomaterials. 2017; 7(8):197. https://doi.org/10.3390/nano7080197
Chicago/Turabian StyleRadtke, Aleksandra, Adrian Topolski, Tomasz Jędrzejewski, Wiesław Kozak, Beata Sadowska, Marzena Więckowska-Szakiel, Magdalena Szubka, Ewa Talik, Lars Pleth Nielsen, and Piotr Piszczek. 2017. "The Bioactivity and Photocatalytic Properties of Titania Nanotube Coatings Produced with the Use of the Low-Potential Anodization of Ti6Al4V Alloy Surface" Nanomaterials 7, no. 8: 197. https://doi.org/10.3390/nano7080197