Electrostatic Assembly of Platinum Nanoparticles along Electrospun Polymeric Nanofibers for High Performance Electrochemical Sensors
Abstract
:1. Introduction
2. Results and Discussion
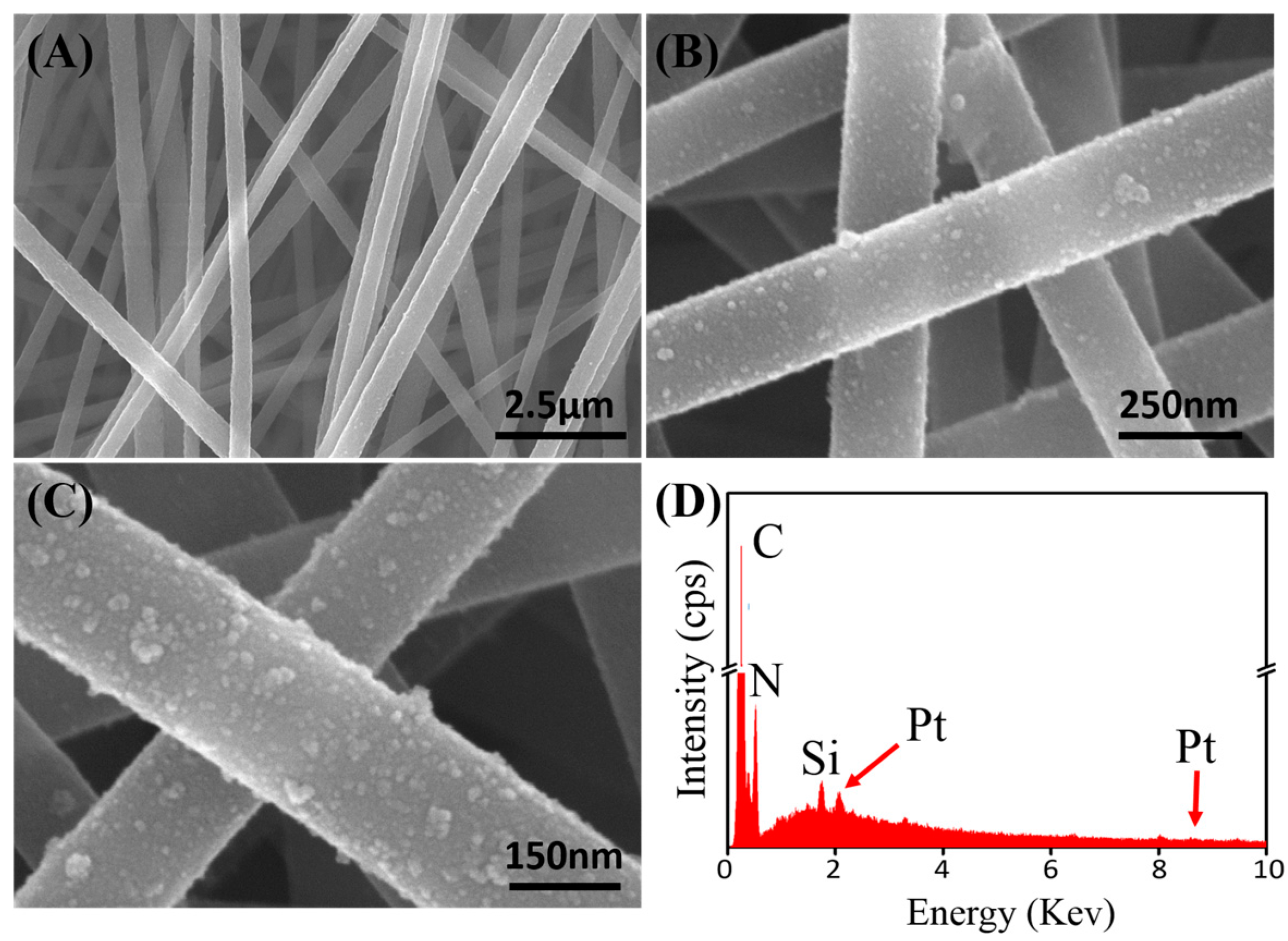
2.1. Morphological Characterizations of PtNPs and PAN–PtNPs Nanofibers
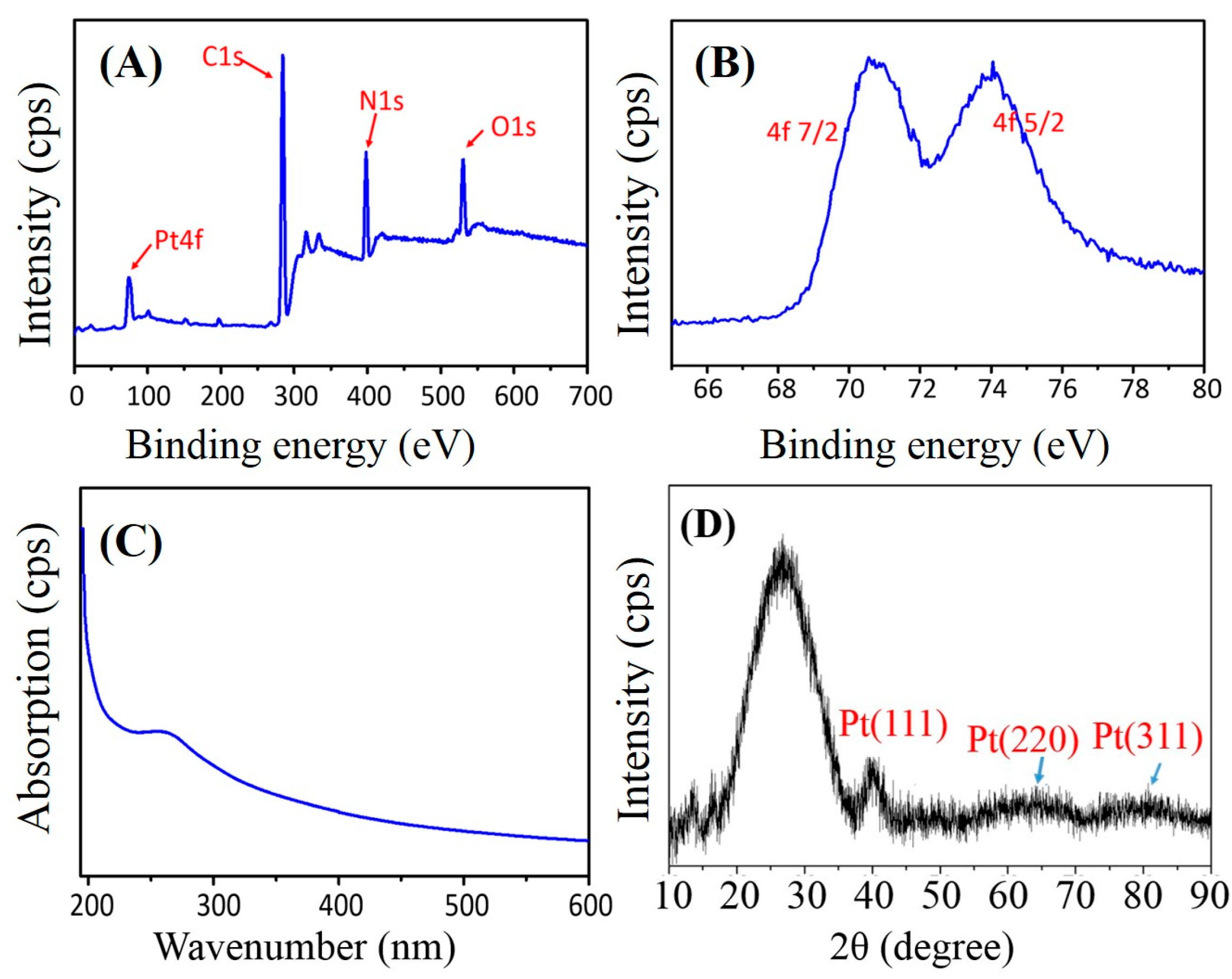
2.2. Property Characterizations of PAN–PtNPs Hybrid Nanofibers
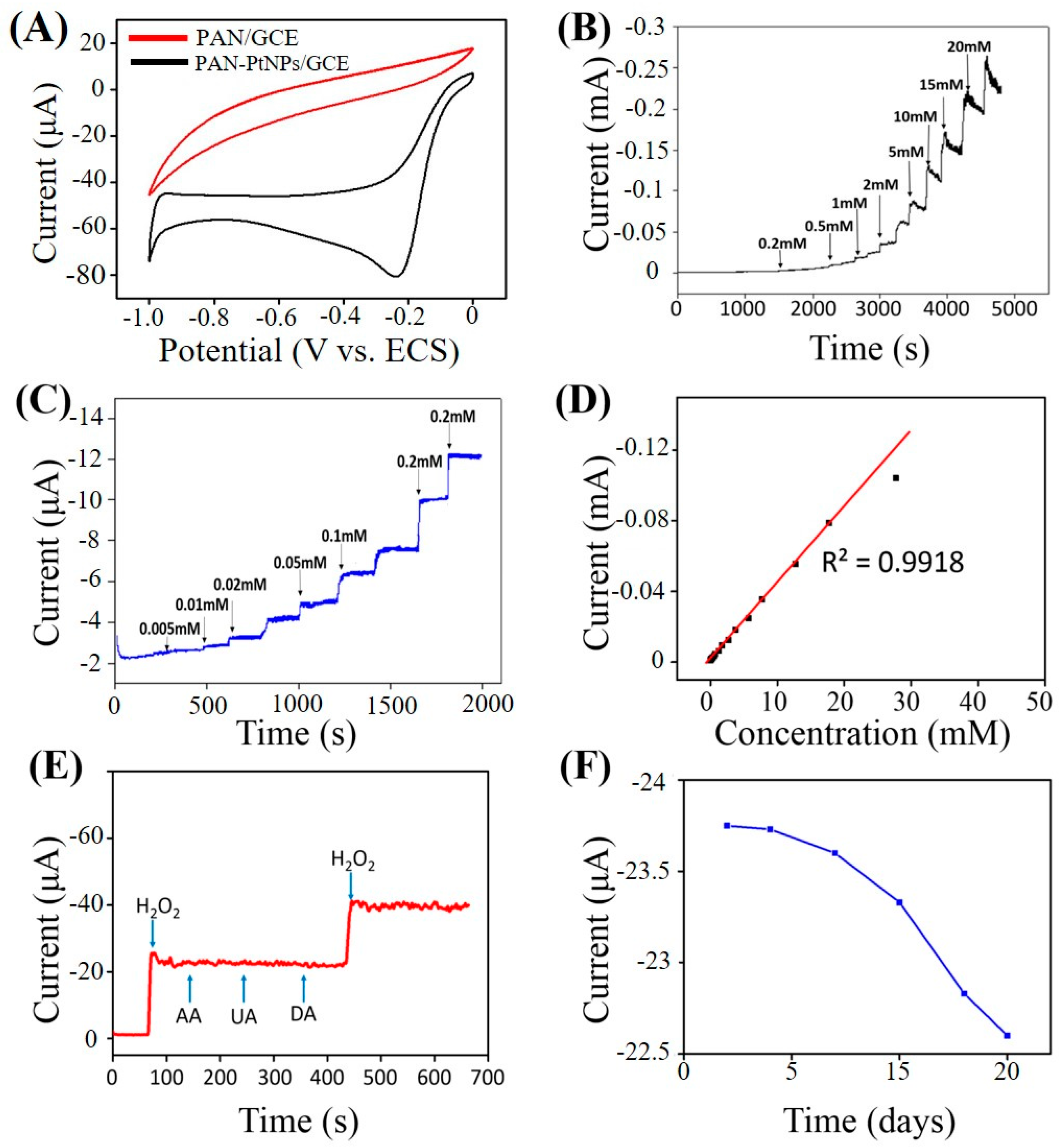
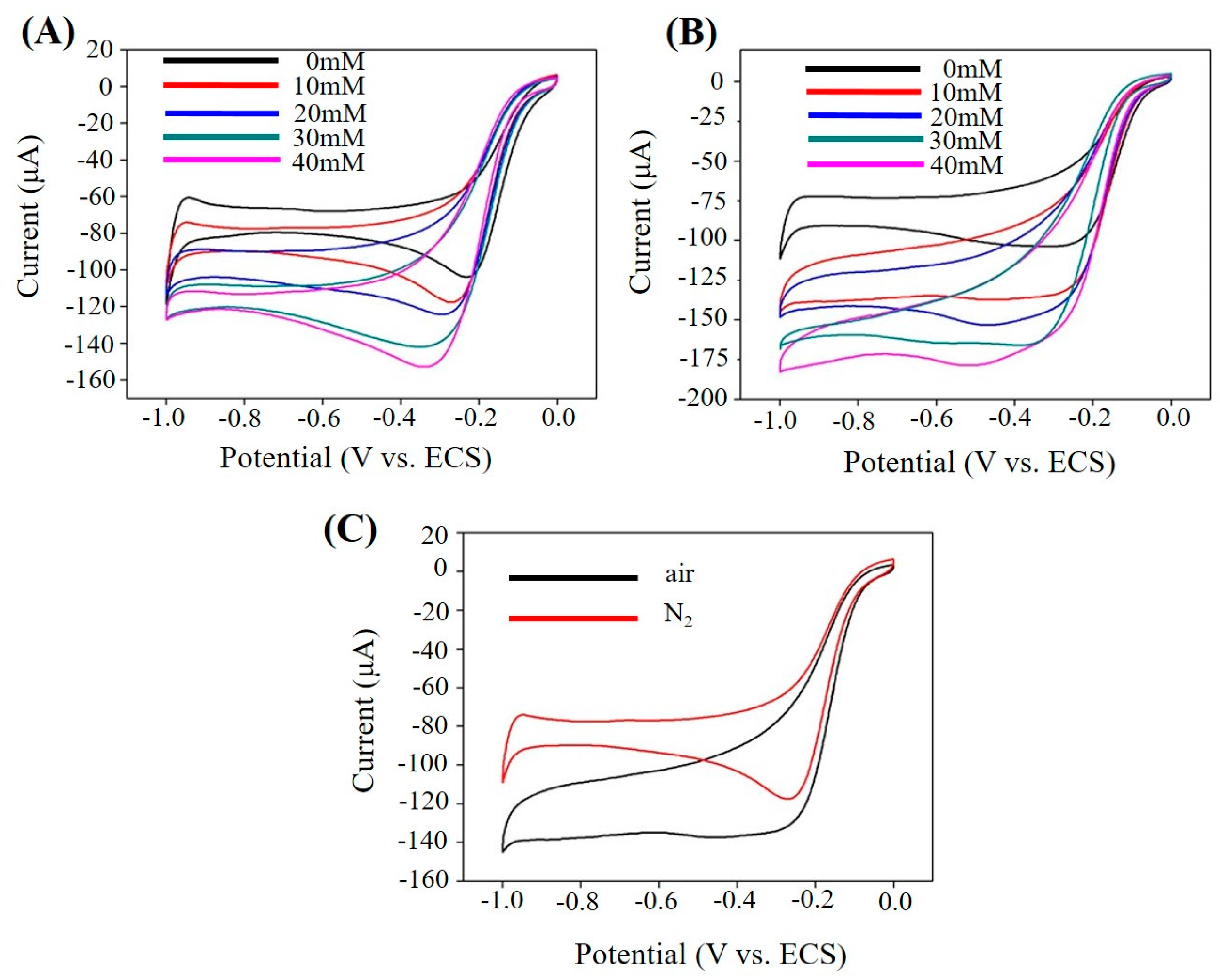
2.3. Electrochemical Sensing H2O2
3. Materials and Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kimmel, D.W.; LeBlanc, G.; Meschievitz, M.E.; Cliffel, D.E. Electrochemical sensors and biosensors. Anal. Chem. 2012, 84, 685–707. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.P.; Zhao, X.N.; Ji, Y.C.; Ouyang, Z.F.; Wen, X.; Li, J.F.; Su, Z.Q.; Wei, G. Electrospinning graphene quantum dots into a nanofibrous membrane for dual-purpose fluorescent and electrochemical biosensors. J. Mater. Chem. B 2015, 3, 2487–2496. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Vij, V.; Kemp, K.C.; Kim, K.S. Engineered carbon-nanomaterial-based electrochemical sensors for biomolecules. ACS Nano 2016, 10, 46–80. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.T.; Pan, Y.L.; Shan, W.Q.; Guo, M.L.; Nie, Z.; Huang, Y.; Yao, S.Z. Enhanced nonenzymatic sensing of hydrogen peroxide released from living cells based on Fe3O4/self-reduced graphene nanocomposites. Anal. Methods 2014, 6, 6073–6081. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, Z.Z.; Zhou, G.Y.; Zhu, L.; Feng, Y.; Lin, T.; Hou, H.Q.; Guo, Q.H. A sensitive hydrogen peroxide sensor based on a three-dimensional n-doped carbon nanotube-hemin modified electrode. Anal. Methods 2015, 7, 8439–8444. [Google Scholar] [CrossRef]
- Lee, K.T.; Liu, D.M.; Liang, Y.Y.; Matsushita, N.; Ikoma, T.; Lu, S.Y. Porous fluorine-doped tin oxide as a promising substrate for electrochemical biosensors-demonstration in hydrogen peroxide sensing. J. Mater. Chem. B 2014, 2, 7779–7784. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Huang, Z.M.; Xu, X.J.; Lim, C.T.; Ramakrishna, S. Preparation of core-shell structured PCL-r-gelatin Bi-component nanofibers by coaxial electrospinning. Chem. Mater. 2004, 16, 3406–3409. [Google Scholar] [CrossRef]
- Arras, M.M.L.; Jana, R.; Muhlstadt, M.; Maenz, S.; Andrews, J.; Su, Z.Q.; Grasl, C.; Jandt, K.D. In situ formation of nanohybrid shish-kebabs during electrospinning for the creation of hierarchical shish-kebab structures. Macromolecules 2016, 49, 3550–3558. [Google Scholar] [CrossRef]
- Lu, W.J.; Sun, J.S.; Jiang, X.Y. Recent advances in electrospinning technology and biomedical applications of electrospun fibers. J. Mater. Chem. B 2014, 2, 2369–2380. [Google Scholar] [CrossRef]
- Lv, C.J.; Di, W.H.; Liu, Z.H.; Zheng, K.Z.; Qin, W.P. Luminescent CePO4: Tb colloids for H2O2 and glucose sensing. Analyst 2014, 139, 4547–4555. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.; Lee, J.S.; Shin, D.H.; Jang, J. Aptamer-functionalized hybrid carbon nanofiber fet-type electrode for a highly sensitive and selective platelet-derived growth factor biosensor. ACS Appl. Mater. Interfaces 2014, 6, 13859–13865. [Google Scholar] [CrossRef] [PubMed]
- Matlock-Colangelo, L.; Baeumner, A.J. Recent progress in the design of nanofiber-based biosensing devices. Lab Chip 2012, 12, 2612–2620. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.W.; Zhu, S.Y.; Zhu, T.; Sun, W.; Li, Q.; Wei, G.; Su, Z.Q. Hydrothermal synthesis of zinc oxide-reduced graphene oxide nanocomposites for an electrochemical hydrazine sensor. RSC Adv. 2015, 5, 22935–22942. [Google Scholar] [CrossRef]
- Wang, J.H.; Zhao, X.J.; Li, J.F.; Kuang, X.; Fan, Y.Q.; Wei, G.; Su, Z.Q. Electrostatic assembly of peptide nanofiber-biomimetic silver nanowires onto graphene for electrochemical sensors. ACS Macro Lett. 2014, 3, 529–533. [Google Scholar] [CrossRef]
- Solanki, P.R.; Kaushik, A.; Agrawal, V.V.; Malhotra, B.D. Nanostructured metal oxide-based biosensors. NPG Asia Mater. 2011, 3, 17–24. [Google Scholar] [CrossRef]
- Su, Z.Q.; Ding, J.W.; Wei, G. Electrospinning: A facile technique for fabricating polymeric nanofibers doped with carbon nanotubes and metallic nanoparticles for sensor applications. RSC Adv. 2014, 4, 52598–52610. [Google Scholar] [CrossRef]
- Zhang, C.L.; Yu, S.H. Nanoparticles meet electrospinning: Recent advances and future prospects. Chem. Soc. Rev. 2014, 43, 4423–4448. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Su, D.F.; Su, Z.Q.; Chen, X.N. Fabrication of multiwalled carbon nanotube/polypropylene conductive fibrous membranes by melt electrospinning. Ind. Eng. Chem. Res. 2014, 53, 2308–2317. [Google Scholar] [CrossRef]
- Su, Z.Q.; Li, J.F.; Li, Q.; Ni, T.Y.; Wei, G. Chain conformation, crystallization behavior, electrical and mechanical properties of electrospun polymer-carbon nanotube hybrid nanofibers with different orientations. Carbon 2012, 50, 5605–5617. [Google Scholar] [CrossRef]
- Zhang, M.F.; Zhao, X.N.; Zhang, G.H.; Wei, G.; Su, Z.Q. Electrospinning design of functional nanostructures for biosensor applications. J. Mater. Chem. B 2017, 5, 1699–1711. [Google Scholar] [CrossRef]
- Zhao, M.G.; Huang, J.Y.; Zhou, Y.; Chen, Q.; Pan, X.H.; He, H.P.; Ye, Z.Z. A single mesoporous ZnO/chitosan hybrid nanostructure for a novel free nanoprobe type biosensor. Biosens. Bioelectron. 2013, 43, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, P.P.; Ouyang, Z.F.; Zhang, M.F.; Lin, Z.J.; Li, J.F.; Su, Z.Q.; Wei, G. Nanoscale graphene doped with highly dispersed silver nanoparticles: Quick synthesis, facile fabrication of 3D membrane-modified electrode, and super performance for electrochemical sensing. Adv. Funct. Mater. 2016, 26, 2122–2134. [Google Scholar] [CrossRef]
- Destaye, A.G.; Lin, C.K.; Lee, C.K. Glutaraldehyde vapor cross-linked nanofibrous PVA mat with in situ formed silver nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 4745–4752. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yao, H.B.; He, D.A.; Zhang, C.L.; Yu, S.H. Facile fabrication of gold nanoparticles-poly(vinyl alcohol) electrospun water-stable nanofibrous mats: Efficient substrate materials for biosensors. ACS Appl. Mater. Interfaces 2012, 4, 1963–1971. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Gao, F.; Gu, Z.Y. Vertically aligned Pt nanowire array/au nanoparticle hybrid structure as highly sensitive amperometric biosensors. Sens. Actuators B 2017, 243, 1092–1101. [Google Scholar] [CrossRef]
- Ao, H.; Qian, Z.S.; Zhu, Y.Y.; Zhao, M.Z.; Tang, C.; Huang, Y.Y.; Feng, H.; Wang, A.J. A fluorometric biosensor based on functional Au/Ag nanoclusters for real-time monitoring of tyrosinase activity. Biosens. Bioelectron. 2016, 86, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.M.; Guo, Y.J.; Wang, L.X.; Luo, F.; Lin, C.Y.; Lin, Z.Y.; Chen, G.A. A sensitive fluorescence biosensor for alkaline phosphatase activity based on the Cu(ii)-dependent dnazyme. Anal. Chim. Acta 2016, 948, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Z.F.; Li, J.F.; Wang, J.H.; Li, Q.; Ni, T.Y.; Zhang, X.Y.; Wang, H.X.; Li, Q.; Su, Z.Q.; Wei, G. Fabrication, characterization and sensor application of electrospun polyurethane nanofibers filled with carbon nanotubes and silver nanoparticles. J. Mater. Chem. B 2013, 1, 2415–2424. [Google Scholar] [CrossRef]
- Zhang, P.P.; Zhao, X.N.; Zhang, X.; Lai, Y.; Wang, X.T.; Li, J.F.; Wei, G.; Su, Z.Q. Electrospun doping of carbon nanotubes and platinum nanoparticles into the beta-phase polyvinylidene difluoride nanofibrous membrane for biosensor and catalysis applications. ACS Appl. Mater. Interfaces 2014, 6, 7563–7571. [Google Scholar] [CrossRef] [PubMed]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic-inorganic nanocomposites—A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, Z.J.; Wan, Q.J.; Wu, K.B.; Yang, N.J. Lithium-doped NiO nanofibers for non-enzymatic glucose sensing. Electrochem. Commun. 2015, 61, 89–92. [Google Scholar] [CrossRef]
- Ji, X.Y.; Wang, P.; Su, Z.G.; Ma, G.H.; Zhang, S.P. Enabling multi-enzyme biocatalysis using coaxial-electrospun hollow nanofibers: Redesign of artificial cells. J. Mater. Chem. B 2014, 2, 181–190. [Google Scholar] [CrossRef]
- Shen, J.H.; Yang, X.L.; Zhu, Y.H.; Kang, H.G.; Cao, H.M.; Li, C.Z. Gold-coated silica-fiber hybrid materials for application in a novel hydrogen peroxide biosensor. Biosens. Bioelectron. 2012, 34, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Moghe, A.K.; Gupta, B.S. Co-axial electrospinning for nanofiber structures: Preparation and applications. Polym. Rev. 2008, 48, 353–377. [Google Scholar] [CrossRef]
- Konishi, Y.; Ohno, K.; Saitoh, N.; Nomura, T.; Nagamine, S.; Hishida, H.; Takahashi, Y.; Uruga, T. Bioreductive deposition of platinum nanoparticles on the bacterium shewanella algae. J. Biotechnol. 2007, 128, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Xu, F.G.; Li, Z.; Jandt, K.D. Protein-promoted synthesis of Pt nanoparticles on carbon nanotubes for electrocatalytic nanohybrids with enhanced glucose sensing. J. Phys. Chem. C 2011, 115, 11453–11460. [Google Scholar] [CrossRef]
- Li, H.H.; Zhao, S.; Gong, M.; Cui, C.H.; He, D.; Liang, H.W.; Wu, L.; Yu, S.H. Ultrathin ptpdte nanowires as superior catalysts for methanol electrooxidation. Angew. Chem. Int. Ed. 2013, 52, 7472–7476. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.Y.; Wang, Y.; Wu, N.Z.; Gui, L.L.; Tang, Y.Q. Shape-selective preparation and properties of oxalate-stabilized Pt colloid. Langmuir 2002, 18, 4619–4624. [Google Scholar] [CrossRef]
- Miao, Y.E.; He, S.X.; Zhong, Y.L.; Yang, Z.; Tjiu, W.W.; Liu, T.X. A novel hydrogen peroxide sensor based on Ag/SnO2 composite nanotubes by electrospinning. Electrochim. Acta 2013, 99, 117–123. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, M.F.; Zhang, X.P.; Xie, G.C.; Su, Z.Q.; Wei, G. Nanoporous carbon nanofibers decorated with platinum nanoparticles for non-enzymatic electrochemical sensing of H2O2. Nanomaterials 2015, 5, 1891–1905. [Google Scholar] [CrossRef] [PubMed]


| Materials | Linear Range | Detection Limit | Reference |
|---|---|---|---|
| PU-AgNPs | 0.5–30 mM | 18.6 μM | [28] |
| PVA-AgNPs | 5 μM–0.6 mM | 5 μM | [38] |
| PAN–PtNPs | 0.1–30 mM | 1.9 μM | [39] |
| Paraffin-Silica-AuNPs | 5 μM–1 mM | 2 μM | [33] |
| PAN–PtNPs | 5 μM–53 mM | 1.46 μM | This work |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Zhang, M.; Liu, X.; Su, Z.; Wei, G. Electrostatic Assembly of Platinum Nanoparticles along Electrospun Polymeric Nanofibers for High Performance Electrochemical Sensors. Nanomaterials 2017, 7, 236. https://doi.org/10.3390/nano7090236
Li P, Zhang M, Liu X, Su Z, Wei G. Electrostatic Assembly of Platinum Nanoparticles along Electrospun Polymeric Nanofibers for High Performance Electrochemical Sensors. Nanomaterials. 2017; 7(9):236. https://doi.org/10.3390/nano7090236
Chicago/Turabian StyleLi, Peng, Mingfa Zhang, Xueying Liu, Zhiqiang Su, and Gang Wei. 2017. "Electrostatic Assembly of Platinum Nanoparticles along Electrospun Polymeric Nanofibers for High Performance Electrochemical Sensors" Nanomaterials 7, no. 9: 236. https://doi.org/10.3390/nano7090236






