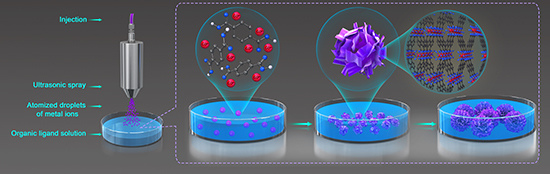
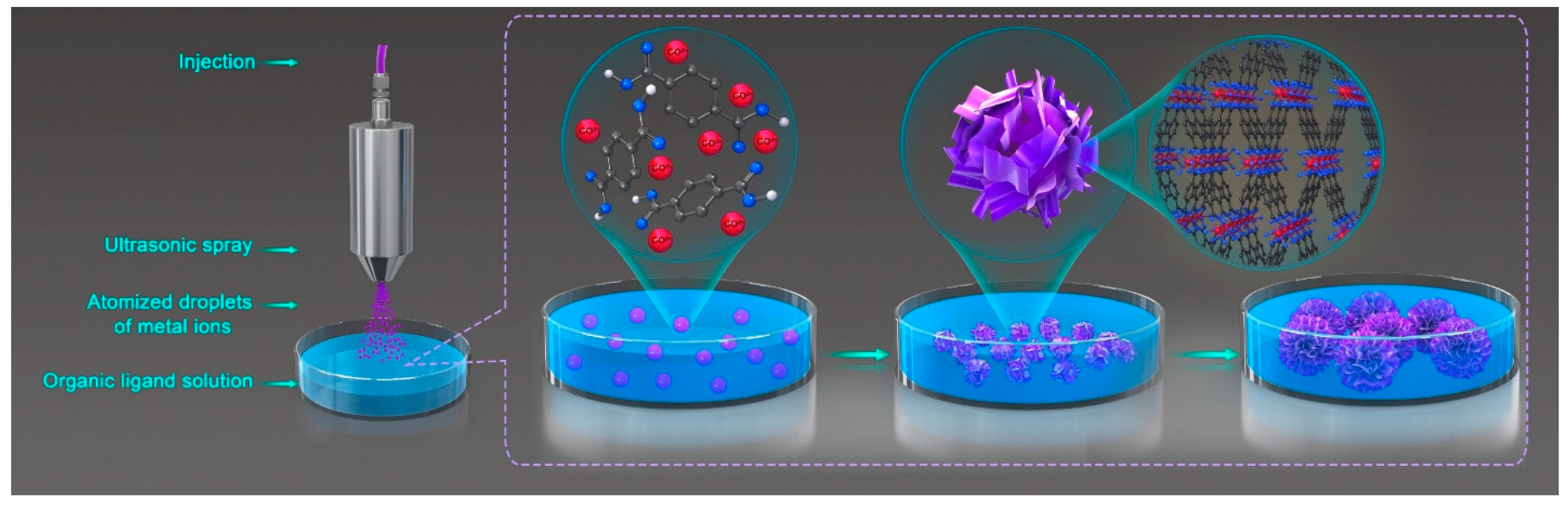
Fabrication of Monodisperse Flower-Like Coordination Polymers (CP) Microparticles by Spray Technique
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Instruments
2.3. Preparation of Co/BDC CP Microparticles by Spray
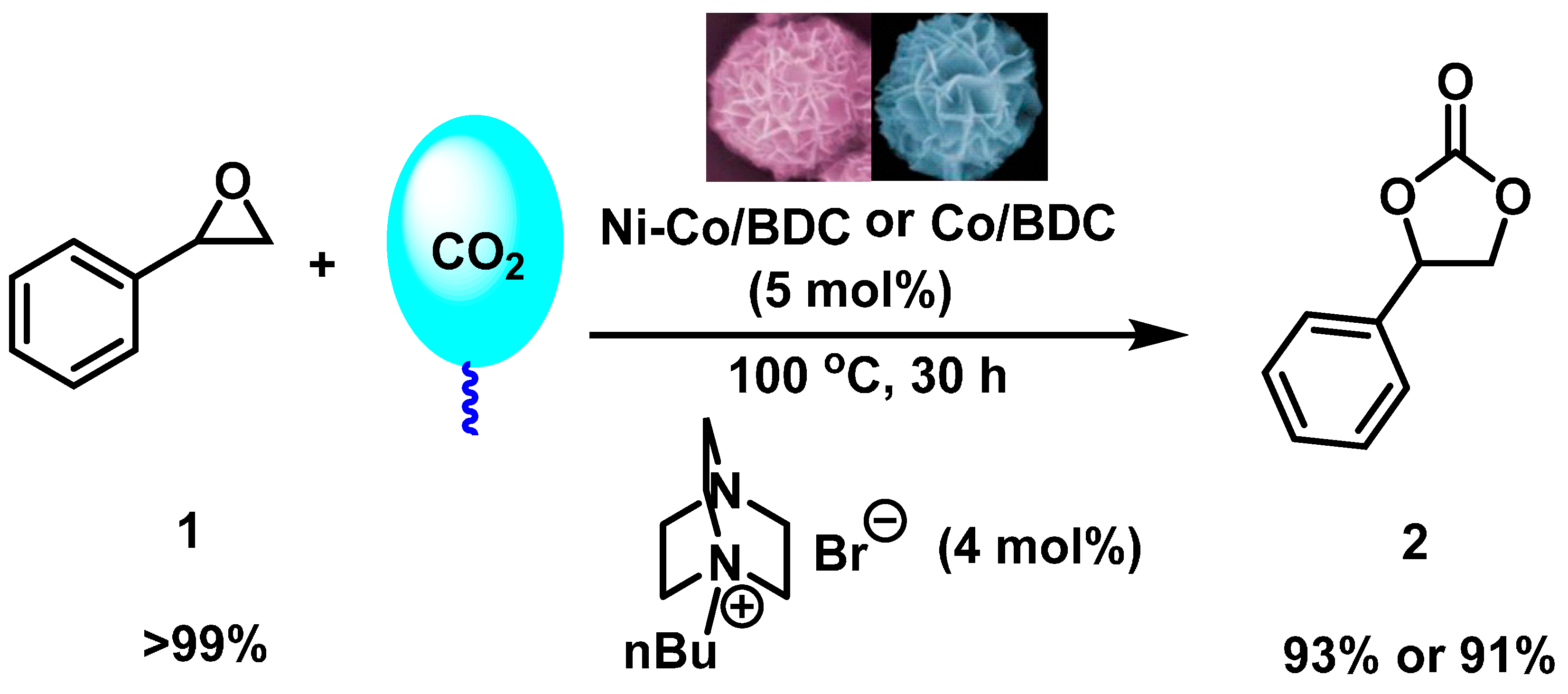
2.4. Catalytic Test
2.5. BET and TGA Tests
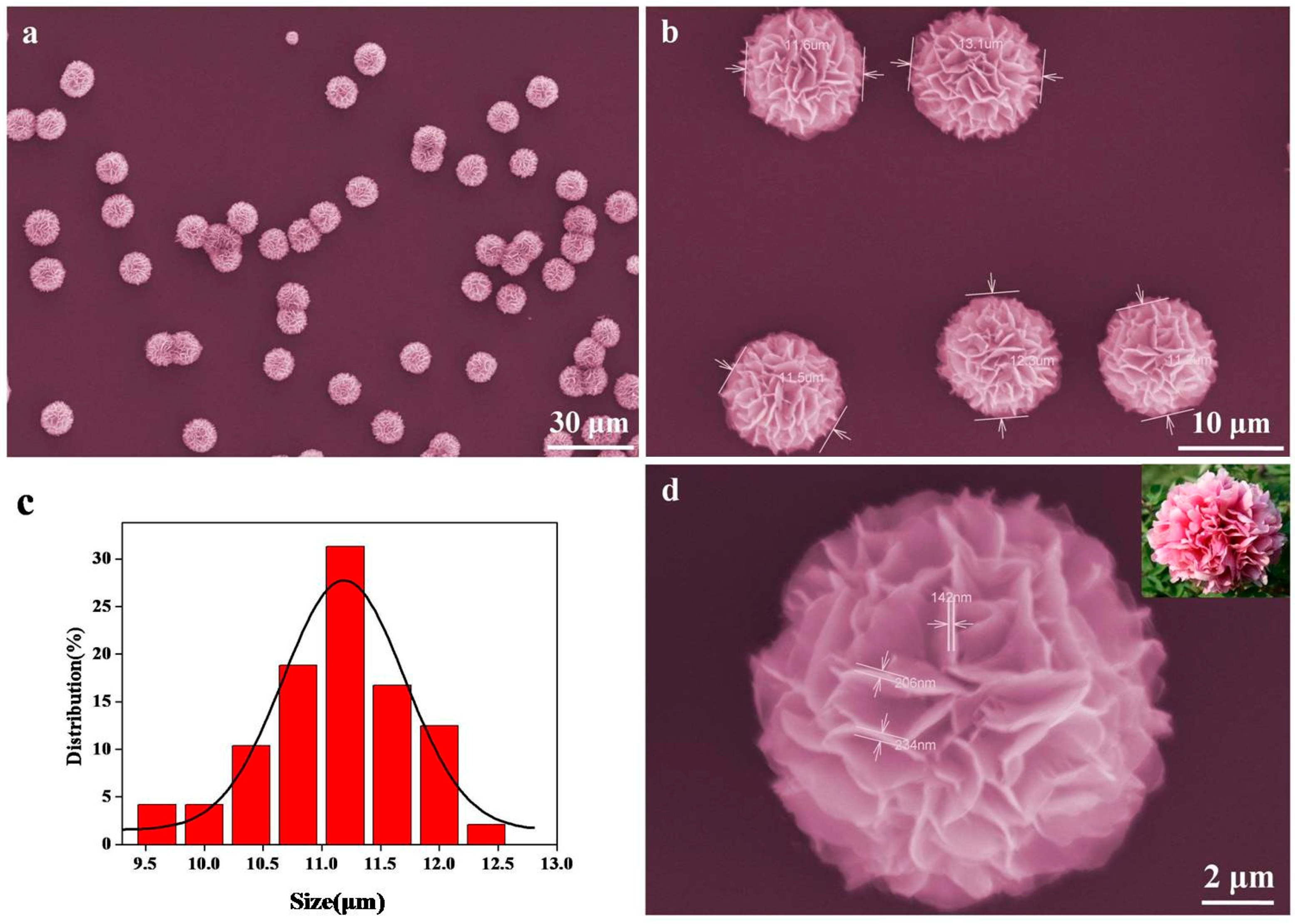
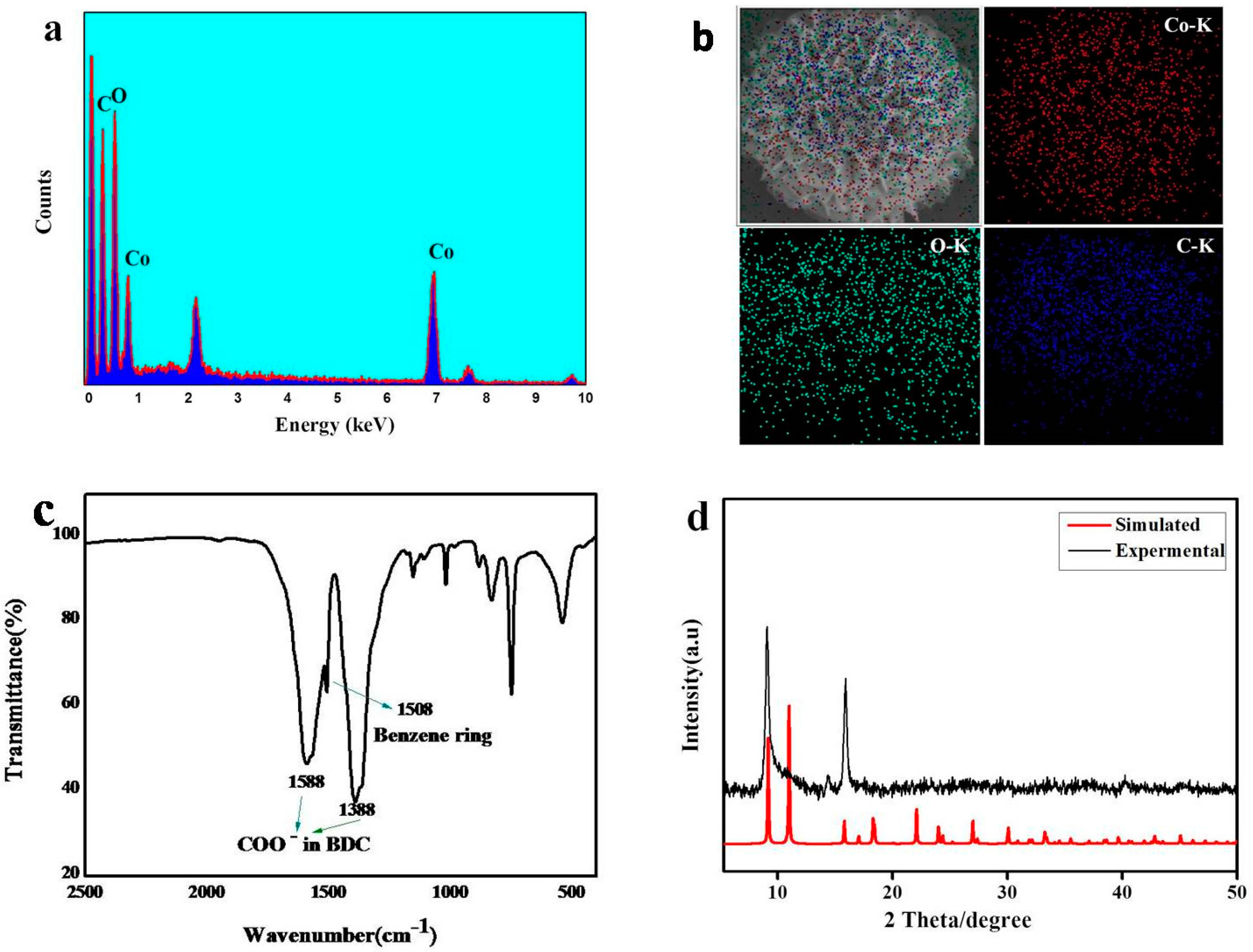
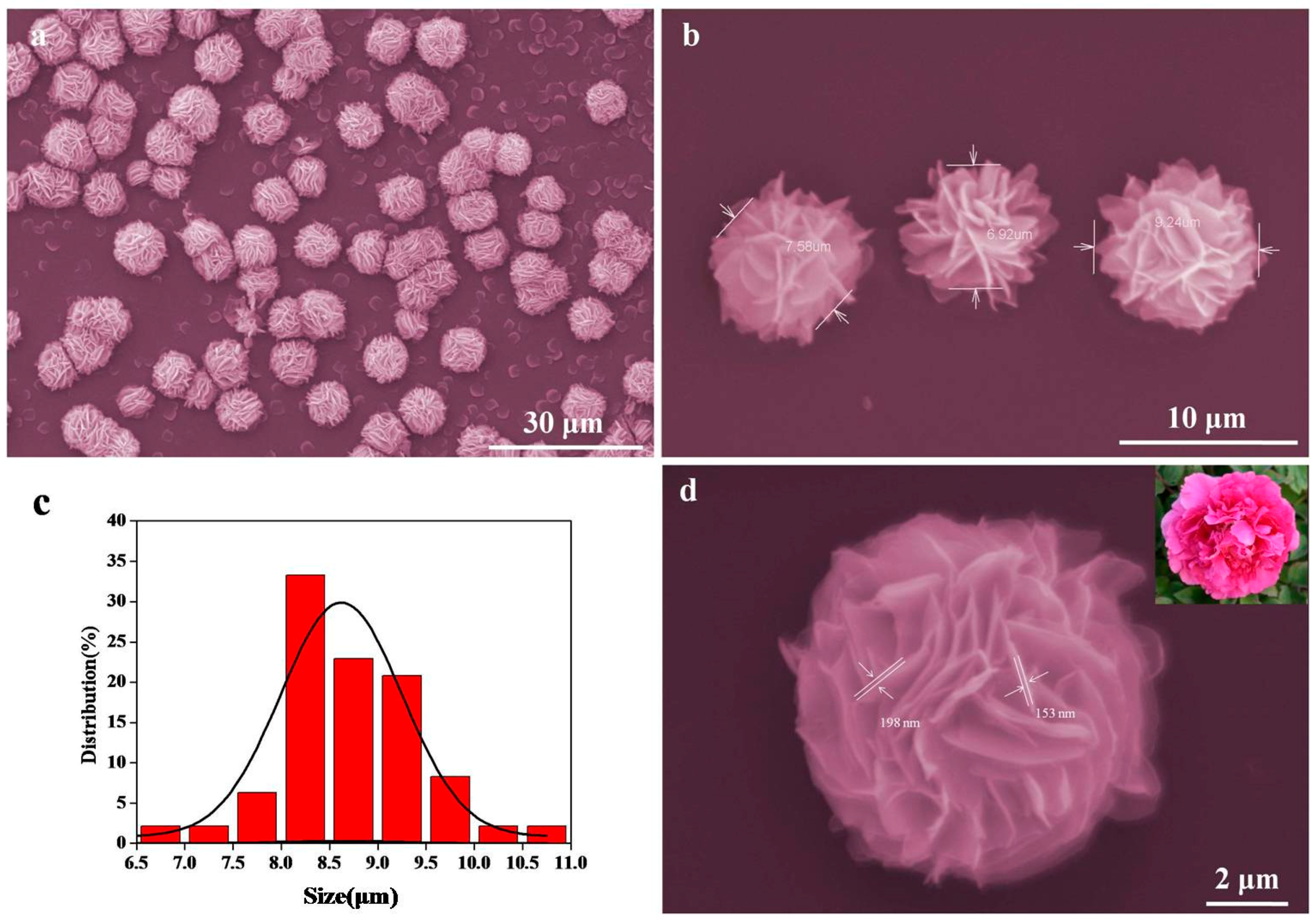
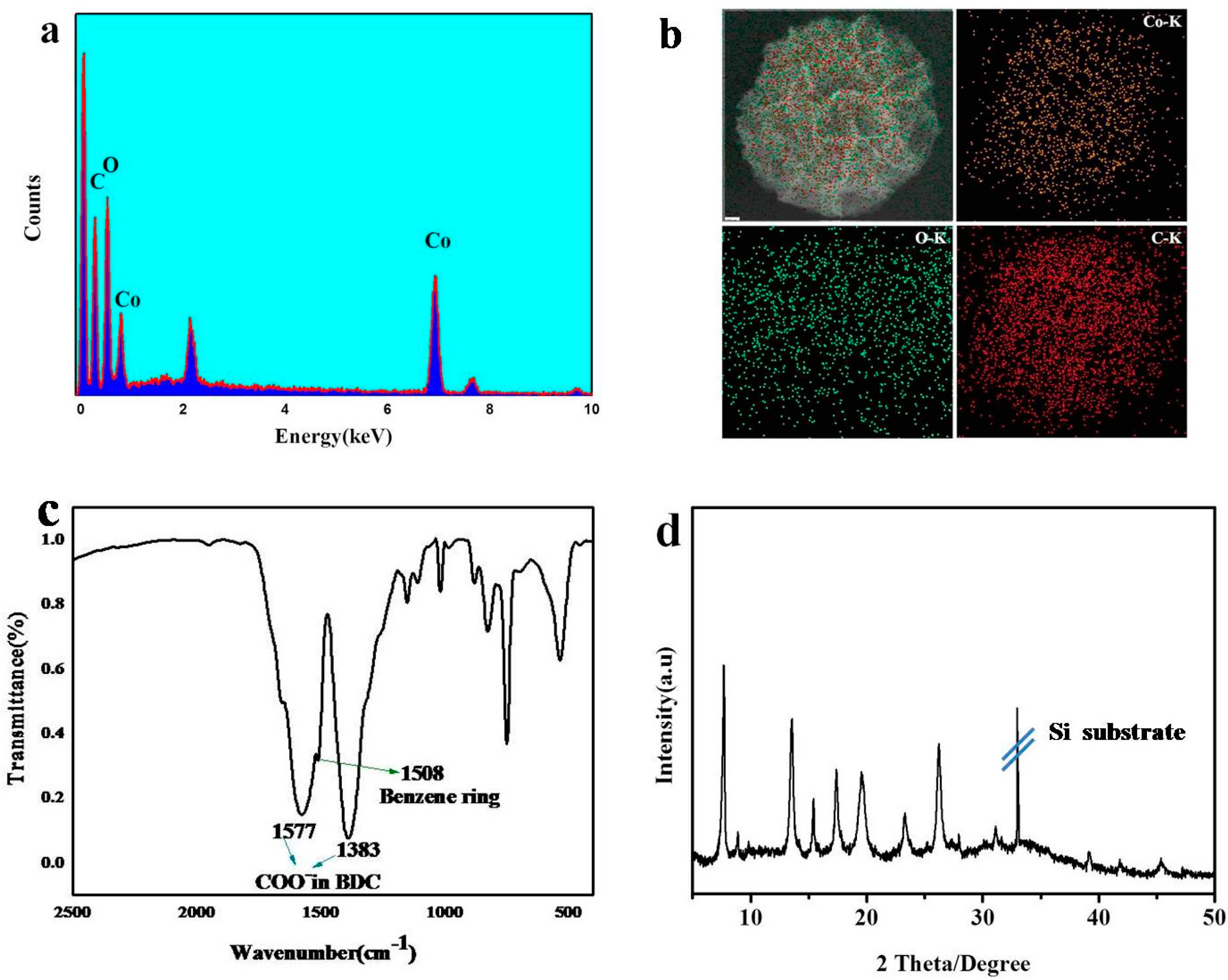
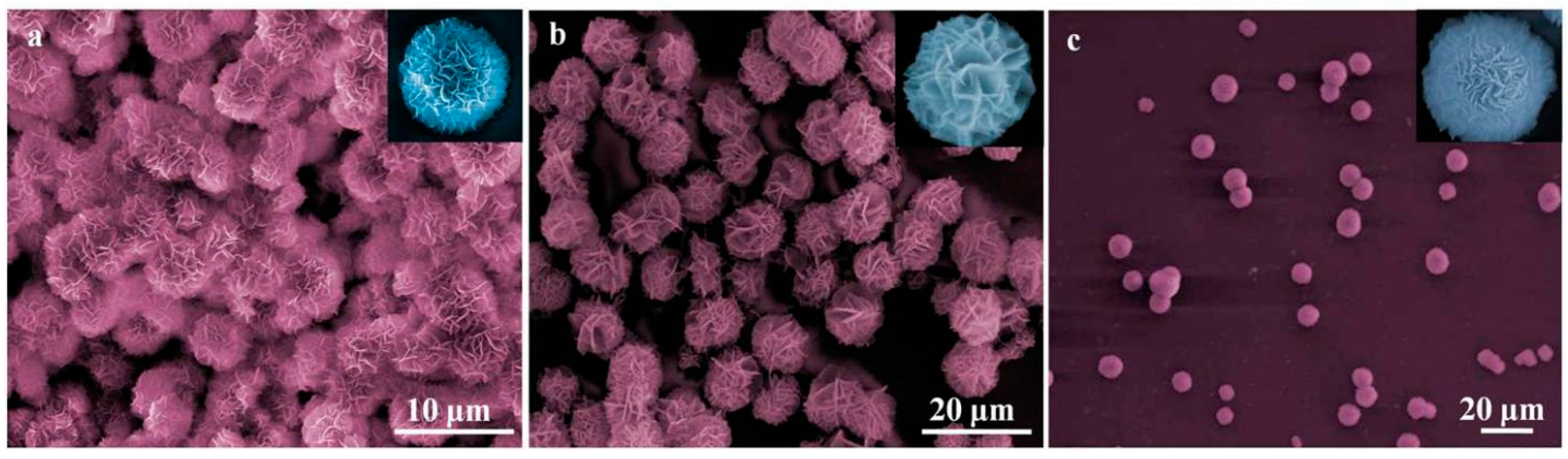
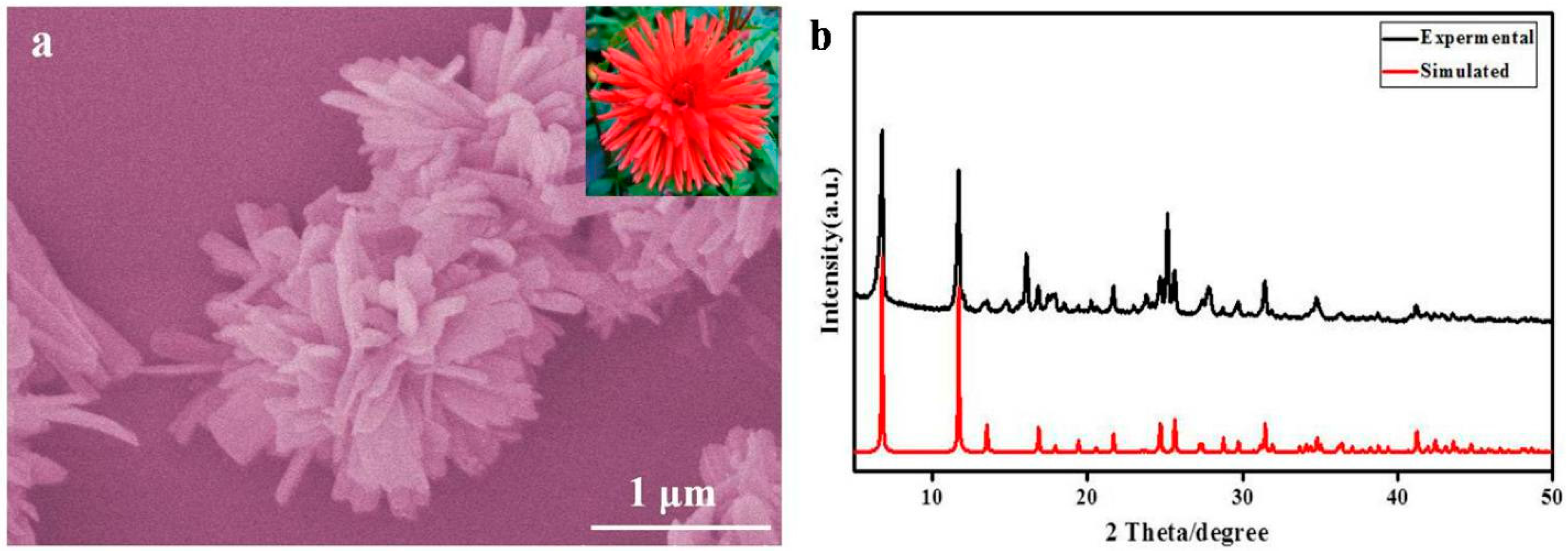
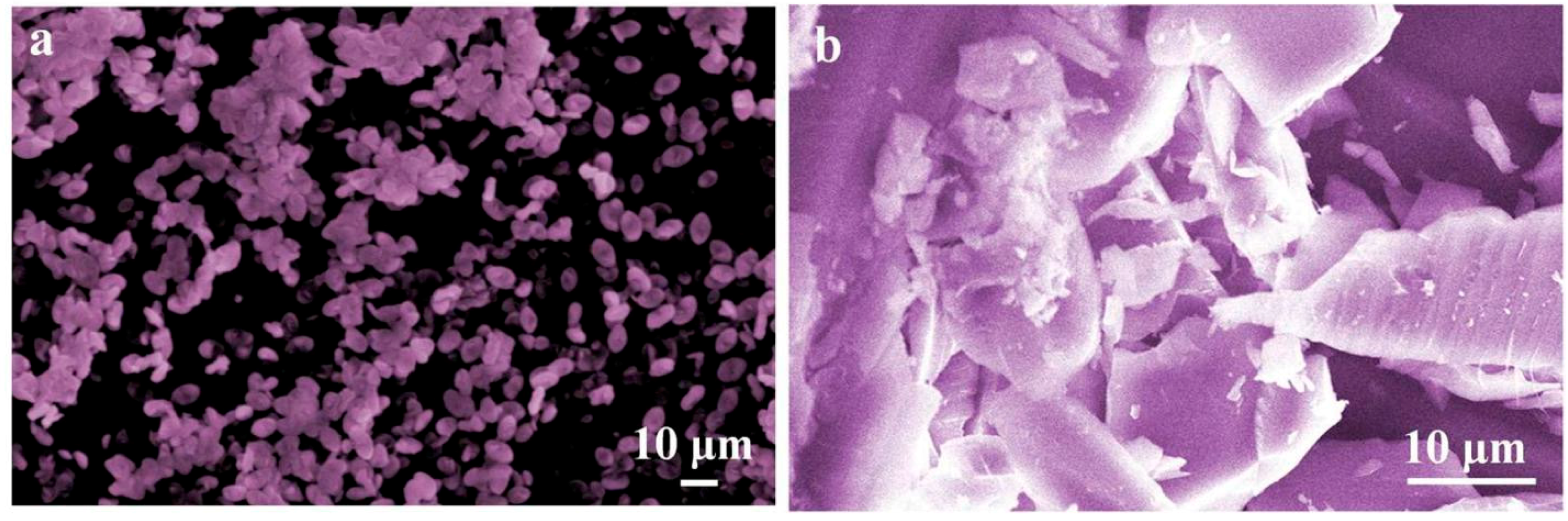
3. Result and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Knobloch, F.W.; Rauscher, W.H. Coordination polymers of copper (II) prepared at liquid-liquid interfaces. J. Polym. Sci. 1959, 38, 261–262. [Google Scholar] [CrossRef]
- Hoskins, B.F.; Robson, R. Design and construction of a new class of scaffolding-like materials comprising infinite polymeric frameworks of 3D-Linked molecular rods. A reappraisal of the Zn(CN)2 and Cd(CN)2 Structures and the synthesis and structure of the diamond-related frameworks [N(CH3)4][CuIZnII(CN)4] and CuI [4,4′,4″,4″′-tetracyanotetraphenylmethane] BF4·xC6H5NO2. J. Am. Chem. Soc. 1990, 112, 1546–1554. [Google Scholar]
- Kitagawa, S.; Matsuyama, S.; Munakata, M.; Emori, T. Synthesis and crystal structures of novel one-dimensional polymers, [{M(bpen)X}∞] [M = CuI, X = PF6−; M = AgI, X =ClO4−; bpen = trans-1,2-bis(2-pyridyl)ethylene] and [{Cu(bpen)(CO)(CH3CN)(PF6)}∞]. J. Chem. Soc. Dalton Trans. 1991, 2869–2874. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, H.L. Hydrothermal synthesis of a metal-organic framework containing large rectangular channels. J. Am. Chem. Soc. 1995, 117, 10401–10402. [Google Scholar] [CrossRef]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Spokoyny, A.M.; Kim, D.; Sumrein, A.; Mirkin, C.A. Infinite coordination polymer nano- and microparticle structures. Chem. Soc. Rev. 2009, 38, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Rodenas, T.; Luz, I.; Prieto, G.; Seoane, B.; Miro, H.; Corma, A.; Kapteijn, F.; LlabresiXamena, F.X.; Gascon, J. Metal-organic framework nanosheets in polymer composite materials for gas separation. Nat. Mater. 2015, 14, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, M.; Kondo, A.; Noguchi, H.; Kojima, N.; Ohba, T.; Kajiro, H.; Hattori, Y.; Kanoh, H. Double-Step Gate Phenomenon in CO2 Sorption of an Elastic Layer-Structured MOF. Langmuir 2016, 32, 9722–9726. [Google Scholar] [CrossRef] [PubMed]
- Leo, P.; Orcajo, G.; Briones, D.; Calleja, G.; Sanchez-Sanchez, M.; Martinez, F. A Recyclable Cu-MOF-74 Catalyst for the Ligand-Free O-Arylation Reaction of 4-Nitrobenzaldehyde and Phenol. Nanomaterials 2017, 7, 149. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Li, S.; Guo, Z.; Farha, O.K.; Hauser, B.G.; Qi, X.; Wang, Y.; Wang, X.; Han, S.; Liu, X.; et al. Imparting functionality to a metal-organic framework material by controlled nanoparticle encapsulation. Nat. Chem. 2012, 4, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Farha, O.K.; Mulfort, K.L.; Thorsness, A.M.; Hupp, J.T. Separating solids: Purification of metal-organic framework materials. J. Am. Chem. Soc. 2008, 130, 8598–8599. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.; Mirkin, C.A. Chemically tailorable colloidal particles from infinite coordination polymers. Nature 2005, 438, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.P.; Dong, S.J.; Wang, E.K. Coordination-induced formation of submicrometer-scale, monodisperse, spherical colloids of organic-inorganic hybrid materials at room temperature. J. Am. Chem. Soc. 2005, 127, 13102–13103. [Google Scholar] [CrossRef] [PubMed]
- Seoane, B.; Castellanos, S.; Dikhtiarenko, A.; Kapteijn, F.; Gascon, J. Multi-scale crystal engineering of metal organic frameworks. Coord. Chem. Rev. 2016, 307, 147–187. [Google Scholar] [CrossRef]
- Masoomi, M.Y.; Morsali, A. Morphological study and potential applications of nano metal-organic coordination polymers. RSC Adv. 2013, 3, 19191–19218. [Google Scholar] [CrossRef]
- Kiyonaga, T.; Higuchi, M.; Kajiwara, T.; Takashima, Y.; Duan, J.G.; Nagashima, K.; Kitagawa, S. Dependence of crystal size on the catalytic performance of a porous coordination polymer. Chem. Commun. 2015, 51, 2728–2730. [Google Scholar] [CrossRef] [PubMed]
- Kojtari, A.; Ji, H.F. Metal Organic Framework Micro/Nanopillars of Cu (BTC)·3H2O and Zn (ADC)·DMSO. Nanomaterials 2015, 5, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Reboul, J.; Diring, S.; Sumida, K.; Kitagawa, S. Structuring of metal-organic frameworks at the mesoscopic/macroscopic scale. Chem. Soc. Rev. 2014, 43, 5700–5734. [Google Scholar] [CrossRef] [PubMed]
- Fluegel, E.A.; Ranft, A.; Haase, F.; Lotsch, B.V. Synthetic routes toward MOF nanomorphologies. J. Mater. Chem. 2012, 22, 10119–10133. [Google Scholar] [CrossRef] [Green Version]
- Tranchemontagne, D.J.; Hunt, J.R.; Yaghi, O.M. Room temperature synthesis of metal-organic frameworks: MOF-5, MOF-74, MOF-177, MOF-199, and IRMOF-0. Tetrahedron 2008, 64, 8553–8557. [Google Scholar] [CrossRef]
- Safarifard, V.; Morsali, A. Applications of ultrasound to the synthesis of nanoscale metal-organic coordination polymers. Coord. Chem. Rev. 2015, 292, 1–14. [Google Scholar] [CrossRef]
- Khazalpour, S.; Safarifard, V.; Morsali, A.; Nematollahi, D. Electrochemical synthesis of pillared layer mixed ligand metal-organic framework: DMOF-1-Zn. RSC Adv. 2015, 5, 36547–36551. [Google Scholar] [CrossRef]
- Ge, C.H.; Du, Y.; Wang, R.; Xue, L.L.; Wu, Z.S.; Xing, T.Z.; Ji, X.C.; Ma, L.; Zhang, X.D. A facile strategy to fabricate carboxyl-rich carbon spheres with copper-based MOF through coordination bond. J. Porous Mater. 2016, 23, 1537–1545. [Google Scholar] [CrossRef]
- Chen, L.Y.; Duan, B.H.; Luo, Q.; Gu, Z.Z.; Liu, J.; Duan, C.Y. Facet-dependent catalytic activity of ZIF-8 nanocubes and rhombic dodecahedra based on tracing substrate diffusion in pores by SERS: A case study for surface catalysis of MOF. Catal. Sci. Technol. 2016, 6, 1616–1620. [Google Scholar] [CrossRef]
- Medina-Velazquez, D.Y.; Alejandre-Zuniga, B.Y.; Loera-Serna, S.; Ortiz, E.M.; Morales-Ramirez, A.D.; Garfias-Garcia, E.; Garcia-Murillo, A.; Falcony, C. An alkaline one-pot reaction to synthesize luminescent Eu-BTC MOF nanorods, highly pure and water-insoluble, under room conditions. J. Nanopart. Res. 2016, 18, 10. [Google Scholar] [CrossRef]
- Liu, J.J.; Guan, Y.F.; Li, L.; Chen, Y.; Dai, W.X.; Huang, C.C.; Lin, M.J. Construction of a bicontinuous donor-acceptor hybrid material at the molecular level by inserting inorganic nanowires into porous MOF. Chem. Commun. 2017, 53, 4481–4484. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.N.; Wang, S.; Zhou, Y.; Bai, X.J.; Song, G.S.; Zhao, X.Y.; Wang, T.Q.; Qi, X.; Zhang, X.M.; Fu, Y. Fabrication of metal-organic framework and infinite coordination polymer nanosheets by the spray technique. Langmuir 2017, 33, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.W.; Hu, M.C.; Li, S.N.; Jiang, Y.C.; Zhai, Q.G. Microporous rod metal-organic frameworks with diverse Zn/Cd-triazolate ribbons as secondary building units for CO2 uptake and selective adsorption of hydrocarbons. Dalton Trans. 2017, 46, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.A.; Guo, H.L.; Wang, S.; Li, Y.Y.; Zhang, H.J.; Liu, C.G. Solvothermal synthesis of luminescent Eu(BTC)(H2O)DMF hierarchical architectures. CrystEngComm 2012, 14, 2914–2919. [Google Scholar] [CrossRef]
- Jia, W.; Su, L.; Lei, Y. Pt nanoflower/polyaniline composite nanofibers based urea biosensor. Biosens. Bioelectron. 2011, 30, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.; Jiang, M.; Camargo, P.H.C.; Cho, E.C.; Tao, J.; Lu, X.; Zhu, Y.; Xia, Y. Pd–Pt bimetallic nanodendrites with high activity for oxygen reduction. Science 2009, 324, 1302–1305. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, A.; Garg, N.; Jin, R. A universal approach to the synthesis of noble metal nanodendrites and their catalytic properties. Angew. Chem. Int. Ed. 2010, 49, 4962–4966. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, D.; Yang, D.; Jiang, M. Fabrication of Flower-Like Silver Structures through Anisotropic Growth. Langmuir 2011, 27, 6211–6217. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Wang, J.; Thomas, D.F.; Chen, A. Synthesis of F-Doped Flower-like TiO2 Nanostructures with High Photoelectrochemical Activity. Langmuir 2008, 24, 3503–3509. [Google Scholar] [CrossRef] [PubMed]
- Li, G.R.; Xie, C.C.; Shen, Z.R.; Chang, Z.; Bu, X.H. Cobalt oxide 2D nano-assemblies from infinite coordination polymer precursors mediated by a multidentatepyridyl ligand. Dalton Trans. 2016, 45, 7866–7874. [Google Scholar] [CrossRef] [PubMed]
- Carne-Sanchez, A.; Imaz, I.; Cano-Sarabia, M.; Maspoch, D. A spray-drying strategy for synthesis of nanoscale metal-organic frameworks and their assembly into hollow superstructures. Nat. Chem. 2013, 5, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Takei, T.; Ii, T.; Kawashima, J.; Ohmura, T.; Ichikawa, M.; Hosoe, M.; Shinya, Y.; Kanoya, I.; Mori, W. Hydrogen-adsorption properties of a novel lantern-type dinuclear Co(BDC)(DABCO)1/2. Chem. Lett. 2007, 36, 1136–1137. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.-Z.; Zhou, Y.; Liu, F.; Li, Y.; Xia, M.-J.; Han, E.-H.; Wang, T.; Zhang, X.; Fu, Y. Fabrication of Monodisperse Flower-Like Coordination Polymers (CP) Microparticles by Spray Technique. Nanomaterials 2017, 7, 237. https://doi.org/10.3390/nano7090237
Li W-Z, Zhou Y, Liu F, Li Y, Xia M-J, Han E-H, Wang T, Zhang X, Fu Y. Fabrication of Monodisperse Flower-Like Coordination Polymers (CP) Microparticles by Spray Technique. Nanomaterials. 2017; 7(9):237. https://doi.org/10.3390/nano7090237
Chicago/Turabian StyleLi, Wen-Ze, Yuan Zhou, Fuchun Liu, Yunong Li, Ming-Jian Xia, En-Hou Han, Tieqiang Wang, Xuemin Zhang, and Yu Fu. 2017. "Fabrication of Monodisperse Flower-Like Coordination Polymers (CP) Microparticles by Spray Technique" Nanomaterials 7, no. 9: 237. https://doi.org/10.3390/nano7090237