Electrochemical Properties of an Na4Mn9O18-Reduced Graphene Oxide Composite Synthesized via Spray Drying for an Aqueous Sodium-Ion Battery
Abstract
:1. Introduction
2. Materials and Methods
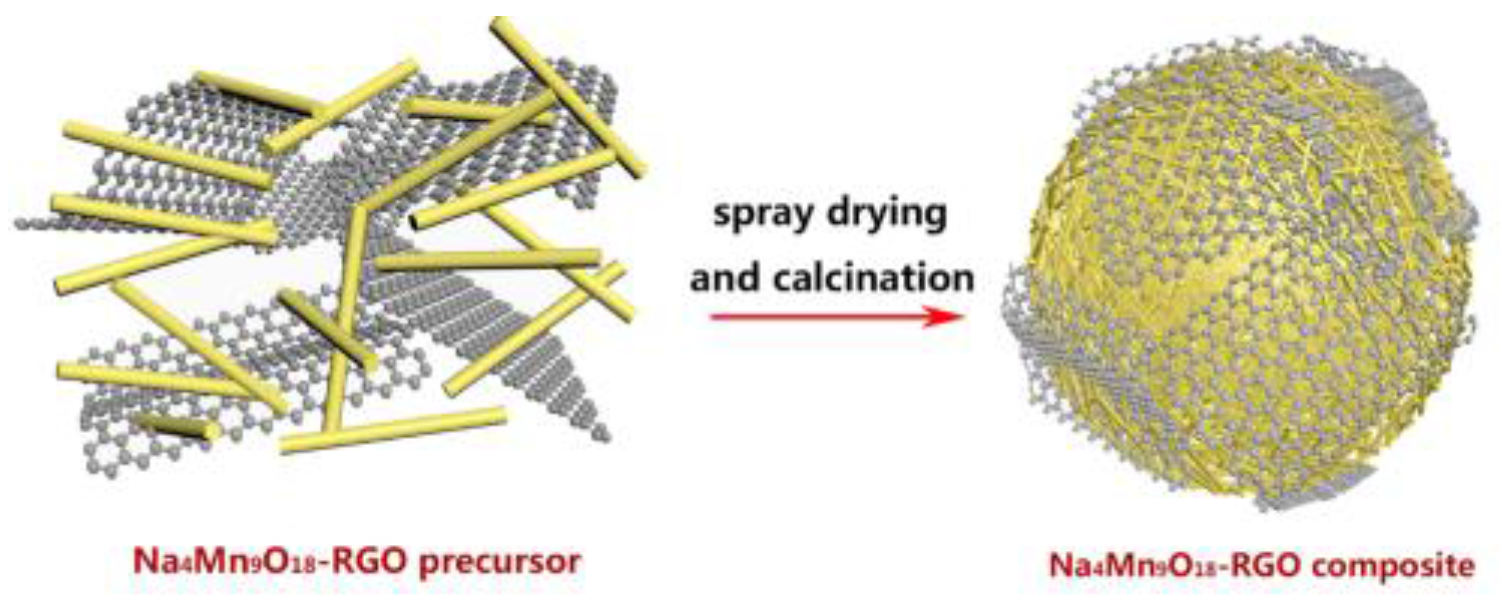
2.1. The Preparation of Na4Mn9O18-RGO Precursors by the HSCR Method
2.2. The Preparation of Na4Mn9O18-RGO by Spray Drying
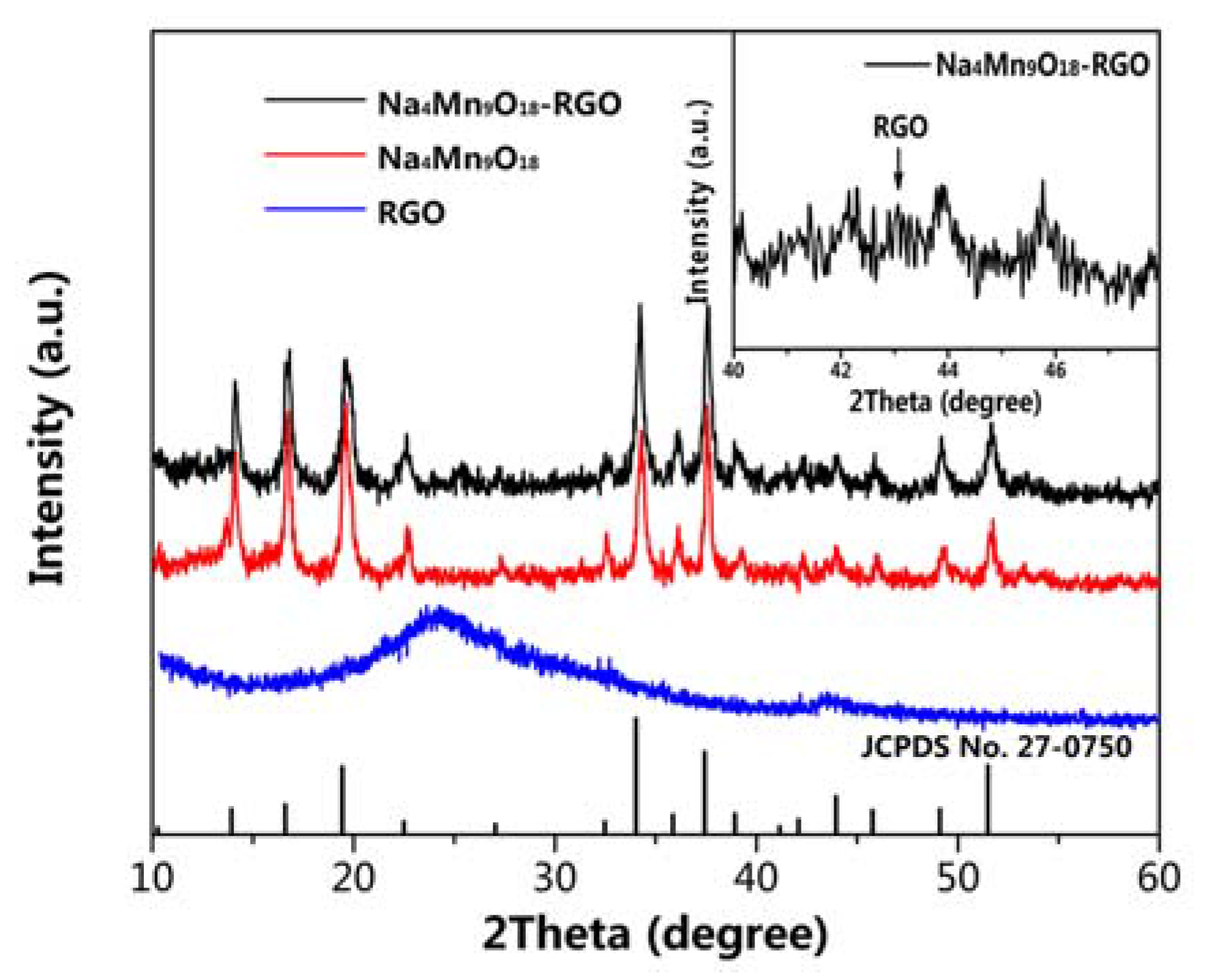
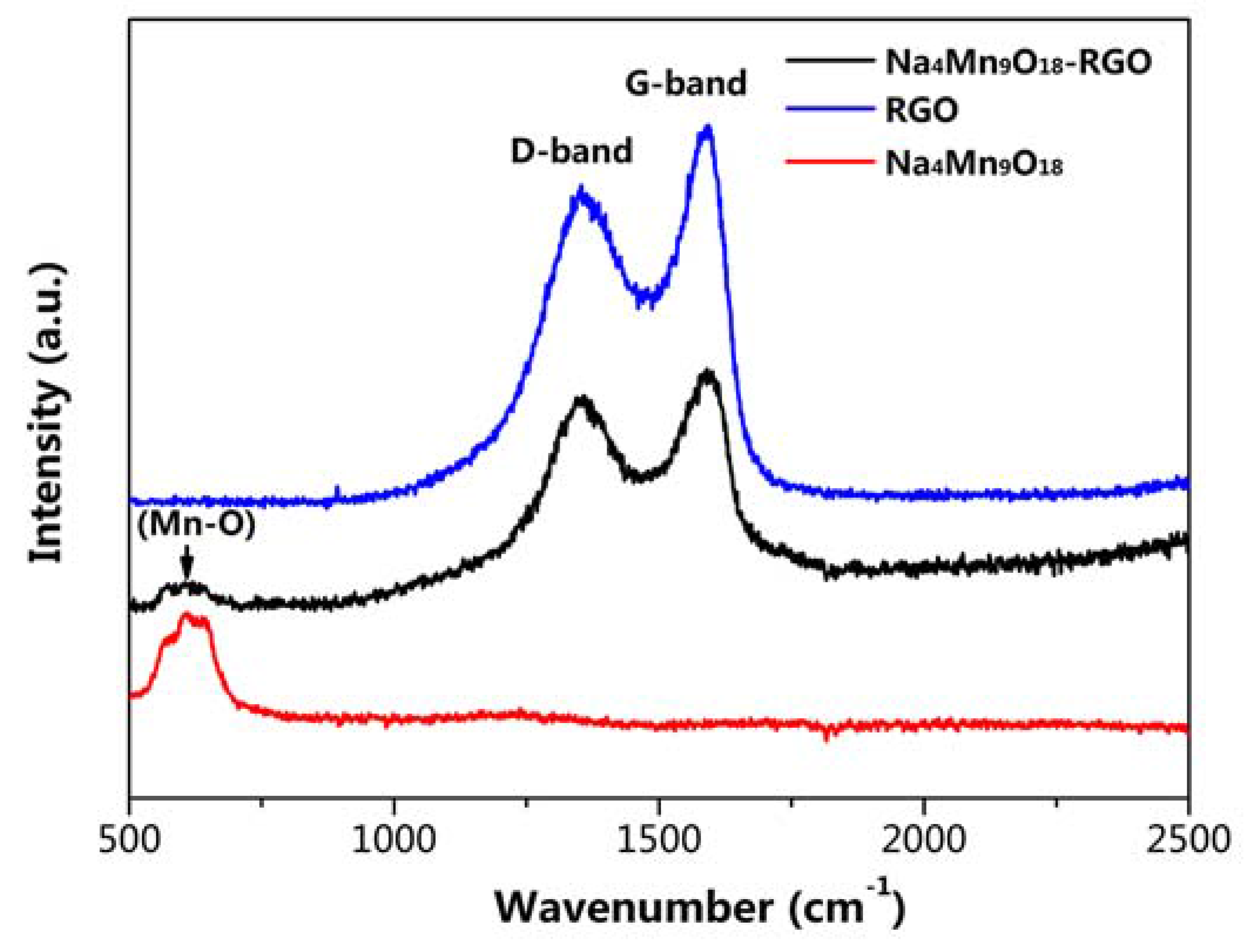
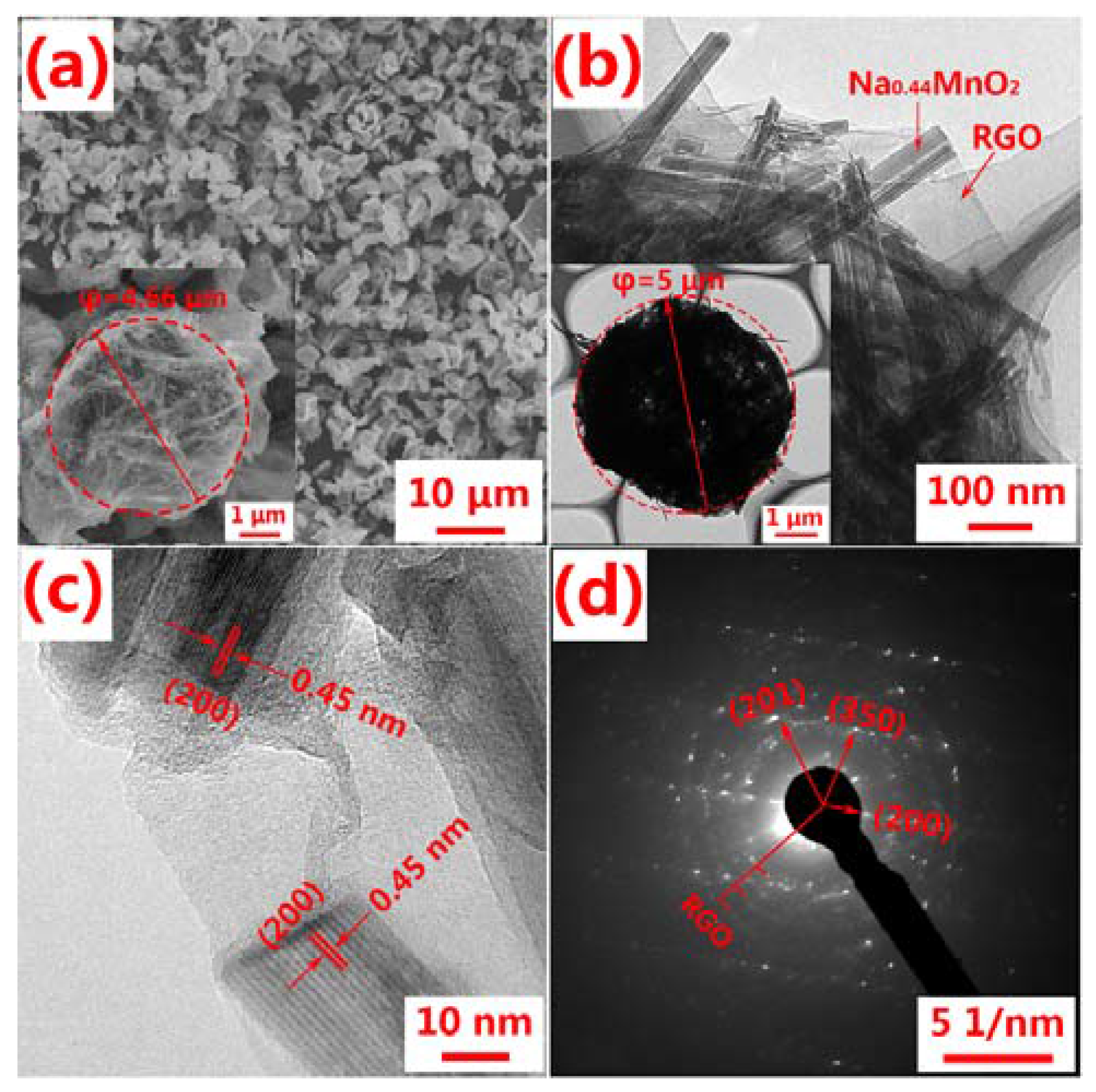
2.3. Materials Characterization
2.4. Electrochemical Measurements
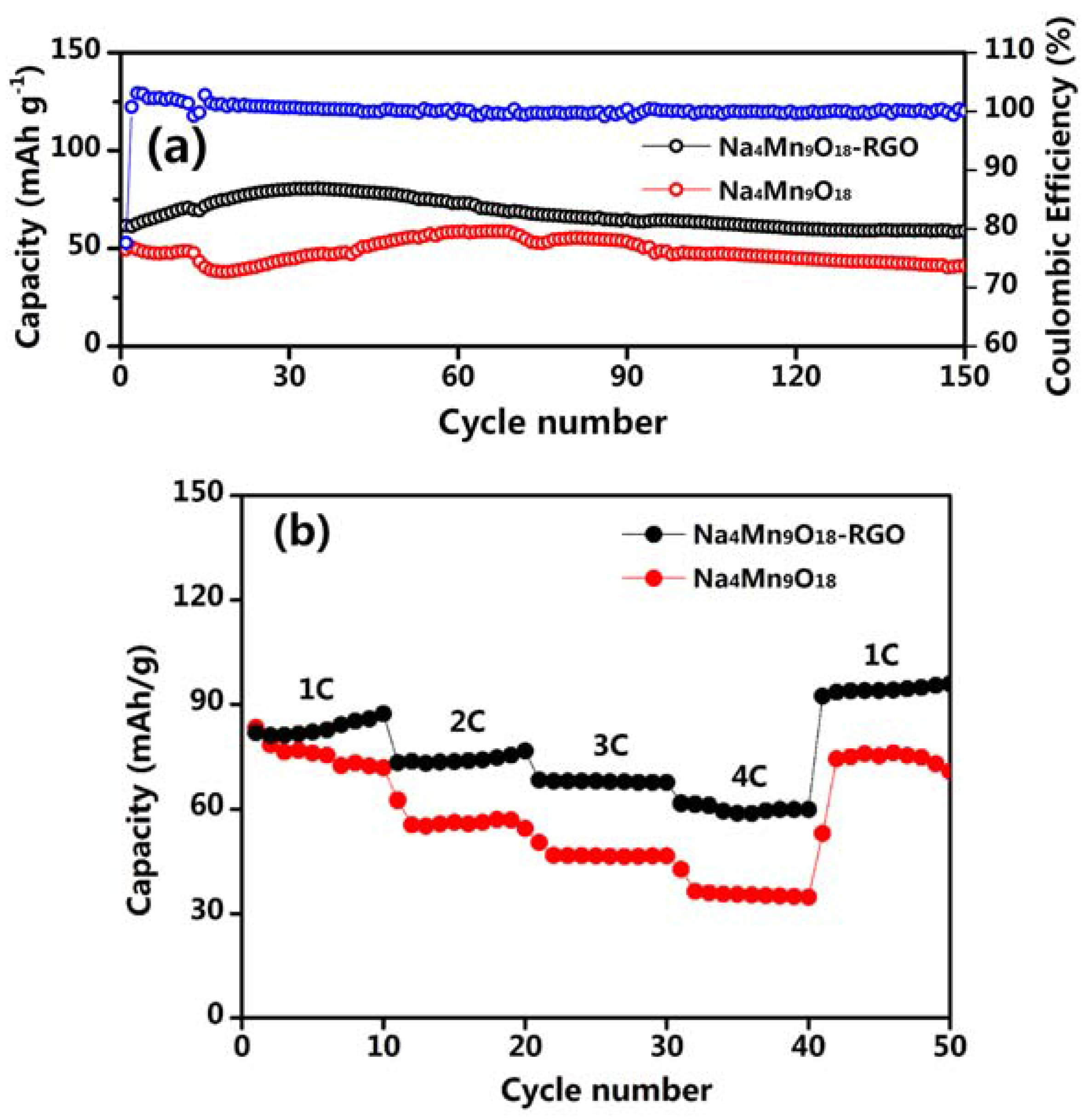
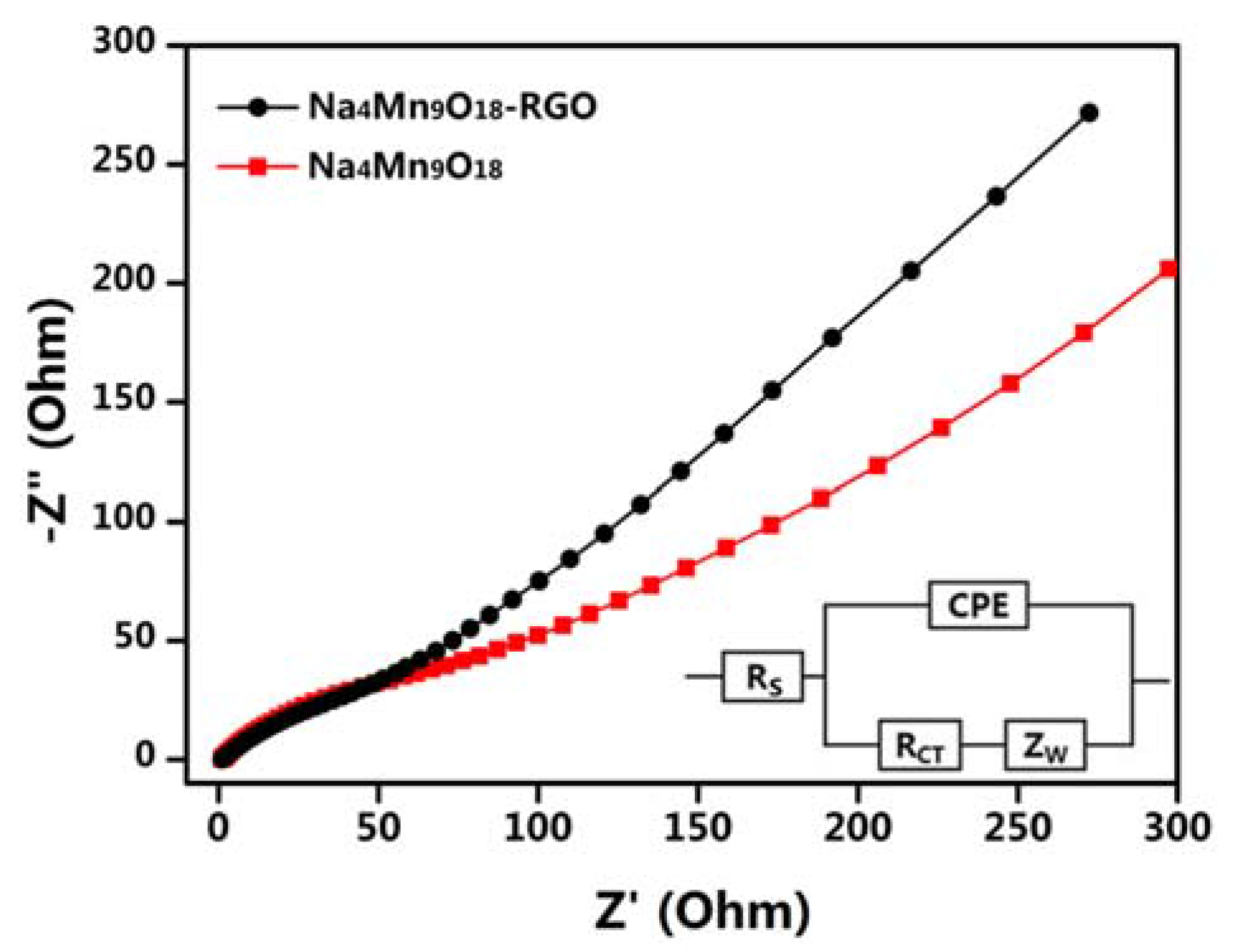
3. Result and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, B.; Liu, Y.; Wu, X.; Yang, Y.; Chang, Z.; Wen, Z.; Wu, Y. Cheminform abstract: An aqueous rechargeable battery based on zinc anode and Na0.95MnO2. Chem. Commun. 2014, 45, 1209–1211. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Young, D.; Xiang, K.; Carter, W.C.; Chiang, Y.M. Towards high power high energy aqueous sodium-ion batteries: The NaTi2(PO4)3/Na0.44MnO2 System. Adv. Energy Mater. 2013, 3, 290–294. [Google Scholar] [CrossRef]
- Li, S.; Dong, Y.; Xu, L.; Xu, X.; He, L.; Mai, L. Effect of carbon matrix dimensions on the electrochemical properties of Na3V2(PO4)3 nanograins for high-performance symmetric sodium-ion batteries. Adv. Mater. 2014, 26, 3545–3553. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.Y.; Kim, Y.; Park, Y.; Choi, A.; Choi, N.S.; Lee, K.T. ChemInform Abstract: Charge Carriers in Rechargeable Batteries: Na Ions vs. Li Ions. Energy Environ. Sci. 2014, 45, 2067–2081. [Google Scholar] [CrossRef]
- Cao, Y.; Xiao, L.; Wang, W.; Choi, D.; Nie, Z.; Yu, J.; Saraf, L.V.; Yang, Z.; Liu, J. Reversible sodium ion insertion in single crystalline manganese oxide nanowires with long cycle life. Adv. Mater. 2011, 23, 3155–3160. [Google Scholar] [CrossRef] [PubMed]
- Minakshi, M.; Meyrick, D.; Appadoo, D. Maricite (NaMn1/3Ni1/3Co1/3PO4)/activated carbon: Hybrid capacitor. Energy Fuel 2013, 27, 3516–3522. [Google Scholar] [CrossRef]
- Minakshi, M. Looking beyond lithium-ion technology-aqueous NaOH battery. Mater. Sci. Eng. B 2012, 177, 1788–1792. [Google Scholar] [CrossRef]
- Liu, S.; Fan, C.Z.; Zhang, Y.; Li, C.H.; You, X.Z. Low-temperature synthesis of Na2Mn5O10 for supercapacitor applications. J. Power Sources 2011, 196, 10502–10506. [Google Scholar] [CrossRef]
- Datta, M.K.; Kuruba, R.; Jampani, P.H.; Chung, S.J.; Saha, P.; Epur, R.; Kadakia, K.; Patel, P.; Gattu, B.; Manivannan, A. Electrochemical properties of a new nanocrystalline NaMn2O4 cathode for rechargeable sodium ion batteries. Mater. Sci. Eng. B-Adv. 2014, 188, 1–7. [Google Scholar] [CrossRef]
- Saint, J.A.; Doeff, M.M.; Wilcox, J. Electrode materials with the Na0.44MnO2 structure: Effect of titanium substitution on physical and electrochemical properties. Chem. Mater. 2008, 20, 3404–3411. [Google Scholar] [CrossRef]
- Whitacre, J.F.; Tevar, A.; Sharma, S. Na4Mn9O18 as a positive electrode material for an aqueous electrolyte sodium-ion energy storage device. Electrochem. Commun. 2010, 12, 463–466. [Google Scholar] [CrossRef]
- Fu, B.; Zhou, X.; Wang, Y. High-rate performance electrospun Na0.44MnO2 nanofibers as cathode material for sodium-ion batteries. J. Power Sources 2016, 310, 102–108. [Google Scholar] [CrossRef]
- Zhao, L.; Ni, J.; Wang, H.; Gao, L. Na0.44MnO2-CNT electrodes for non-aqueous sodium batteries. RSC. Adv. 2013, 3, 6650–6655. [Google Scholar] [CrossRef]
- Yan, J.; Wang, J.; Liu, H.; Bakenov, Z.; Gosselink, D.; Chen, P. Rechargeable hybrid aqueous batteries. J. Power Sources 2012, 216, 222–226. [Google Scholar] [CrossRef]
- Lee, J.S.; Sun, T.K.; Cao, R.; Choi, N.S.; Liu, M.; Lee, K.T.; Cho, J. Metal-air batteries: Metal-air batteries with high energy density: Li-air versus Zn-air. Adv. Energy Mater. 2011, 1, 34–50. [Google Scholar] [CrossRef]
- Minakshi, M.; Mitchell, D.R.G.; Prince, K. Incorporation of TiB2 additive into MnO2 cathode and its influence on rechargeability in an aqueous battery system. Solid State Ion. 2008, 179, 355–361. [Google Scholar] [CrossRef]
- Minakshi, M.; Pandey, A.; Blackford, M.; Ionescu, M. Effect of TiS2 additive on LiMnPO4 cathode in aqueous solutions. Energy Fuel 2010, 24, 6193–6197. [Google Scholar] [CrossRef]
- Zou, H.; Li, S.; Wu, X.; Mcdonald, M.J.; Yang, Y. Spray-drying synthesis of pure Na2CoPO4F as cathode material for sodium ion batteries. ECS Electrochem. Lett. 2015, 4, A53–A55. [Google Scholar] [CrossRef]
- Wu, F.; Wang, Z.; Li, X.; Guo, H.; Peng, Y.; Xiong, X.; He, Z.; Zhang, Q. Characterization of spherical-shaped Li4Ti5O12 prepared by spray drying. Electrochim. Acta 2012, 78, 331–339. [Google Scholar] [CrossRef]
- Liu, C.; Guo, W.L.; Wang, Q.H.; Li, J.G.; Yang, X.P. Parametric study of hydrothermal soft chemical synthesis and application of Na0.44MnO2 nanorods for Li-ion battery cathode materials: Synthesis conditions and electrochemical performance. J. Alloys Compd. 2016, 658, 588–594. [Google Scholar] [CrossRef]
- Guerrero-Contreras, J.; Caballero-Briones, F. Graphene oxide powders with different oxidation degree, prepared by synthesis variations of the Hummers method. Mater. Chem. Phys. 2015, 153, 209–220. [Google Scholar] [CrossRef]
- Ju, S.H.; Yun, C.K. Effects of drying control chemical additive on properties of Li4Ti5O12 negative powders prepared by spray pyrolysis. J. Power Sources 2010, 195, 4327–4331. [Google Scholar] [CrossRef]
- Bai, S.; Song, J.; Wen, Y.; Cheng, J.; Cao, G.; Yang, Y.; Li, D. Effects of zinc and manganese ions in aqueous electrolytes on structure and electrochemical performance of Na0.44MnO2 cathode material. RSC Adv. 2016, 6, 40793–40798. [Google Scholar] [CrossRef]
- Yesibolati, N.; Umirov, N.; Koishybay, A.; Omarova, M.; Kurmanbayeva, I.; Zhang, Y.; Zhao, Y.; Bakenov, Z. High performance Zn/LiFePO4 aqueous rechargeable battery for large scale applications. Electrochim. Acta 2015, 152, 505–511. [Google Scholar] [CrossRef]
- Dong, J.K.; Ponraj, R.; Kannan, A.G.; Lee, H.W.; Fathi, R.; Ruffo, R.; Mari, C.M.; Kim, D.K. Diffusion behavior of sodium ions in Na0.44MnO2 in aqueous and non-aqueous electrolytes. J. Power Sources 2013, 244, 758–763. [Google Scholar]
- Liu, C.; Li, J.; Zhao, P.; Guo, W.; Yang, X. Fast preparation of Na0.44MnO2 nanorods via a high NaOH concentration hydrothermal soft chemical reaction and their lithium storage properties. J. Nanopart. Res. 2015, 17, 142. [Google Scholar] [CrossRef]
- Veerappan, G.; SunyoungYoo; Zhang, K.; Ma, M.; Kang, B.; Park, J.H. High-reversible capacity of perovskite BaSnO3/rGO composite for lithium-ion battery anodes. Electrochim. Acta 2016, 214, 31–37. [Google Scholar] [CrossRef]
- Bharathidasan, P.; Kim, D.W.; Devaraj, S.; Sivakkumar, S.R. Supercapacitive characteristics of carbon-based graphene composites. Electrochim. Acta 2016, 204, 146–153. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Jorio, A.; Hofmann, M.; Dresselhaus, G.; Saito, R. Perspectives on carbon nanotubes and graphene raman spectroscopy. Nano Lett. 2010, 10, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, D.; Wu, H.; Duan, J.; Zhang, Y. Hydrothermal self-assembly of manganese dioxide/manganese carbonate/reduced graphene oxide aerogel for asymmetric supercapacitors. Electrochim. Acta 2015, 164, 154–162. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, F.; Liu, Z.; Zhao, Y.; Feng, Y.; Zhang, Y. Electrochemical Properties of an Na4Mn9O18-Reduced Graphene Oxide Composite Synthesized via Spray Drying for an Aqueous Sodium-Ion Battery. Nanomaterials 2017, 7, 253. https://doi.org/10.3390/nano7090253
Yin F, Liu Z, Zhao Y, Feng Y, Zhang Y. Electrochemical Properties of an Na4Mn9O18-Reduced Graphene Oxide Composite Synthesized via Spray Drying for an Aqueous Sodium-Ion Battery. Nanomaterials. 2017; 7(9):253. https://doi.org/10.3390/nano7090253
Chicago/Turabian StyleYin, Fuxing, Zhengjun Liu, Yan Zhao, Yuting Feng, and Yongguang Zhang. 2017. "Electrochemical Properties of an Na4Mn9O18-Reduced Graphene Oxide Composite Synthesized via Spray Drying for an Aqueous Sodium-Ion Battery" Nanomaterials 7, no. 9: 253. https://doi.org/10.3390/nano7090253



