4.2.1. Compressive Strength
The characteristic for cement composites is that the smaller
w/
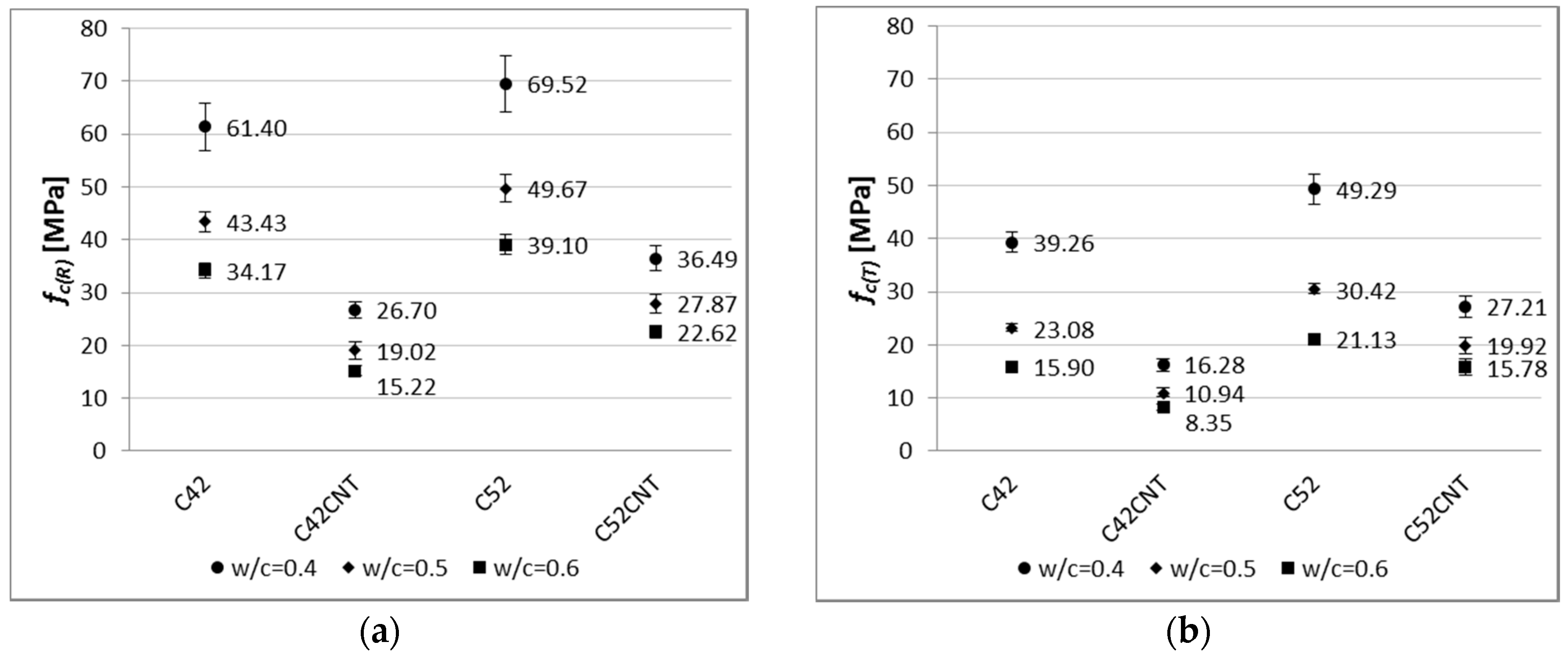
c ratio, the higher values of mechanical parameters. This has been confirmed in this study, for both the reference and post-temperature samples (
Figure 8). The reference samples with
w/
c = 0.5 and 0.4 achieved higher
fc(R) values by an average of 26% and 75%, respectively, than samples with
w/
c = 0.6. Considering this dependence after thermal shock, these differences are even greater—38% and 116%, respectively.
By analyzing the results of compressive strength in the cement used criterion, it was found that the higher values (both before and after the influence of temperature) were obtained by samples made of the higher class cement, i.e., CEM I 52.5R (series C52 and C52CNT). The effect of the cement’s class is more visible for the CNT material, as the C52CNT samples have reached higher fc(R) and fc(T) values by an average of 43% and 77%, respectively, than the series C42CNT. On the other hand, the above relationship for the classical cement paste (C52 compared to C42) is much lower—14% and 29%, respectively.
The experience of other researchers indicates that the introduction of additional porosity into the material’s volume is beneficial, in terms of some physical properties, e.g., density, heat transfer coefficient, capillary rise. However, the presence of extra pores in the material, which cause discontinuity of the structure, usually results in a deterioration in mechanical properties [
49,
50,
51]. It is similar in the case of the cement pastes tested, in which series C42CNT reached 56% and 55% of compressive strength of the series C42, for
fc(R) and
fc(T), respectively. For the series in which CEM I 52.5R was used (C52CNT compared to C52), these differences are equal to 45% and 38%, respectively. The negative strength effect is reduced with the use of the higher class cement. This is a valuable guideline for practical use of the CNT cement matrix with SDS.
By analyzing the decrease in compressive strength (
Table 3) after thermal load, it was noted that with the increase in the
w/
c ratio, the degree of material degradation also increased. These results confirm the generally well-known fact that a cement matrix with a higher compressive strength is more resistant to elevated temperatures (also thermal shock).
It is noteworthy that smaller decreases in compressive strength were observed for the CNT samples. This phenomenon is especially visible for the series made of CEM I 52.5R; the
fc decrease for the series C52 was in the range of 29–46%, and for the series C52CNT, 25–30%. This indicates the positive effect of the CNT on the mechanical strength of cement matrix under the influence of elevated temperature, despite the increased porosity of the material. CNTs (well dispersed in a cement matrix) may form bridges between hydrates, which result in an increase in local stiffness of the CSH phase [
21,
22]. Also, other phenomenon occur, such as crack bridging [
52,
53,
54], delay in micro crack propagation [
52], and chemical binding of hydrated phases [
23,
54,
55]. Thus, all the above mentioned mechanisms may result in an increase in the coherence of the cementitious material subjected to thermal shock.
The use of the above described method of the temperature loading caused the formation of thermal cracks due to the volume deformation of sample—in the heating phase, the thermal expansion of the material; in the cooling phase, the shrinkage. The steam pressure created in the pores and capillaries of cement paste causes crack propagation, and transformation of the technological cracks that are present in the structure of the material before applying service loads, into macro-cracks visible on the sample’s surface. In this way, the cement paste structure defects were extracted without excessive deterioration of the mechanical properties of the tested material.
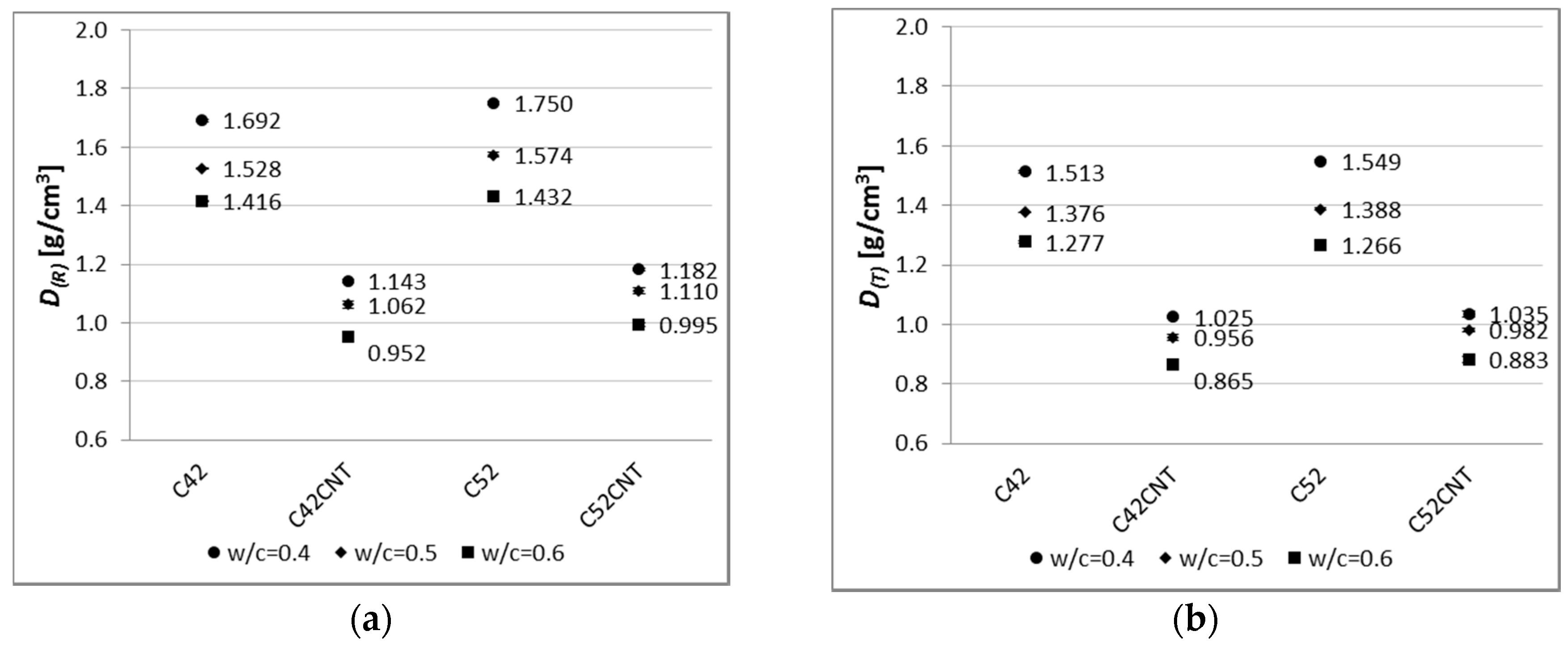
A relationship between compressive strength and apparent density can be observed (
Figure 15). This dependence was described by linear regression equations, separately for the classical cement pastes (C42, C52) and the cement matrix containing CNT (C42CNT, C52CNT). The determination coefficient (
R2) of the equation describing the relationship for the classical cement pastes was equal to 0.98 and 0.91, respectively, for the reference and heat-treated samples. It means that the applied model of regression covers more than 90% of achieved data. For the cement pastes with CNT, the
R2 was equal to 0.78 and 0.52. In the case of the reference samples, the good fit of the regression line to the empirical data is observed. The
R2 value for samples after thermal load is not very high, but it should be kept in mind that the equation describes the dependence on materials made of two different cements.
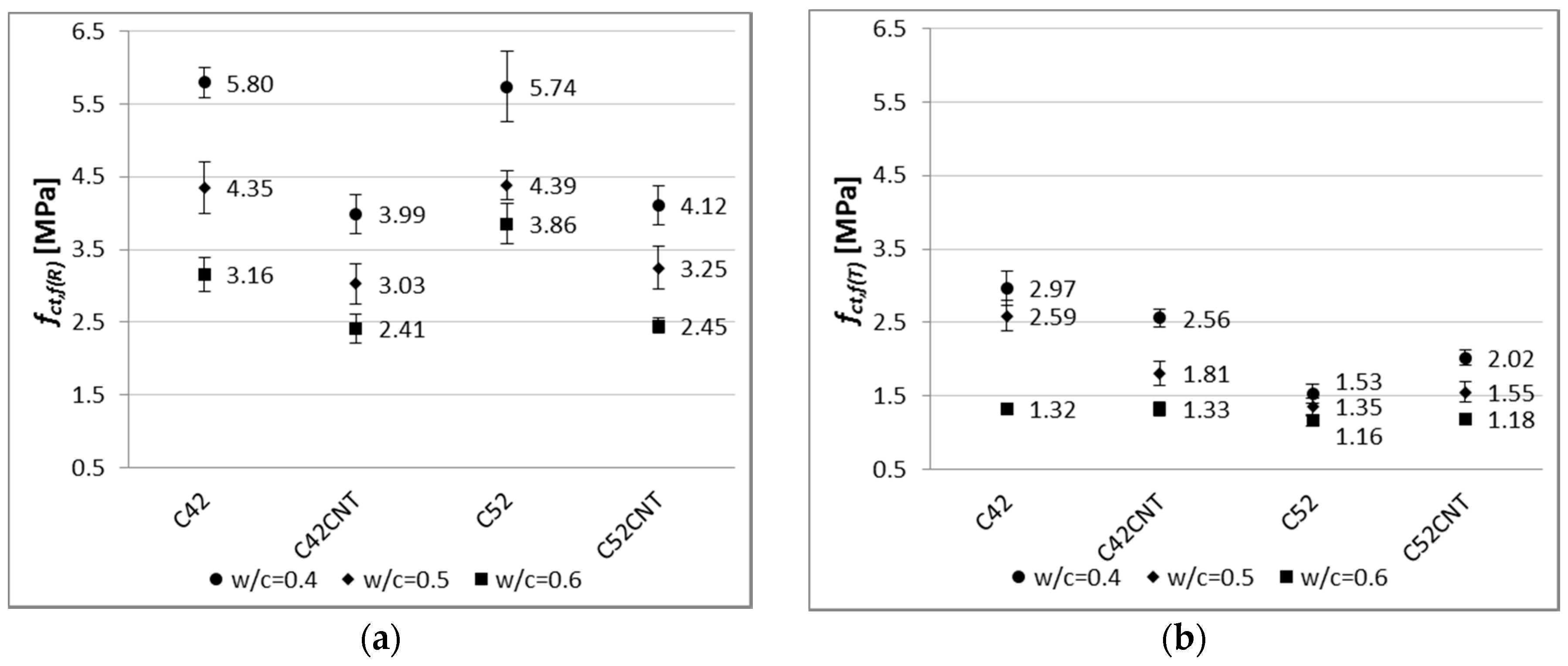
4.2.2. Flexural Strength
As with compressive strength, one of the major factors which shapes bending tensile strength, is the
w/
c ratio. Based on the results shown in
Figure 9, the cement pastes with
w/
c = 0.5 obtained higher
fct,f(R) and
fct,f(T) values by an average of 26% and 46%, respectively, than samples with
w/
c = 0.6. The same relation between samples, both for
w/
c = 0.4 and 0.6; 65% and 82%. Clearly, the difference between the results obtained is higher for the heat-treated samples, in terms of
w/
c. Cement paste with
w/
c = 0.6 contains the most water, which results in the highest vapor pressure during heating. This phenomenon, combined with the thermal deformation of the material, results in a greater decrease in the thermal shock resistance with the higher
w/
c ratio.
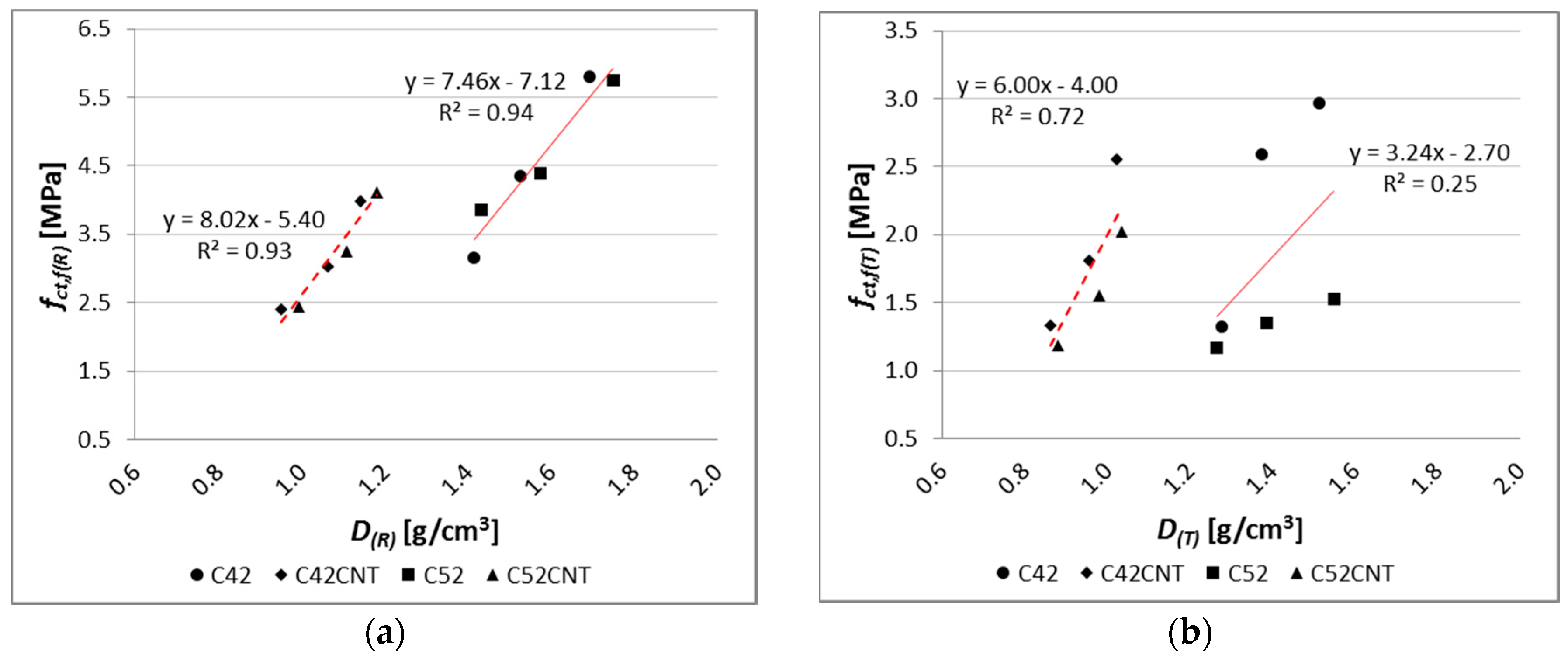
The differences in fct,f(R) between samples with and without CNTs are less than in the case of fc(R). The bending tensile strength of the reference samples of series C52CNT and C42CNT is reduced by approximately 30%, in comparison to the series C52 and C42. It should be remembered that the CNT cement pastes have much higher porosity than conventional cement matrix, which is the main cause of reduced strength.
On the other hand, after exposure to the elevated temperature, particularly in the case of samples containing CEM I 52.5R, the C52CNT samples had higher fct,f(T) values, by an average of 18%, than series C52. The beneficial effect of using CNTs is larger, the lower the w/c ratio is. It can be stated, as in the case of fc, that the CNT forms mechanical connections with the cement hydration products in the material’s nano-structure. This translates into increased cohesion of cement paste in the aspect of its stretching. It is so important that when using the CNT application method, the foam effect was achieved, which translates into a significant reduction in weight of the cementitious material.
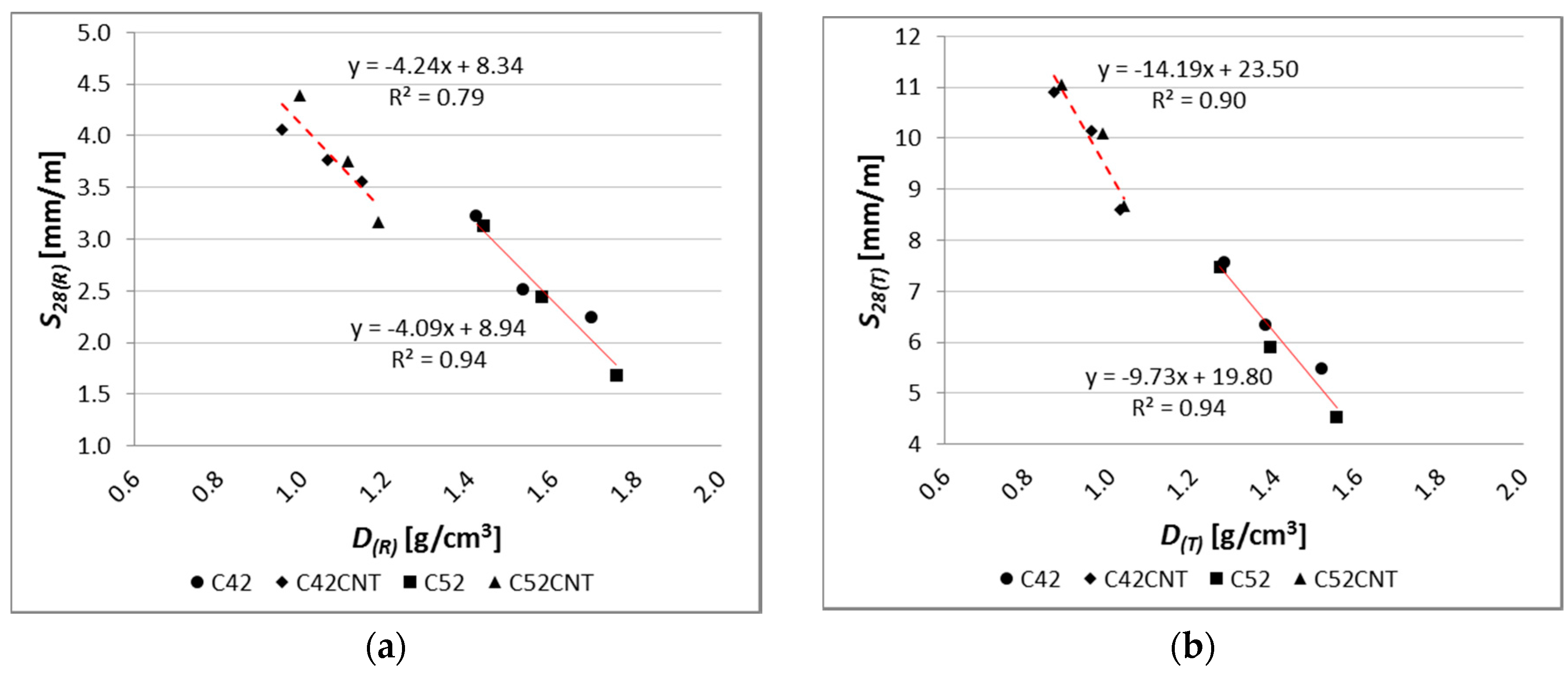
There is a very strong relationship between bending tensile strength and apparent density of the modified cement pastes (
Figure 16), but only for the reference samples. The coefficient of determination of the linear regression equation reaches a very high value, equal to 0.93 and 0.94, respectively, for pastes with and without CNT. On the other hand, after the influence of the elevated temperature, the regression model no longer reflects empirical data in such a good way (
R2 = 0.72 for the CNT matrix,
R2 = 0.25 for the classical cement paste). In addition, on the
fct,f and
D graph, the main advantage of the CNT cementitious material is visible—the reduction in weight without loss of bending tensile strength (mainly after the influence of the elevated temperature).
By analyzing the results shown in
Figure 9, it can be observed that the class of cement has very little effect on the
fct,f(R) results. The cement pastes made of CEM I 52.5R reached higher
fct,f(R) values, only by 5%, than the samples with CEM I 42.5R. The effect of the cement’s class is much higher in the case of cementitious materials with aggregate (mortars and concretes), because the resultant mechanical strength of the material is also determined by the interfacial transition zone (ITZ). In the case of presence of aggregate in the material, the strength of ITZ depends to a large extent on the cement’s class [
56].
The effect of the cement’s class is clearly visible after the thermal load. Samples made of the cement of higher class (CEM I 52.5R) achieved lower
fct,f(T) values by an average of 30% from the CEM I 42.5R specimens. This is due to the material’s fragility. As the compressive strength of cement composite increases, the material’s fragility also increases. Fragility, as a physical property of a material, consists in its cracking under the influence of external forces. Materials that are fragile absorb relatively little energy before cracking. The fragility [
1] of cement matrix is a ratio of bending tensile strength (
fct,f) to compressive strength (
fc). Then, the fragile material is such, for which the ratio is less than 0.125.
Results in
Table 5 confirms that cement pastes with the highest compressive strength are usually the most fragile (
w/
c = 0.4). The class of cement has a much greater effect on the fragility than, for example, the
w/
c ratio. For example, the fragility of the reference samples made of CEM I 52.5R is higher by an average of 19%, than cement pastes made of CEM I 42.5R. After the temperature load, this difference is over 2.5 times bigger, and is equal to 52%.
The presence of CNTs in the cement matrix has reduced fragility both before and after exposure to the elevated temperature by 45% and 77%, respectively. As the length to width ratio of the CNTs applied is significant (length 1500 times larger than diameter), the CNT anchorage in the hydrates is locally large in length. It is likely that during tensile stress growth in the cementitious material, the CNTs slide in the anchor, which results in the change of fragility of the CNT pastes towards the materials with plastic properties [
21,
22]. The CNTs’ tensile strength is several tens of thousands of times greater than the cement paste, so it is not possible to break CNTs at the stresses present in cement matrix.
When analyzing the decrease in bending tensile strength (
Table 3) after temperature load, no strict relationship between this parameter and the
w/
c ratio was observed.
As in the case of fc, the increased resistance of the CNT pastes to the thermal shock was observed because the decrease of fct,f was in the range of 36–52%, and for the samples of the classical cement paste, 40–73%. Smaller losses in bending tensile strength showed samples made of CEM I 42.5R, which is indirectly related to the fragility phenomenon described above.