1. Introduction
Titanium dioxide (TiO
2) can act as a photosensitizer and is known to generate reactive oxygen species (ROS), including OH and HO
2 radicals, superoxide anions (O
2−), hydrogen peroxide (H
2O
2), and
1O
2, under ultraviolet (UV) irradiation (less than 390 nm) [
1,
2,
3,
4]. UV-irradiated TiO
2 nanoparticles (NPs) have shown a cell-killing effect toward HeLa cells [
5]. However, the clinical use of TiO
2 NPs is hampered because UV light cannot deeply penetrate human tissue, and TiO
2 NPs have poor dispersion stability at physiological pH [
6,
7]. Shimizu et al. found that TiO
2 generates ROS under ultrasound irradiation (39 kHz) [
8], although the ultrasound frequency (39 kHz) was too low for clinical applications. Additionally, sonicating TiO
2 NPs at a clinically appropriate frequency that allows deep body invasion (1 MHz) also showed an effective decrease in cell viability and inhibited tumor growth in vivo when TiO
2 NP suspension was directly injected into tumor [
9]. This indicated the availability of TiO
2 NPs in sonodynamic therapy (SDT) and showed that developing a carrier system that could deliver TiO
2 NPs into cells by improving their dispersion stability under physiological conditions was required for effective SDT.
We have focused on the charge properties of surface OH groups on TiO
2 NPs. The isoelectric point of TiO
2 NPs with an anatase crystal structure is 6.2, meaning that TiO
2 NPs are negatively charged at neutral pH [
10]. Polyion complex (PIC) micelles incorporating TiO
2 NPs (TiO
2 NP-PIC micelles) were successfully prepared using polyallylamine bearing poly(ethylene glycol) grafts (PAA-g-PEG) [
11], in which the micelles were formed through electrostatic interaction as a driving force, and van der Waals force and hydrophobic interaction were also stabilized as a result of polyion complex formation. Although bare TiO
2 NPs have poor solubility against water at physiological pH, the incorporation of TiO
2 NPs into the micelles provided a remarkable improvement in dispersion stability. It was confirmed that ultrasound irradiation to HeLa cells treated by TiO
2 NP-PIC micelles induced a decrease in cell viability through
1O
2 generation. The cell viability decreased as irradiation time increased. When other irradiation conditions were kept constant, the decrease in cell viability was dependent on irradiation time. Furthermore, this decrease in cell viability was completely inhibited by the presence of glutathione, which is a radical scavenger, demonstrating that the cell-killing effect was due to ROS generated by ultrasound irradiation of the TiO
2 NP-PIC micelles. In this study, we evaluated the effects of polymer composition and sonication time on the cell-killing effect of the TiO
2 NP-PIC micelles. Furthermore, it was confirmed that the cell-killing effect of the TiO
2 NP-PIC micelles was induced by apoptotic cell death.
2. Results and Discussion
TiO
2 NP-PIC micelles were prepared using four types of PAA-g-PEG bearing PEGs of different molecular weights (Mn = 2000 and 5000) and grafting densities (13 and 26 mol % for PEG2000, and 12 and 21 mol % for PEG5000), named 2k13, 2k26, 5k12, and 5k21, respectively. The mean diameter, polydispersity index, zeta potential, and composition (polymer/TiO
2 w/
w) were determined, as summarized in
Table 1. For the mean diameter and zeta-potential, it was difficult to compare with bare TiO
2 NPs due to their poor dispersion stability at physiological pH. The compositions were controlled by the molecular weight (Mn) of the PEG grafts, with PEG grafts of Mn 2000 and 5000 giving polymer/TiO
2 w/
w ratios of 2 and 4, respectively. The mean diameter tended to grow large, such that the PEG graft Mn was also large and the PEG graft content was high. Importantly, all prepared TiO
2 NP-PIC micelles had almost neutral zeta potentials, suggesting that electrically neutral PEG grafts surrounded the micellar surface.
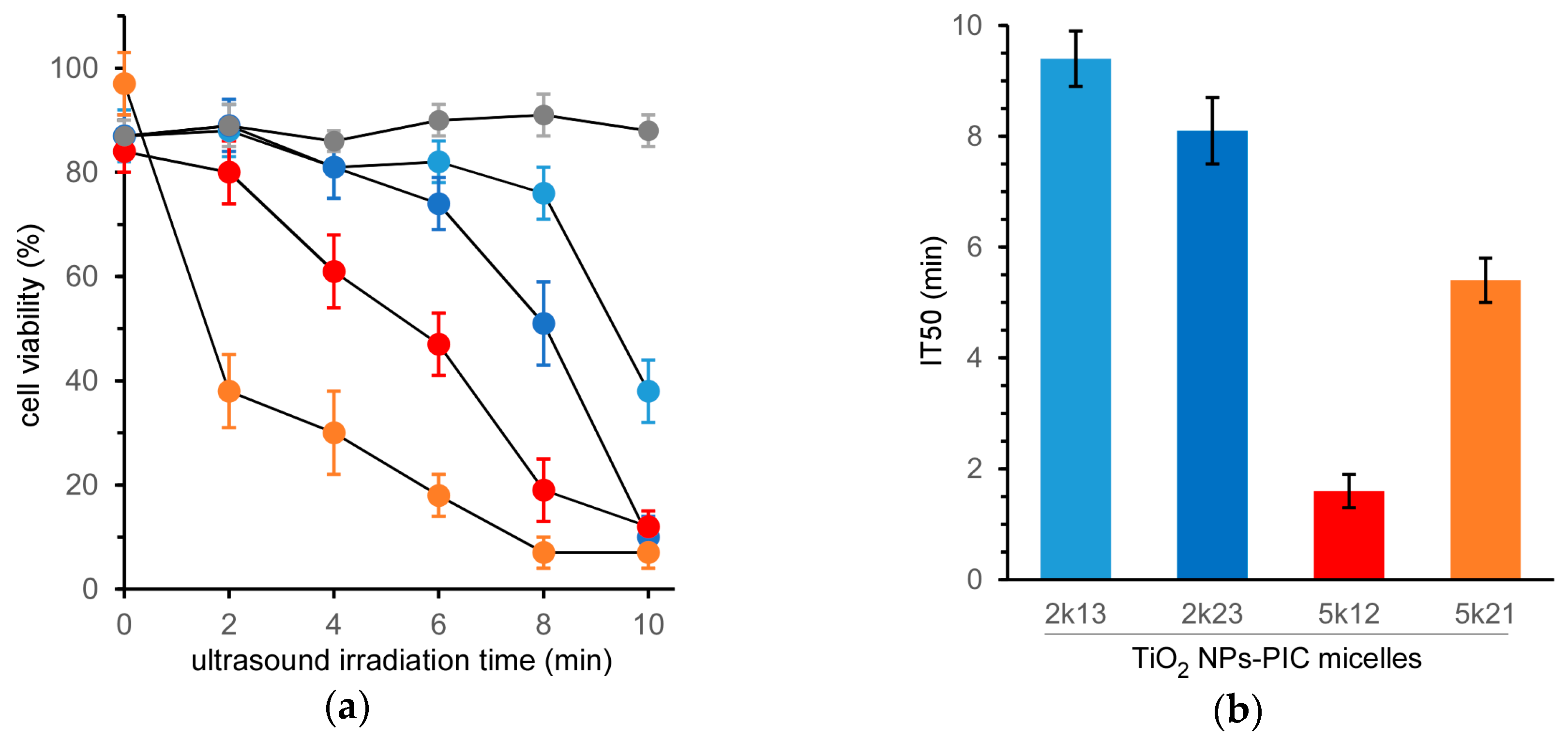
The cell-killing effect of the TiO
2 NP-PIC micelles toward HeLa cells was evaluated by MTT (3-(4,5-di-methylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay at various ultrasound irradiation times (
Figure 1a). Micelles without ultrasound irradiation (ultrasound irradiation time = 0) maintained high cell viability and demonstrated negligible cytotoxicity. Prolonging ultrasound irradiation induced a decrease in cell viability, showing an obvious difference in the cell-killing effect among the micelles. Additionally, ultrasound irradiation to the cells without the treatment of the TiO
2 NP-PIC micelles did not induce the decrease in cell viability as shown in
Figure 1a, although ultrasound irradiation to a solvent without TiO
2 NPs induce the generation of solvent radicals, i.e., H and OH radicals that can combine to give hydrogen and hydrogen peroxide in the case of water [
12,
13]. This suggests that the main cytotoxic species in ROS generated by ultrasound irradiation might be singlet oxygen. The half maximal inhibitory time of ultrasound irradiation (IT50) values, as an indication of the cell-killing effect under ultrasound irradiation, were determined from
Figure 1a for each micelle, as shown in
Figure 1b. There was a 5.7-fold difference between the IT50 values of the most effective 5k12 micelles and the least effective 2k13 micelles.
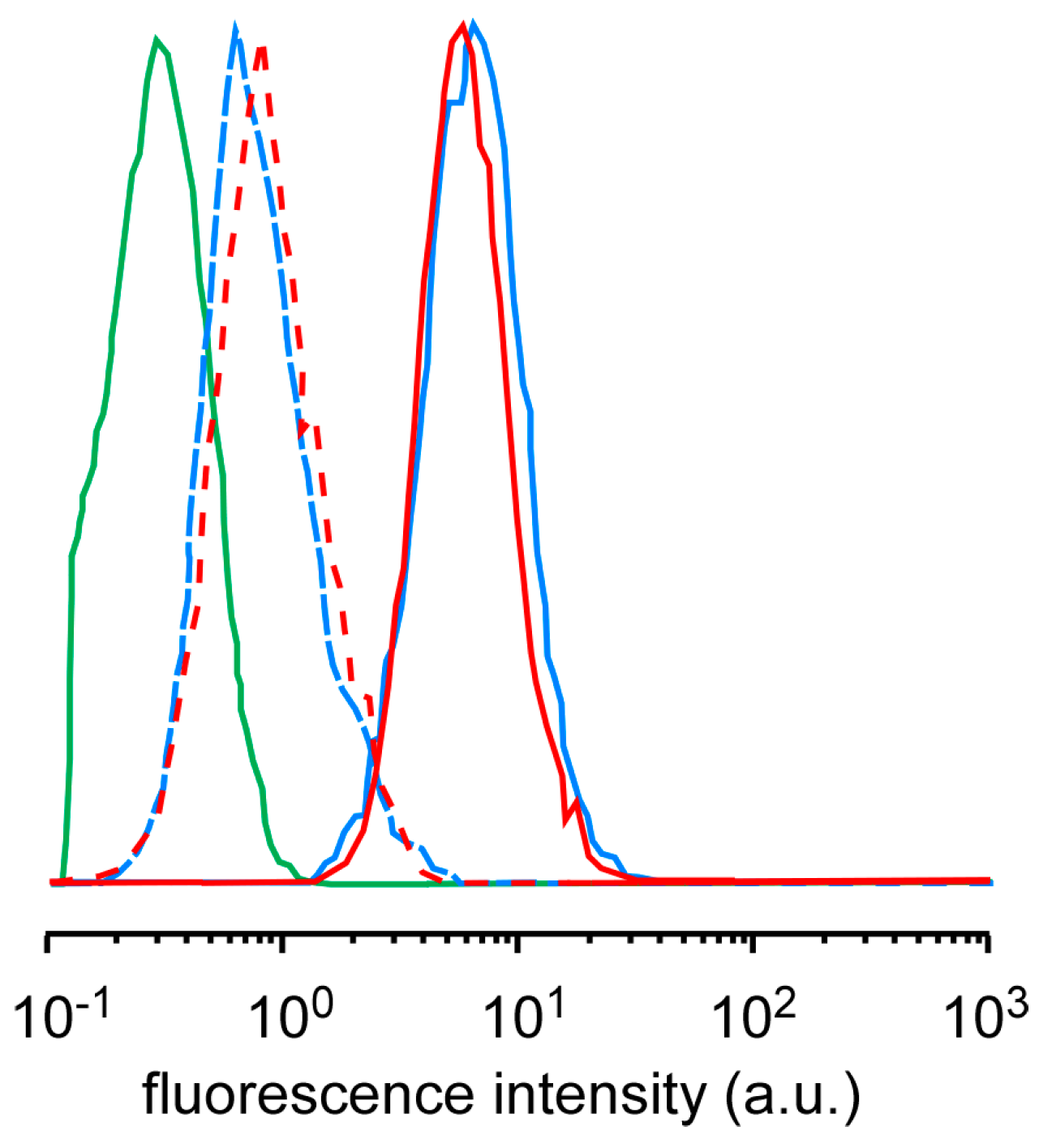
The difference in the cell-killing effect among the micelles might be due to the difference in ROS generation, cellular uptake, and intracellular distribution. Singlet oxygen sensor green (SOSG) has been used as a probe to confirm that the ultrasound irradiation of TiO
2 NP-PIC micelles increased the amount of
1O
2 generation in proportion to the irradiation time and the ultrasound power, and that there was no difference among the micelles [
11]. The amounts of
1O
2 generation for 5k12 micelles and 2k13 micelles were compared using SOSG by flow cytometry (
Figure 2). For both micelles, the fluorescence intensity of HeLa cells treated by the mixture of micelles and SOSG were slightly increased even without ultrasound irradiation, suggesting that the comparable amount of SOSG were taken up into the cells. The ultrasound irradiation to HeLa cells treated with the mixture of micelles and SOSG provided significant increase in fluorescence intensity, indicating that TiO
2 NP-PIC micelles could generate
1O
2 in the cells, and there was no difference in the fluorescence intensity between 5k12 micelles and 2k13 micelles. Furthermore, the cellular uptake of micelles was already evaluated by flow cytometry [
11]. The TiO
2 NPs uptake increased in an incubation time-dependent manner, suggesting that cellular uptake occurred via an endocytosis pathway, with no meaningful difference in TiO
2 NPs uptake among the micelles. Consequently, TiO
2 NP-PIC micelles could generate
1O
2 in the cells even after 24 hours of incubation with HeLa cells, and the generated amount of
1O
2, which is the main cytotoxic species among ROS, might be comparable among micelles. Therefore, to explain the difference in the cell-killing effect among the micelles, the intracellular distribution of the micelles, especially their distribution to mitochondria, was compared between the most effective 5k12 micelles and least effective 2k13 micelles using fluorescein 5-isothiocyanate (FITC)-labeled TiO
2 NPs. ROS damage to mitochondria is known to effectively induce cellular apoptosis [
14]. The intracellular distributions of the micelles were compared by laser scanning microscopy (
Figure 3), in which the mitochondria were stained and observed. Mitochondria, identified by red fluorescence, were widely distributed in the cytoplasm. For both micelles, green fluorescence dots were observed in the cytoplasm, with most green fluorescence overlapping with red fluorescence. However, it should be noted that 5k12 micelles were more widely distributed in the cytoplasm than the 2k13 micelles despite the comparable amount of TiO
2 NP-PIC micelle uptake into the cells. This meant that 5k12 micelles were likely to cause ROS damage to more mitochondria. The difference in the cell-killing effect among the micelles shown in
Figure 1 might be due to the difference in intracellular distribution of the TiO
2 NP-PIC micelles.
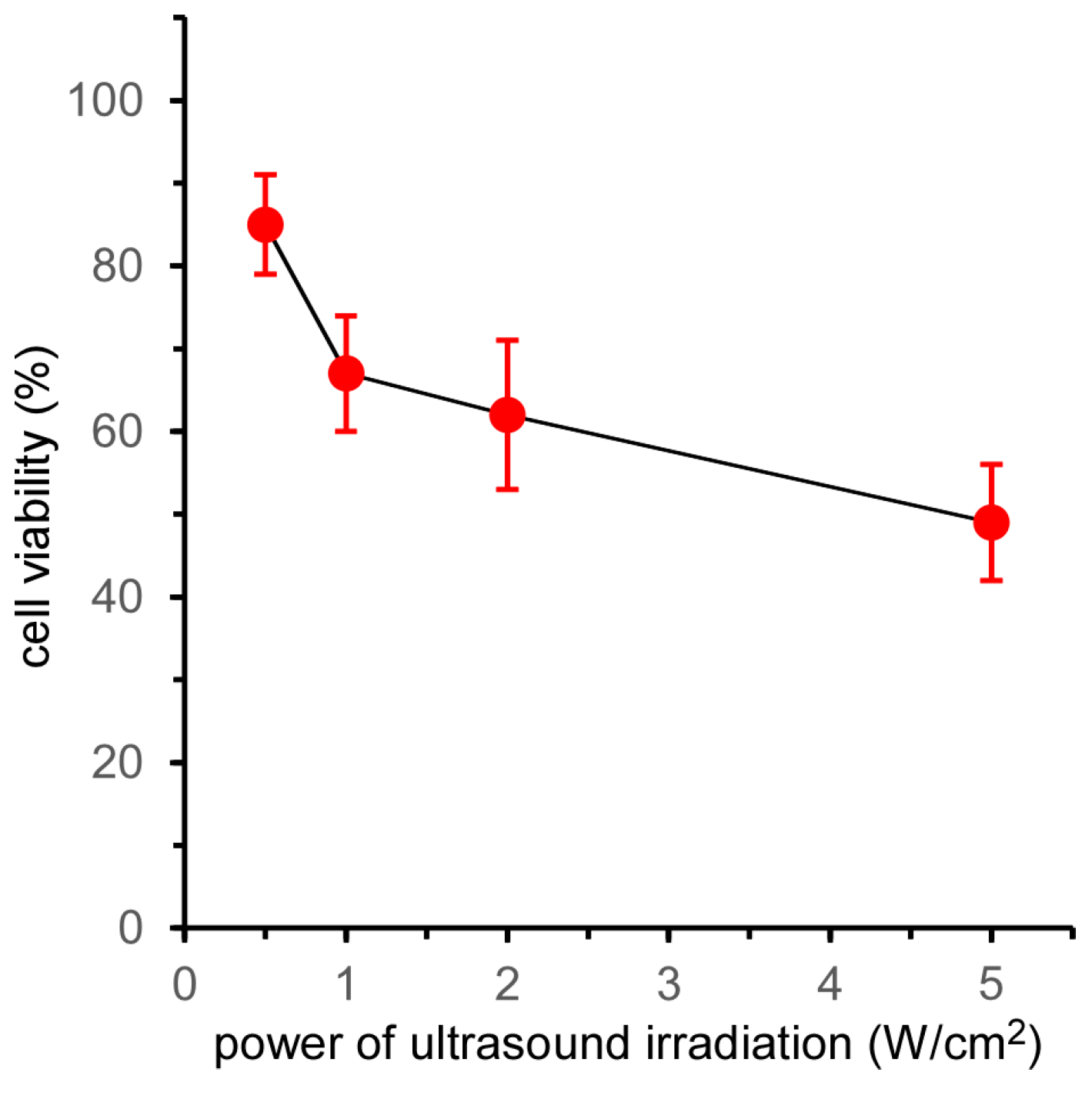
The effect of ultrasound power on the cell-killing effect of 5k21 micelles was evaluated by MTT assay (
Figure 4). The cell viability at a power of 0.5 W/cm
2 after 2 min of irradiation was the same as that shown in
Figure 1a. Increasing the ultrasound power resulted in a decrease in cell viability. However, the effect of ultrasound power was weak compared with that of irradiation time, with half the cells remaining alive at an ultrasound power of 5.0 W/cm
2. As described above, the amount of
1O
2 generated by ultrasound irradiation to TiO
2 NP-PIC micelles increased in proportion with both the irradiation time and the ultrasound power [
11]. By increasing the ultrasound power from 0.5 to 5.0 W/cm
2, the generated amount of
1O
2 increased 10-fold, but the cell viability decreased by approx. 50%. Furthermore, the cell viability in
Figure 4 stopped falling at approx. 50%, with little decrease in cell viability observed when further increasing the ultrasound power. In contrast, prolonging the irradiation time from 2 to 10 min increased the amount of
1O
2 generated five-fold and effectively decreased the cell viability to approx. 10%. These results indicated that prolonging the ultrasound irradiation time was more effective than increasing the ultrasound power to increase the sonodynamic therapeutic effect of TiO
2 NP-PIC micelles. The diffusion of the micelles in the cytoplasm might participate in this difference between the effects of irradiation time and power. Due to high reactivity, the lifetime and diffusion distance of ROS in the cytoplasm are 10–40 ns and 10–20 nm, respectively [
15], and these values were determined for ROS generated by photo-irradiation to photosensitizer. The reactivity of ROS is the same as those generated by ultrasound irradiation to TiO
2 NPs, and ROS generated by ultrasound irradiation to TiO
2 NP-PIC micelles inside the cells have comparable lifetime and diffusion distance of ROS generated by photo-irradiation to photosensitizer. Therefore, TiO
2 NP-PIC micelles can only damage the limited mitochondria existing near them during ultrasound irradiation. Accordingly, prolonging the irradiation time, which increases the diffusion area, might result in an increased cell-killing effect, while the increase in the ultrasound power might not be effective. This agreed with the results shown in
Figure 1 and
Figure 3, in which micelles distributed widely in the cytoplasm exhibited effective cell-killing.
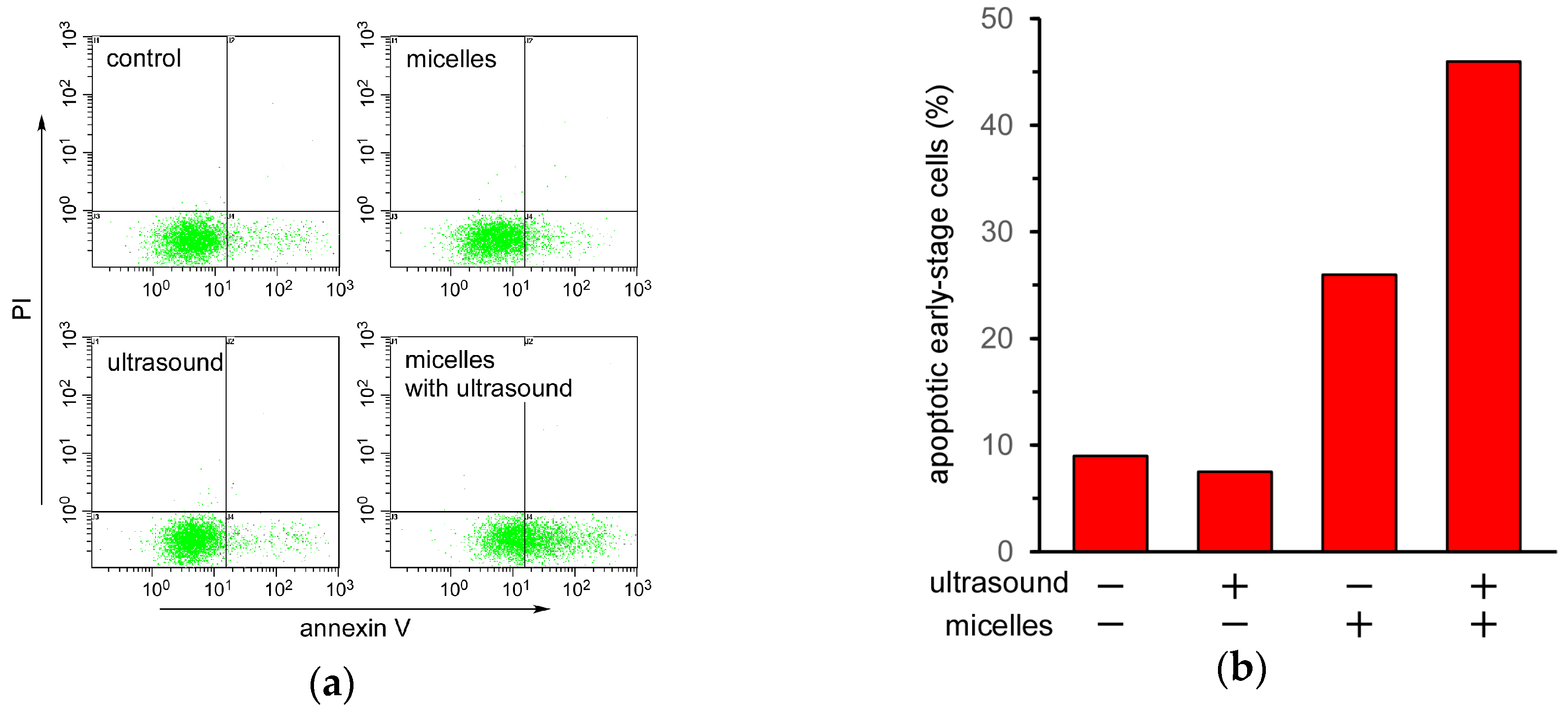
Finally, the mechanism of cell death induced by ultrasound irradiation of the TiO
2 NP-PIC micelles was evaluated by an annexin V and propidium iodide (PI) double staining assay using flow cytometry. In the case of apoptotic cells, their phospholipid membrane asymmetry is lost, leading to the exposure of phosphatidylserine (PS) at the cellular surface, a process that can be monitored using annexin V. Annexin V can identify apoptotic cells with the exposed PS, since annexin V is a Ca
2+-dependent phospholipid-binding protein with a high affinity for PS. The stage of apoptosis can be distinguished using both FITC-labeled annexin V and PI. At the late stage of apoptosis, the permeability of the plasma membrane increases, and PI can bind to cellular DNA by moving across the cell membrane. Therefore, late-stage cells are stained with both PI and annexin V, whereas early-stage cells are stained with only annexin V.
Figure 5a shows the flow cytometry of HeLa cells under various treatments. The cell count in the lower right region, in which the cells were stained with only annexin V, increased when treated with the micelles and further increased under ultrasound irradiation. This increase in cell count in the lower right region under ultrasound irradiation indicated that ultrasound irradiation induced apoptosis in HeLa cells treated with TiO
2 NP-PIC micelles. Cell death induced by a combination of TiO
2 NPs and ultrasound irradiation has been reported to be due to apoptosis. Yamaguchi et al. reported that water-dispersed TiO
2-PEG induced apoptotic cell death in human glioblastoma cell line U251 under ultrasound irradiation [
16]. Furthermore, Ninomiya et al. reported that TiO
2 NPs modified with pre-S1/S2 proteins, which are part of the L protein from the hepatitis B virus with a high affinity toward hepatocyte, induced apoptotic cell death in human hepatoma HepG2 cells under ultrasound irradiation [
17]. Therefore, it is fitting that cell death induced by TiO
2 NP-PIC micelles under ultrasound irradiation was due to apoptosis, not necrosis.