Uniformly Dispersed and Re-Agglomerated Graphene Oxide-Based Cement Pastes: A Comparison of Rheological Properties, Mechanical Properties and Microstructure
Abstract
:1. Introduction
2. Materials and Experimental Procedure
2.1. Raw Materials
2.2. Test Methods
2.2.1. Characterization Techniques
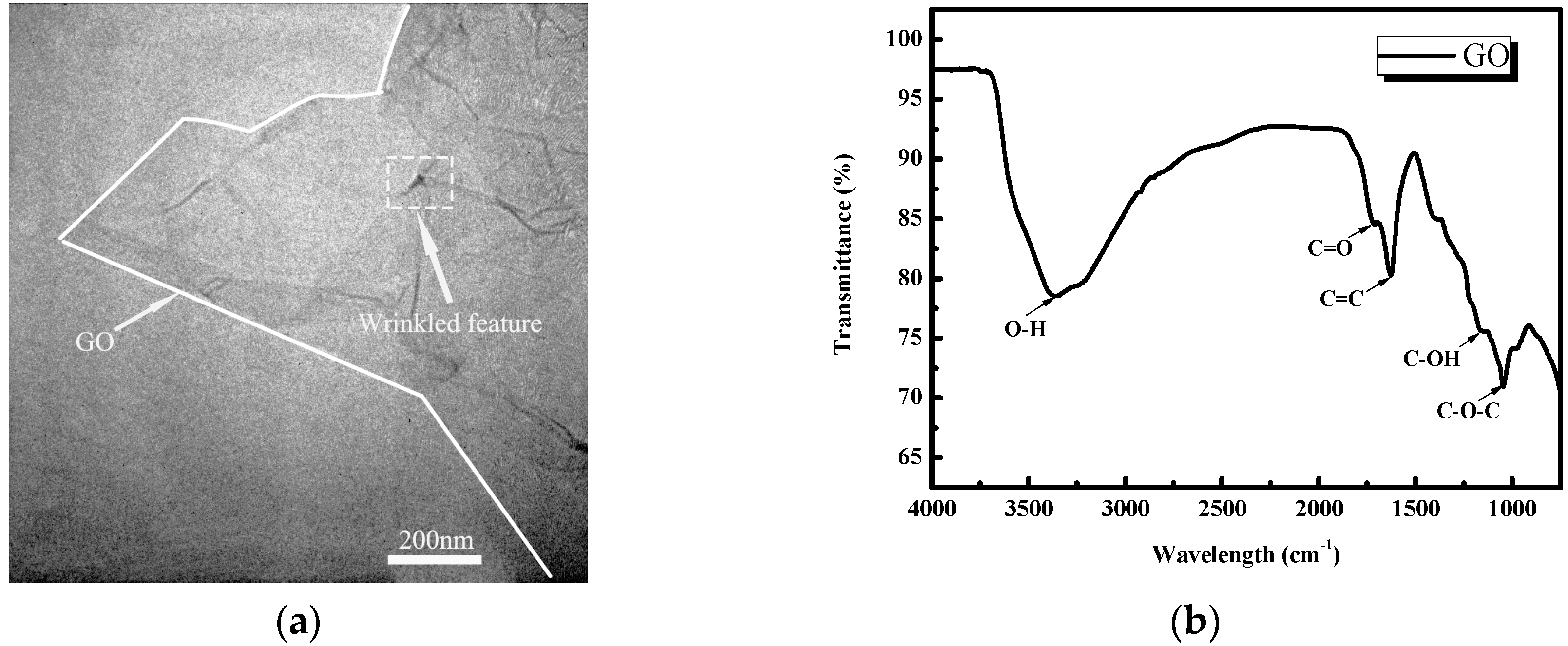
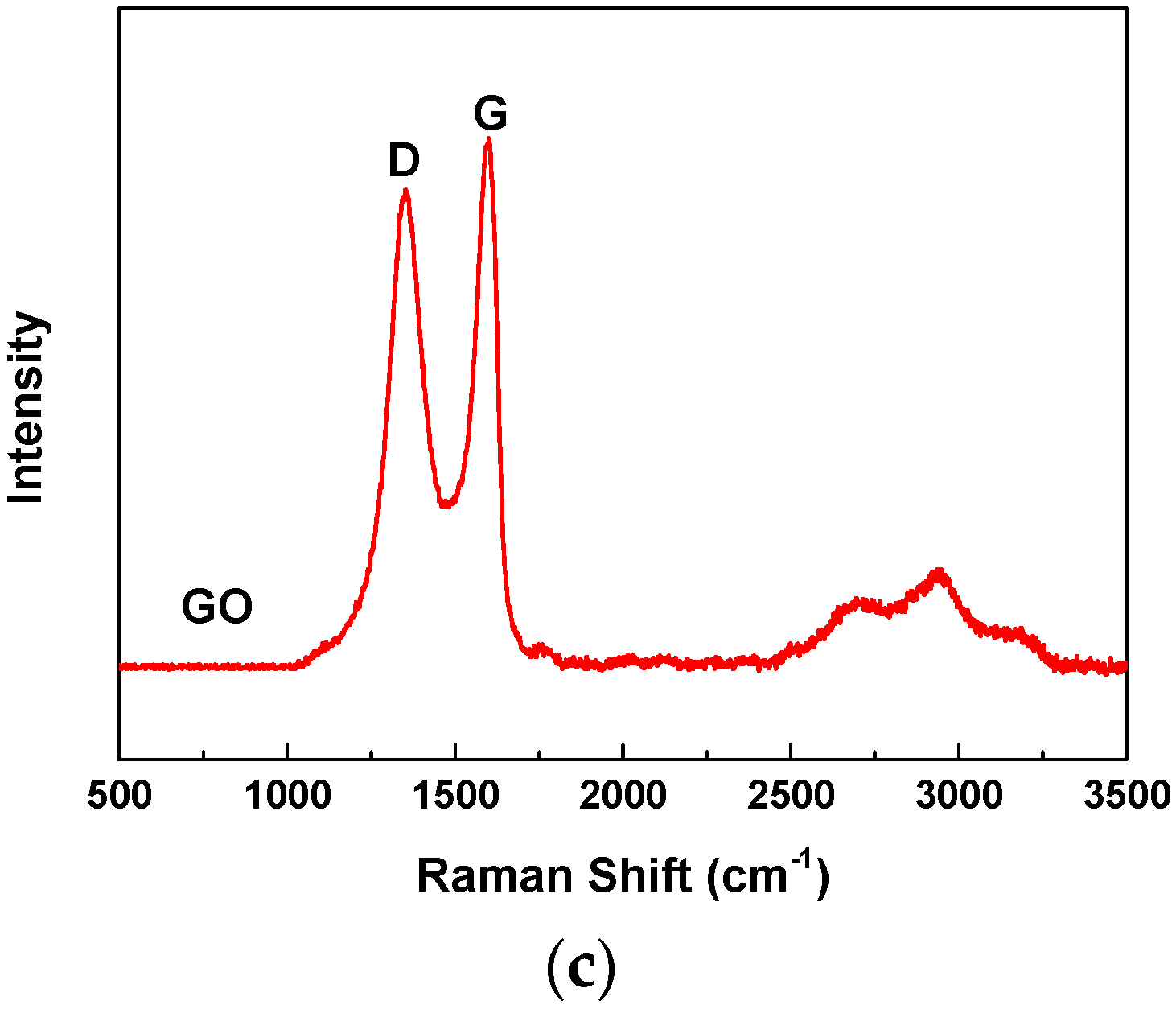
2.2.2. Characterization of the As-Received Graphene Oxide (GO)
2.2.3. Rheological Measurements
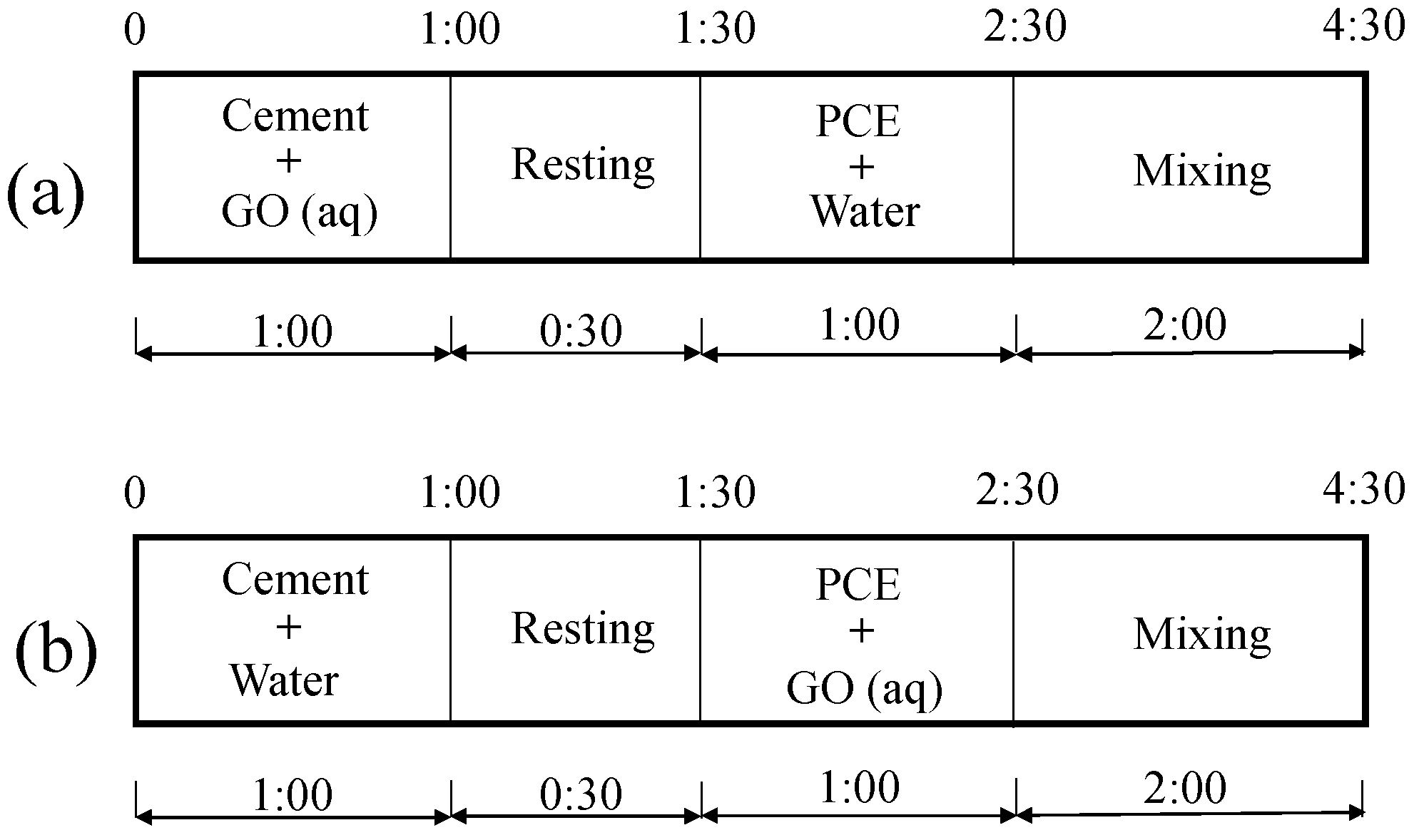
2.2.4. Mix Proportions
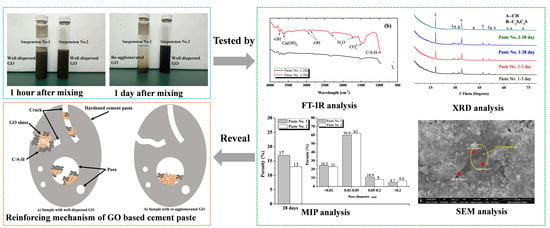
3. Results and Discussion
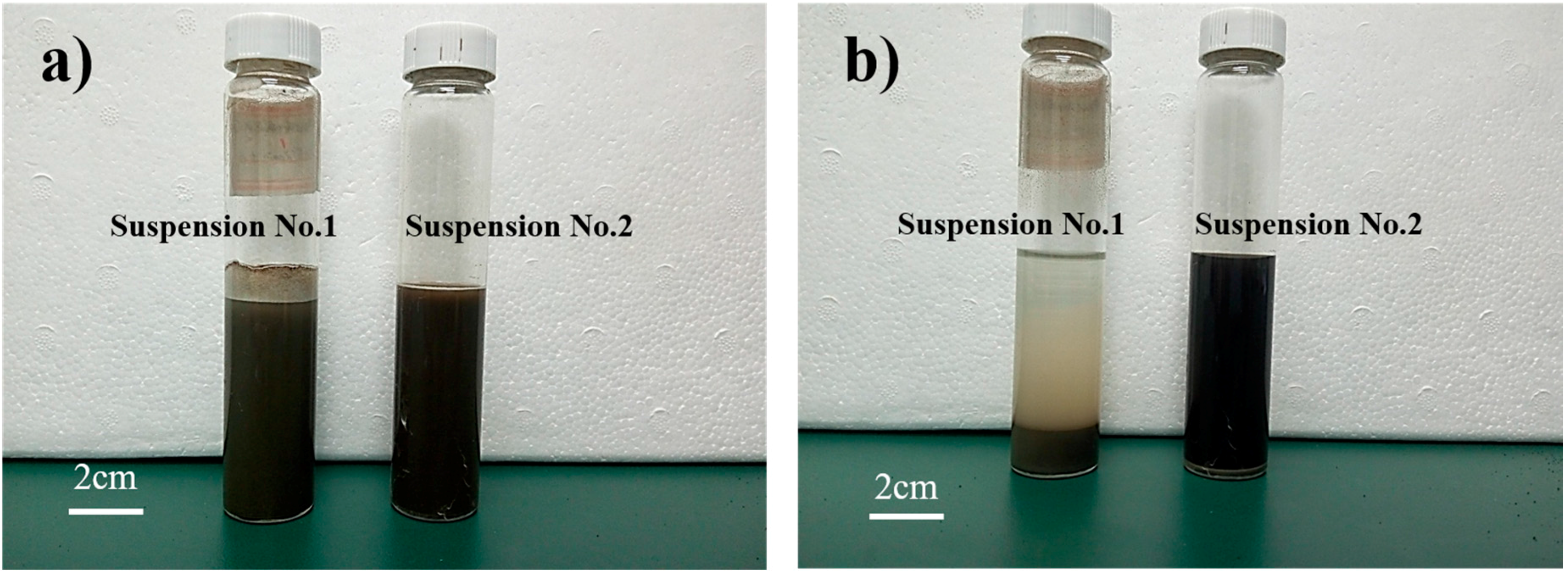
3.1. Characterization of GO State in the C-GO-PCE Suspensions
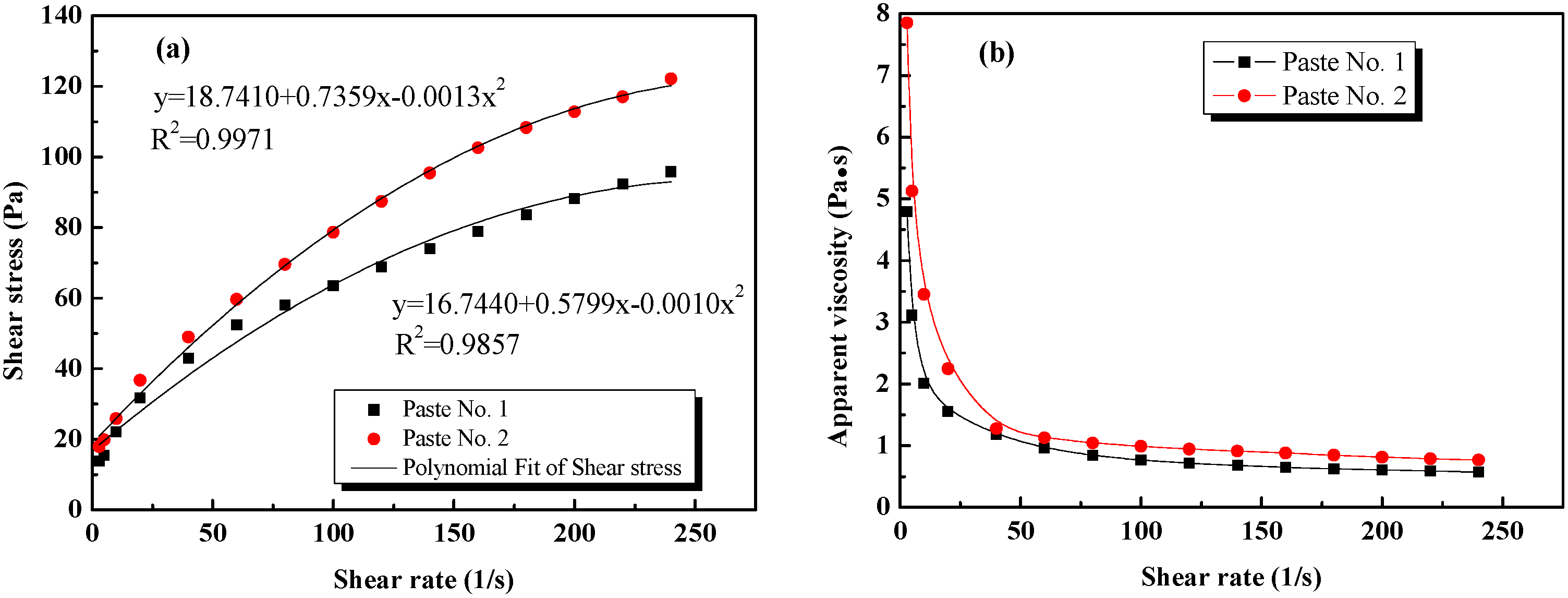
3.2. Effect of Different GO Dispersion States on the Rheological Properties of Cement Pastes
3.3. Mechanical Properties
3.4. Microstructural Properties
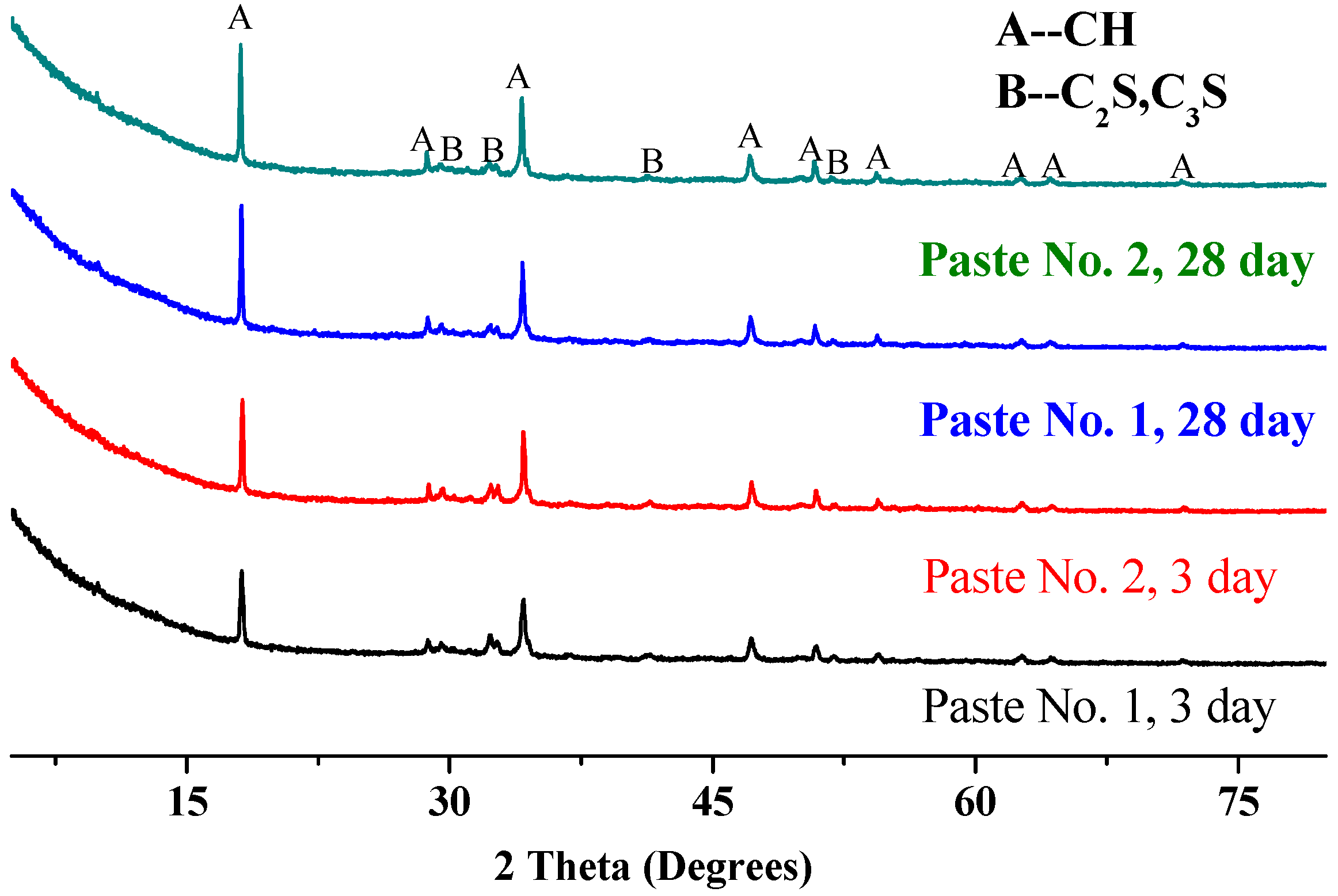
3.4.1. Analysis Based on X-ray Diffraction (XRD)
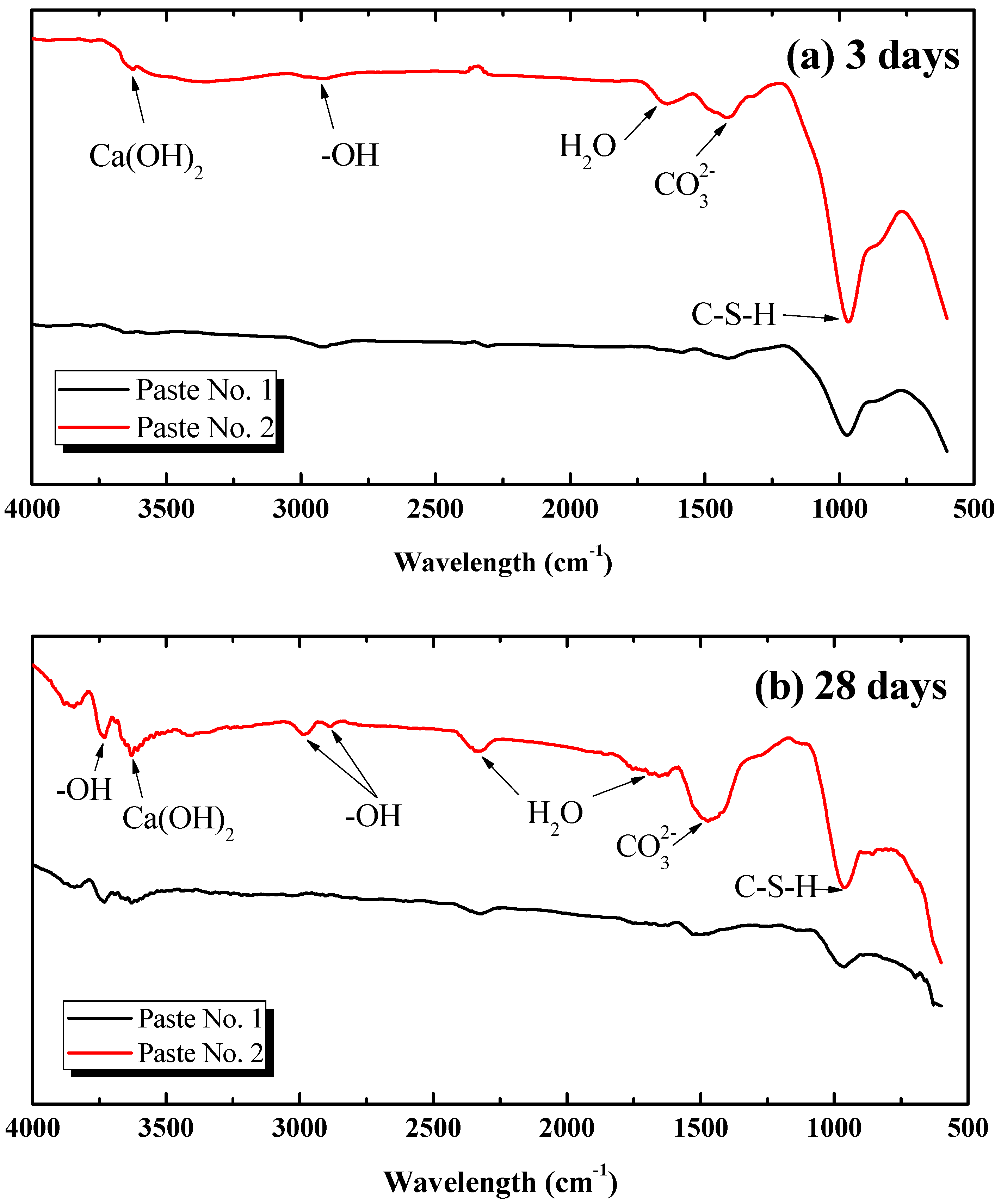
3.4.2. Analysis Based on FT-IR
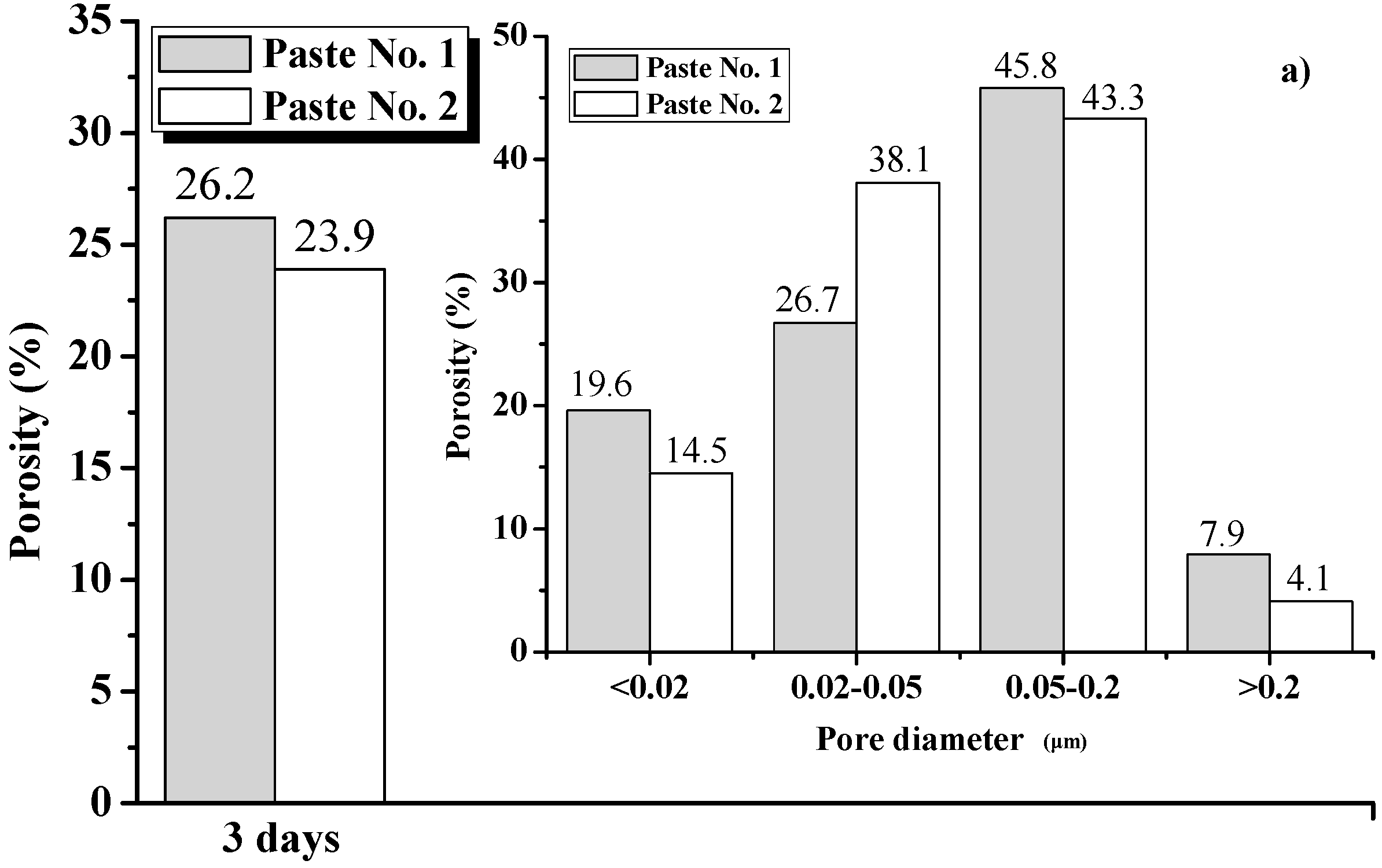
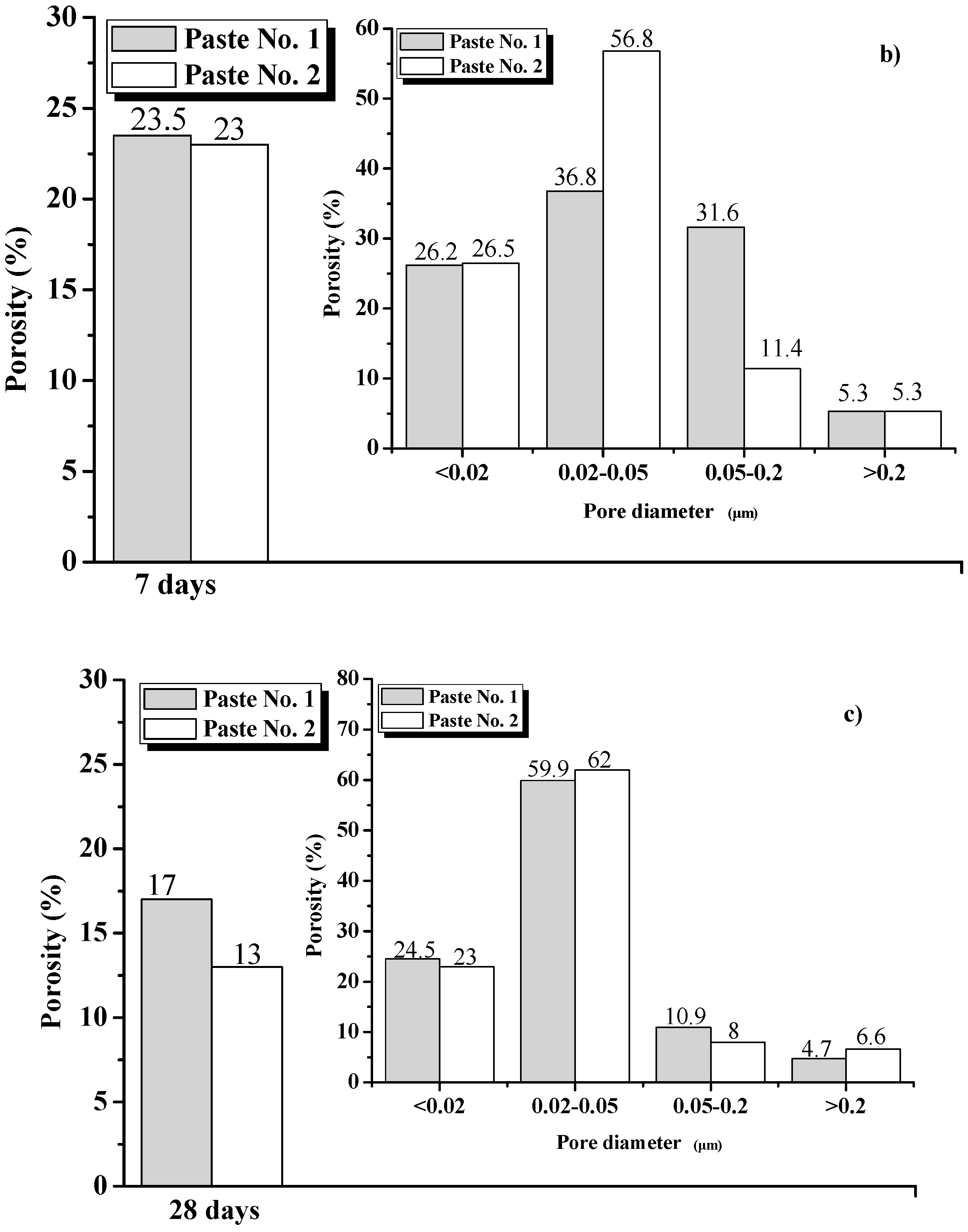
3.4.3. Analysis Based on Mercury Intrusion Porosimetry (MIP)
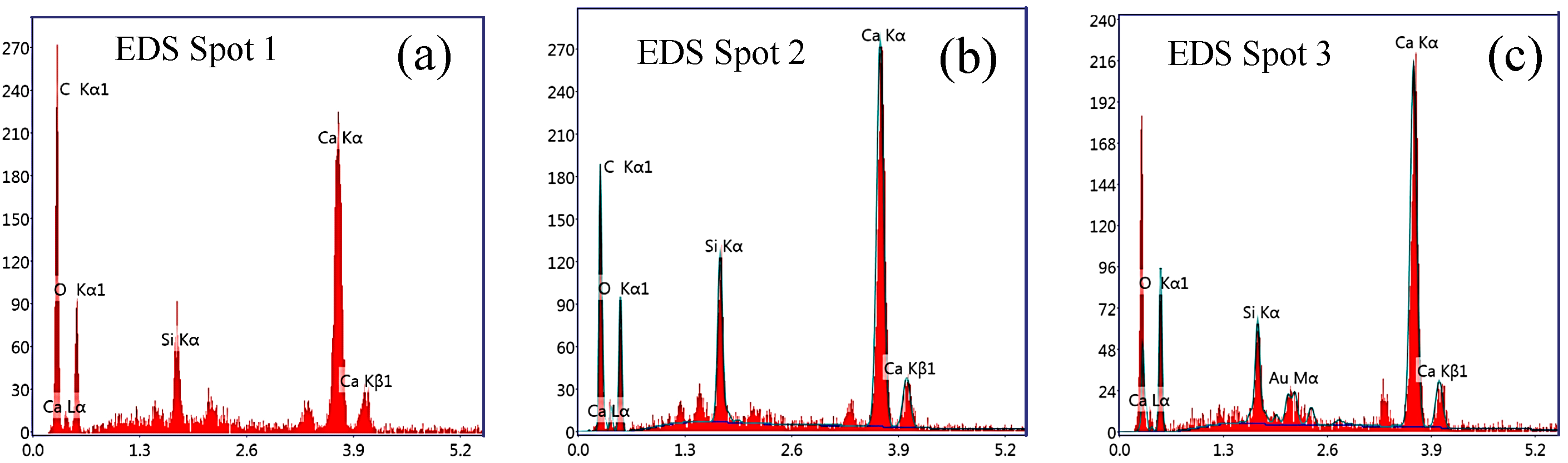
3.4.4. Analysis Based on Scanning Electron Microscopy (SEM) and Energy Dispersive Spectrometry (EDS)
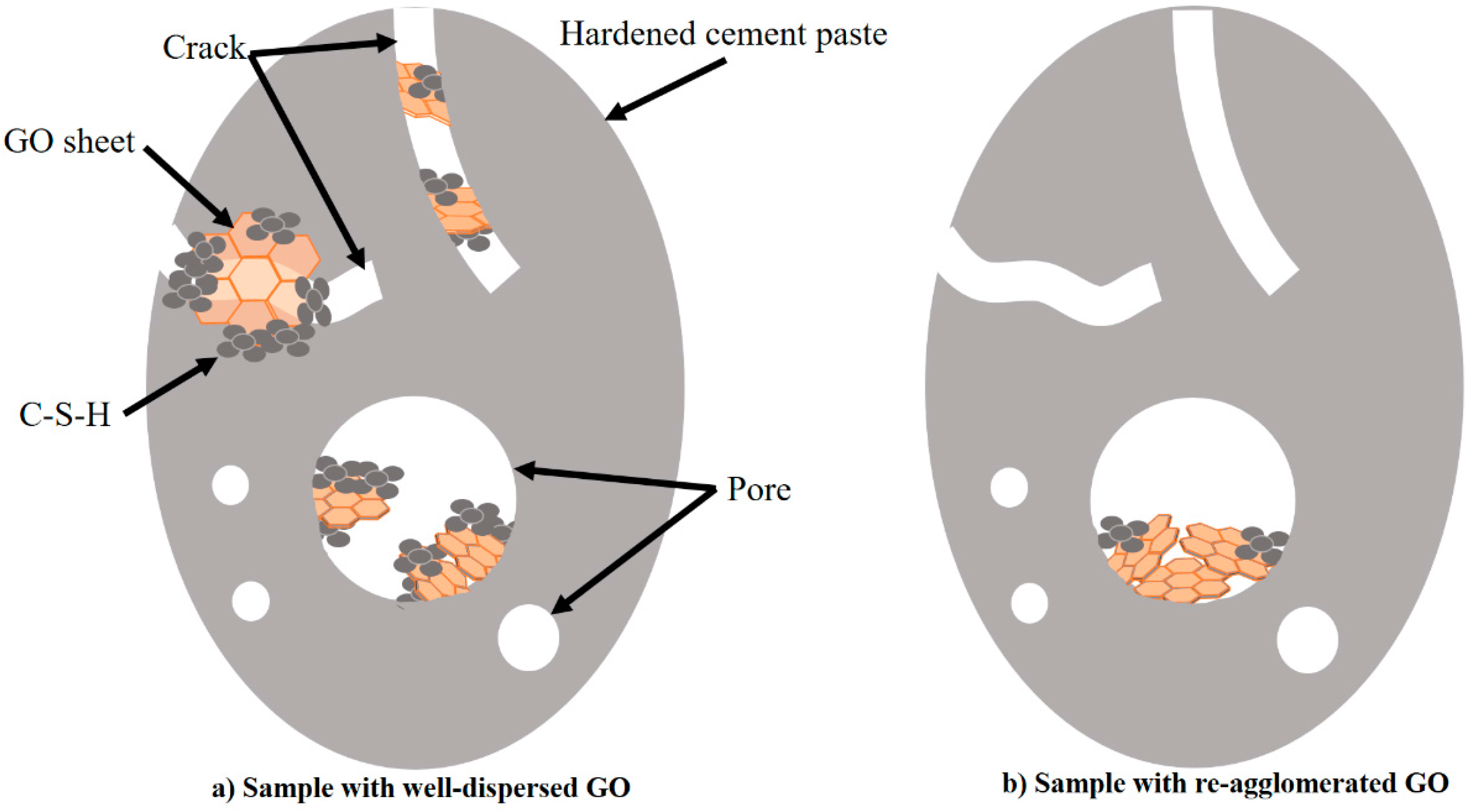
3.5. Mechanism of GO Reinforcement of Hardened Cement Paste
4. Conclusions
- (1)
- The yield stress and plastic viscosity is higher in the cement paste containing well-dispersed GO (Paste No. 2) than in that containing re-agglomerated GO (Paste No. 1). This phenomenon can be attributed to two actions. First, the uniform dispersion of GO increases the water requirement of paste. Second, the GO encourages and enhances the connections between flocculated structures of hydration products, thus makes them larger and more solid.
- (2)
- The addition of GO in cement paste not only can reduce pores in the cement matrix but also can constrain the development of cracks. The comparison of Paste No. 1 and Paste No. 2 demonstrates that the mechanical properties of Paste No. 2, including compressive and flexural strengths, were improved since the uniform dispersion of GO facilitates the hydration process of cement paste and the GO nanosheets fill the inner pores and increase the density of the cement matrix.
- (3)
- The XRD, FT-IR and SEM results show that there was an enhancement in the Ca(OH)2 content in Paste No. 2, which is strongly related to the hydration process. Although the hydration products contained in Pastes No. 1 and No. 2 are essentially the same, the hydration process of Paste No. 2 was notably faster. Quantitative analysis by MIP indicates that the total porosity of Paste No. 2 was lower than that of Paste No. 1 and contains lower percentages of harmful pores. Therefore, it can be considered that the uniform dispersion of GO is able to improve the ratio of different pore size and optimize the pore size distribution in cement pastes.
- (4)
- The observed improvement in the mechanical properties of the hardened cement paste accompanying the use of GO is achieved by limiting crack development, reinforcing the weak areas of the cement matrix and promoting C-S-H gel formation inside the pores.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Silvestre, J.; Silvestre, N.; Brito, J.D. Review on concrete nanotechnology. Eur. J. Environ. Civ. Eng. 2015, 20, 1–31. [Google Scholar] [CrossRef]
- Sumesh, M.; Alengaram, U.J.; Jumaat, M.Z.; Mo, K.H.; Alnahhal, M.F. Incorporation of nano-materials in cement composite and geopolymer based paste and mortar—A review. Constr. Build. Mater. 2017, 148, 62–84. [Google Scholar] [CrossRef]
- Pérez-Nicolás, M.; Navarro-Blasco, Í.; Fernández, J.M.; Alvarez, J.I. The Effect of TiO2 Doped Photocatalytic Nano-Additives on the Hydration and Microstructure of Portland and High Alumina Cements. Nanomaterials 2017, 7, 329. [Google Scholar] [CrossRef] [PubMed]
- Konsta-Gdoutos, M.S.; Aza, C.A. Self-sensing carbon nanotube (CNT) and nanofiber (CNF) cementitious composites for real time damage assessment in smart structures. Cem. Concr. Compos. 2014, 53, 162–169. [Google Scholar] [CrossRef]
- Stephens, C.; Brown, L.; Sanchez, F. Quantification of the re-agglomeration of carbon nanofiber aqueous dispersion in cement pastes and effect on the early age flexural response. Carbon 2016, 107, 482–500. [Google Scholar] [CrossRef]
- Ji, X.; Xu, Y.; Zhang, W.; Cui, L.; Liu, J. Review of functionalization, structure and properties of graphene/polymer composite fibers. Compos. Part A 2016, 87, 29–45. [Google Scholar] [CrossRef]
- Lu, L.; Ouyang, D. Properties of Cement Mortar and Ultra-High Strength Concrete Incorporating Graphene Oxide Nanosheets. Nanomaterials 2017, 7, 187. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; He, P.; Jia, D.; Duan, X.; Yang, Z.; Wang, S.; Zhou, Y. In-situ preparation of fully stabilized graphene/cubic-leucite composite through graphene oxide/geopolymer. Mater. Des. 2016, 101, 301–308. [Google Scholar] [CrossRef]
- Hou, D.; Lu, Z.; Li, X.; Ma, H.; Li, Z. Reactive molecular dynamics and experimental study of graphene-cement composites: Structure, dynamics and reinforcement mechanisms. Carbon 2017, 115, 188–208. [Google Scholar] [CrossRef]
- Zhang, R.; Gao, B.; Ma, Q.; Zhang, J.; Cui, H.; Liu, L. Directly grafting graphene oxide onto carbon fiber and the effect on the mechanical properties of carbon fiber composites. Mater. Des. 2016, 93, 364–369. [Google Scholar] [CrossRef]
- Song, Y.; Chen, Y.; Liu, W.; Li, W.; Wang, Y.; Zhao, D.; Liu, X. Microscopic mechanical properties of titanium composites containing multi-layer graphene nanofillers. Mater. Des. 2016, 109, 256–263. [Google Scholar] [CrossRef]
- Paredes, J.I.; Villarrodil, S.; Martínezalonso, A.; Tascón, J.M. Graphene oxide dispersions in organic solvents. Langmuir ACS J. Surf. Colloids 2008, 24, 10560–10564. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, R.; Yao, H.; Wang, Z.; Zheng, S. Adsorption characteristics of graphene oxide nanosheets on cement. RSC Adv. 2016, 6, 63365–63372. [Google Scholar] [CrossRef]
- Lu, Z.; Hou, D.; Meng, L.; Sun, G.; Lu, C. Mechanism of cement paste reinforced by graphene oxide/carbon nanotubes composites with enhanced mechanical properties. RSC Adv. 2015, 5, 100598–100605. [Google Scholar] [CrossRef]
- Shang, Y.; Zhang, D.; Yang, C.; Liu, Y. Effect of graphene oxide on the rheological properties of cement pastes. Constr. Build. Mater. 2015, 96, 20–28. [Google Scholar] [CrossRef]
- Lv, S.; Ma, Y.; Qiu, C.; Sun, T.; Liu, J. Effect of graphene oxide nanosheets of microstructure and mechanical properties of cement composites. Constr. Build. Mater. 2013, 49, 121–127. [Google Scholar] [CrossRef]
- Johnson, D.W.; Dobson, B.P.; Coleman, K.S. A manufacturing perspective on graphene dispersions. Curr. Opin. Colloid Interface Sci. 2015, 20, 367–382. [Google Scholar] [CrossRef] [Green Version]
- Rana, S.; Fangueiro, R. A Review on nanomaterial dispersion, microstructure and mechanical properties of carbon nanotube and nanofiber reinforced cementitious composites. J. Nanomater. 2013, 2013, 1–19. [Google Scholar]
- Saafi, M.; Tang, L.; Fung, J.; Rahman, M.; Liggat, J. Enhanced properties of graphene/fly ash geopolymeric composite cement. Cem. Concr. Res. 2015, 67, 292–299. [Google Scholar] [CrossRef] [Green Version]
- Gao, R.J.; Yao, Y.; Wu, H.; Wang, L. Effect of amphoteric dispersant on the dispersion properties of nano-SiO2 particles. J. Appl. Polym. Sci. 2017, 134, 45075. [Google Scholar] [CrossRef]
- Korayem, A.H.; Tourani, N.; Zakertabrizi, M.; Sabziparvar, A.M.; Duan, W.H. A review of dispersion of nanoparticles in cementitious matrices: Nanoparticle geometry perspective. Constr. Build. Mater. 2017, 153, 346–357. [Google Scholar] [CrossRef]
- Parveen, S.; Rana, S.; Fangueiro, R.; Paiva, M.C. Microstructure and mechanical properties of carbon nanotube reinforced cementitious composites developed using a novel dispersion technique. Cem. Concr. Res. 2015, 73, 215–227. [Google Scholar] [CrossRef]
- Gao, Y.; Ren, X.; Tan, X.; Hayat, T.; Alsaedi, A. Insights into key factors controlling GO stability in natural surface waters. J. Hazard. Mater. 2017, 335, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.H.; Ying, L.I.; Zhao, H.R.; Ya, H.E.; Deng, L.J. Dispersity of Graphene Oxide in Cement Matrix and Impact on Its Structure and Properties. Fine Chem. 2016, 33. [Google Scholar] [CrossRef]
- Zhao, L.; Guo, X.; Ge, C.; Li, Q.; Guo, L. Mechanical behavior and toughening mechanism of polycarboxylate superplasticizer modified graphene oxide reinforced cement composites. Compos. Part B Eng. 2017, 113, 308–316. [Google Scholar] [CrossRef]
- Zhao, L.; Guo, X.; Liu, Y.; Ge, C.; Guo, L. Synergistic effects of silica nanoparticles/polycarboxylate superplasticizer modified graphene oxide on mechanical behavior and hydration process of cement composites. RSC Adv. 2017, 7, 16688–16702. [Google Scholar] [CrossRef]
- Gao, D.G.; Ma, Y.J. Preparation and Properties of Copolymer of Graphene Oxide and Monomers of Polycarboxylate Superplasticizer. Fine Chem. 2015, 32. [Google Scholar] [CrossRef]
- Lu, Z.; Hanif, A.; Ning, C.; Shao, H.; Yin, R. Steric stabilization of graphene oxide in alkaline cementitious solutions: Mechanical enhancement of cement composite. Mater. Des. 2017, 127, 154–161. [Google Scholar] [CrossRef]
- Yan, B.L.; Jiang, L.Z.; Xiao, Z.M.; Liu, C.; Zhang, Q.Y.; Chen, P.; Huo, C.M.; Xi, J.S.; Song, L.C.; Wang, X.; et al. Common Portland Cement; Chinese National Standard GB/T 175-2007; Standards Press of China: Beijing, China, 2007. [Google Scholar]
- Guo, Y.W.; Zhao, X.L.; Guo, J.Y. Polycarboxylates High Performance Water-Reducing Admixture; Chinese Construction Industry Standard JG/T 223-2007; Standards Press of China: Beijing, China, 2007. [Google Scholar]
- Perrot, A.; Lecompte, T.; Khelifi, H.; Brumaud, C.; Hot, J. Yield stress and bleeding of fresh cement pastes. Cem. Concr. Res. 2012, 42, 937–944. [Google Scholar] [CrossRef]
- Zingg, A.; Holzer, L.; Kaech, A.; Winnefeld, F.; Pakusch, J. The microstructure of dispersed and non-dispersed fresh cement pastes—New insight by cryo-microscopy. Cem. Concr. Res. 2008, 38, 522–529. [Google Scholar] [CrossRef]
- Zhang, D.T.; Wang, W.Y.; Bai, X.M.; Yang, J.D.; Xiao, Z.M.; Yan, B.L.; Wang, T.; Chen, P.; Diao, Z.J.; Jiang, L.Z.; et al. Method of Testing Cements-Determination of Strength; Chinese National Standard GB/T17671-1999; Standards Press of China: Beijing, China, 2007. [Google Scholar]
- Adam, W.; Stegmann, V.R. Electrochemical preparation, characterization and application of electrodes modified with nickel–cobalt hex cyanoferrate/graphene oxide–carbon nanotubes. J. Electroanal. Chem. 2015, 755, 197–202. [Google Scholar]
- Wang, M.; Wang, R.; Yao, H.; Farhan, S.; Zheng, S. Study on the three dimensional mechanism of graphene oxide nano-sheets modified cement. Constr. Build. Mater. 2016, 126, 730–739. [Google Scholar] [CrossRef]
- Sharma, S.; Kothiyal, N.C. Comparative effects of pristine and ball-milled graphene oxide on physico-chemical characteristics of cement mortar nanocomposites. Constr. Build. Mater. 2016, 115, 256–268. [Google Scholar] [CrossRef]
- Kudin, K.N.; Ozbas, B.; Schniepp, H.C.; Prud’Homme, R.K.; Aksay, I.A. Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett. 2007, 8, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Zhang, Q.; Yang, M.G.; Huang, C.H.; Yang, Y.G. Structural evolution during annealing of thermally reduced graphene nano-sheets for application in supercapacitors. Carbon 2012, 50, 3572–3584. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B Condens. Matter 2008, 61, 14095–14107. [Google Scholar] [CrossRef]
- Guo, T.; Wang, L.; Wang, N.; Tian, P.; Song, Y.L.; Yao, L.J.; Yu, W.Q.; Lang, J.L.; Liu, H.K.; Chen, G.Z.; et al. Method for Testing Uniformity of Concrete Admixture; Chinese National Standard GB/T 8077-2012; Standards Press of China: Beijing, China, 2012. [Google Scholar]
- Celik, F.; Canakci, H. An investigation of rheological properties of cement-based grout mixed with rice husk ash (RHA). Constr. Build. Mater. 2015, 91, 187–194. [Google Scholar] [CrossRef]
- Yahia, A.; Khayat, K.H. Analytical models for estimating yield stress of high-performance pseudo plastic grout. Cem. Concr. Res. 2001, 31, 731–738. [Google Scholar] [CrossRef]
- Khayat, K.H.; Yahia, A. Effect of wetland gum-high-range water reducer combinations on rheology of cement grout. ACI Mater. J. 1997, 94, 365–372. [Google Scholar]
- Konsta-Gdoutos, M.S.; Metaxa, Z.S.; Shah, S.P. Highly dispersed carbon nanotube reinforced cement based materials. Cem. Concr. Res. 2016, 40, 1052–1059. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, Q.; Yu, Q.; Gao, R.; Tong, T. Experimental investigation on mechanical and piezoresistive properties of cementitious materials containing graphene and graphene oxide nanoplatelets. Constr. Build. Mater. 2016, 127, 565–576. [Google Scholar] [CrossRef]
- Wang, Q.; Cui, X.; Wang, J.; Li, S.; Lv, C. Effect of fly ash on rheological properties of graphene oxide cement paste. Constr. Build. Mater. 2017, 138, 35–44. [Google Scholar] [CrossRef]
- Lu, Z.; Li, X.; Hanif, A.; Chen, B.; Parthasarathy, P. Early-age interaction mechanism between the graphene oxide and cement hydrates. Constr. Build. Mater. 2017, 152, 232–239. [Google Scholar] [CrossRef]
- Lv, S.H.; Sun, T.; Ma, Y.J.; Qiu, C.C.; Ding, H.D.; Liu, J.J. Regulation of grapheme oxide on microstructure of hydration crystals of cement composites and its impact on reinforcing toughness. Concrete 2013, 25, 105–112. [Google Scholar]
- Yang, H.; Monasterio, M.; Cui, H.; Han, N. Experimental study of the effects of graphene oxide on microstructure and properties of cement paste composite. Composites Part A 2017, 102, 263–272. [Google Scholar] [CrossRef]
- Wu, Z.W.; Lian, H.Z. High Performance Concrete; China Railway Publishing House: Beijing, China, 1999. [Google Scholar]
- Li, X.; Korayem, A.H.; Li, C.; Liu, Y.; He, H. Incorporation of graphene oxide and silica fume into cement paste: A study of dispersion and compressive strength. Constr. Build. Mater. 2016, 123, 327–335. [Google Scholar] [CrossRef]
- Long, W.J.; Wei, J.J.; Ma, H.; Xing, F. Dynamic Mechanical Properties and Microstructure of Graphene Oxide Nanosheets Reinforced Cement Composites. Nanomaterials 2017, 7, 407. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Yuan, B.; Wang, X.; Hu, Y. Enhanced mechanical properties, water stability and repeatable shape recovery behavior of Ca2+ crosslinking graphene oxide-based nacre-mimicking hybrid film. Mater. Des. 2017, 115, 46–51. [Google Scholar] [CrossRef]

| CaO | SiO2 | Al2O3 | Fe2O3 | SO3 | MgO | SrO | Na2O | Cl− | Loss on Ignition |
|---|---|---|---|---|---|---|---|---|---|
| 62.65 | 21.88 | 4.49 | 3.45 | 2.44 | 2.36 | 0.90 | 0.51 | 0.01 | 1.31 |
| Appearance | Solid Content (% by Mass) | pH | Viscosity | Absorbance Ratio A230/A600 | Carbon (% by Mass) | Molar Ratio (O/C) |
|---|---|---|---|---|---|---|
| Brown paste | 43 ± 1 | ≥1.2 | ≥2000 | ≥45 | 47 ± 5 | 0.6 ± 1 |
| Mix No. | Cement (g) | GO (g) | PCE (g) | Water (g) | GO/PCE | pH |
|---|---|---|---|---|---|---|
| Suspension No. 1 | 2 | 0.3 | 0.3 | 99.7 | 1:1 | 12.4 |
| Suspension No. 2 | 2 | 0.3 | 0.3 | 99.7 | 1:1 | 12.4 |
| Mix No. | Cement (g) | Water (g) | W/C | GO (g) | GO/Cement | PCE/GO |
|---|---|---|---|---|---|---|
| Paste No. 1 | 3600 | 1440 | 0.4 | 2.16 | 0.06% | 1.0 |
| Paste No. 2 | 3600 | 1440 | 0.4 | 2.16 | 0.06% | 1.0 |
| Mix No. | Sample Style (Diameter) | Solvent (Density) | Particle Weight Concentration | Density of Particle (g/mL) | Special Surface (m2/g) |
|---|---|---|---|---|---|
| Suspension No. 1 | GO (350 nm) | Water (1 g/mL) | 0.0003 | 2.2 | 915.4922 |
| Suspension No. 2 | GO (350 nm) | Water (1 g/mL) | 0.0003 | 2.2 | 2217.6372 |
| Mix No. | Fitting Equation | (Pa) | (Pa·s) | Correlation Coefficient |
|---|---|---|---|---|
| Paste No. 1 | y = 16.7440 + 0.5799x − 0.0010x2 | 16.7440 | 0.5799 | 0.9857 |
| Paste No. 2 | y = 18.7410 + 0.7359x − 0.0013x2 | 18.7410 | 0.7359 | 0.9971 |
| Age (Days) | Compressive Strength (% Increase) | Flexural Strength (% Increase) | ||
|---|---|---|---|---|
| Paste No. 1 | Paste No. 2 | Paste No. 1 | Paste No. 2 | |
| 3 | 43.1 | 46.7 (8%) | 2.2 | 2.8 (27%) |
| 7 | 58.5 | 62.0 (5%) | 5.0 | 6.3 (26%) |
| 28 | 62.3 | 64.8 (4%) | 7.5 | 8.9 (19%) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, W.-J.; Li, H.-D.; Fang, C.-L.; Xing, F. Uniformly Dispersed and Re-Agglomerated Graphene Oxide-Based Cement Pastes: A Comparison of Rheological Properties, Mechanical Properties and Microstructure. Nanomaterials 2018, 8, 31. https://doi.org/10.3390/nano8010031
Long W-J, Li H-D, Fang C-L, Xing F. Uniformly Dispersed and Re-Agglomerated Graphene Oxide-Based Cement Pastes: A Comparison of Rheological Properties, Mechanical Properties and Microstructure. Nanomaterials. 2018; 8(1):31. https://doi.org/10.3390/nano8010031
Chicago/Turabian StyleLong, Wu-Jian, Hao-Dao Li, Chang-Le Fang, and Feng Xing. 2018. "Uniformly Dispersed and Re-Agglomerated Graphene Oxide-Based Cement Pastes: A Comparison of Rheological Properties, Mechanical Properties and Microstructure" Nanomaterials 8, no. 1: 31. https://doi.org/10.3390/nano8010031