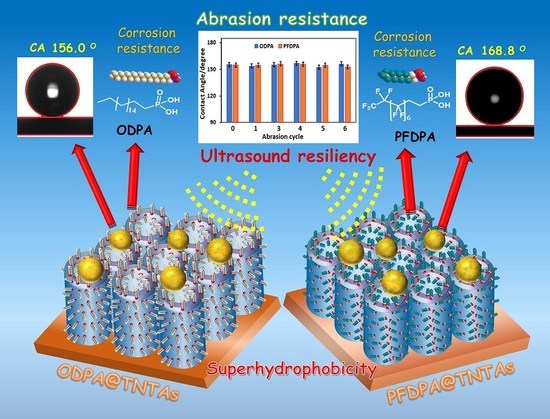
Resistance of Superhydrophobic Surface-Functionalized TiO2 Nanotubes to Corrosion and Intense Cavitation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of TiO2 Nanotubes
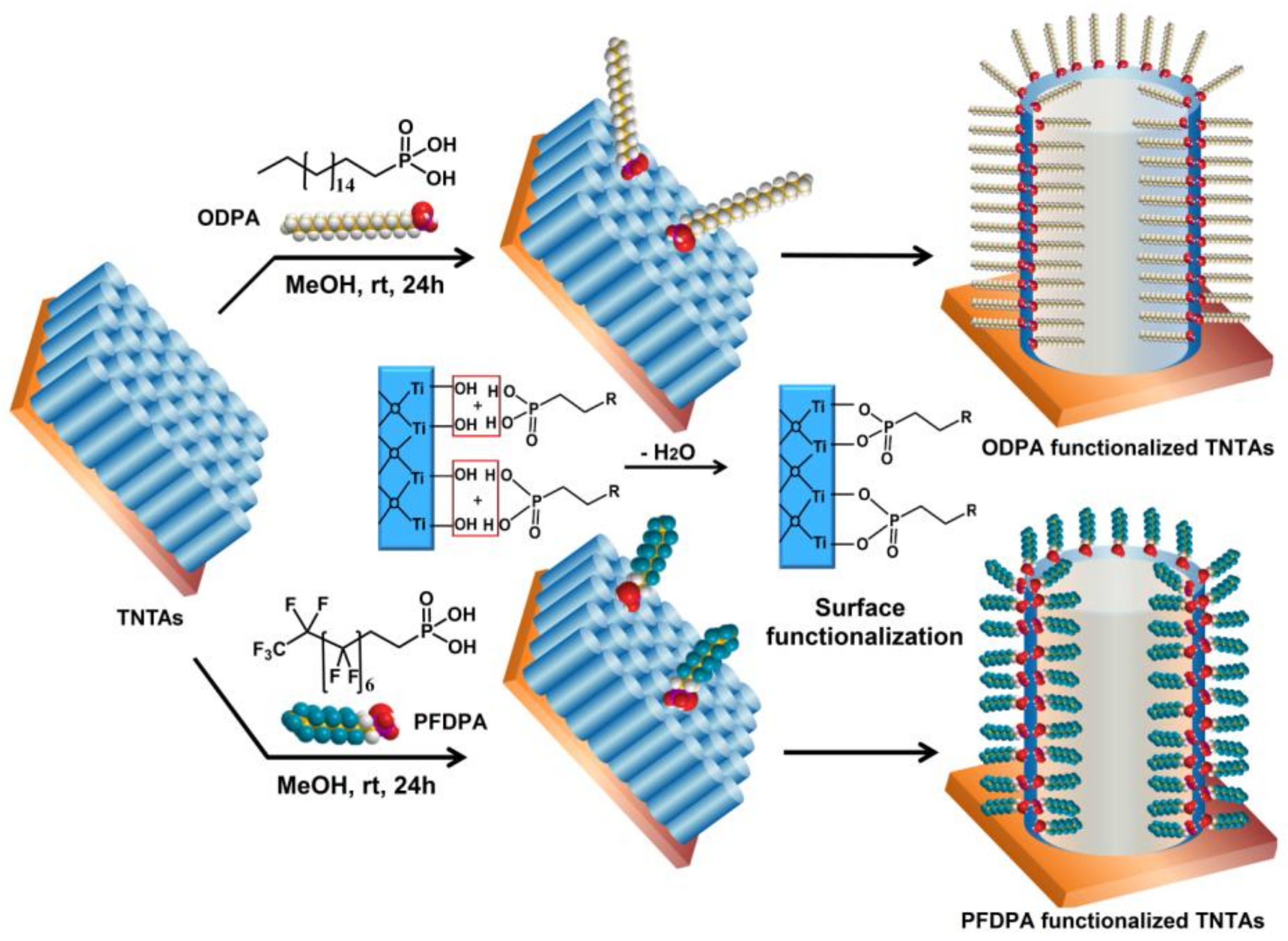
2.3. Surface Functionalization
2.4. Characterization and Testing
3. Results and Discussion
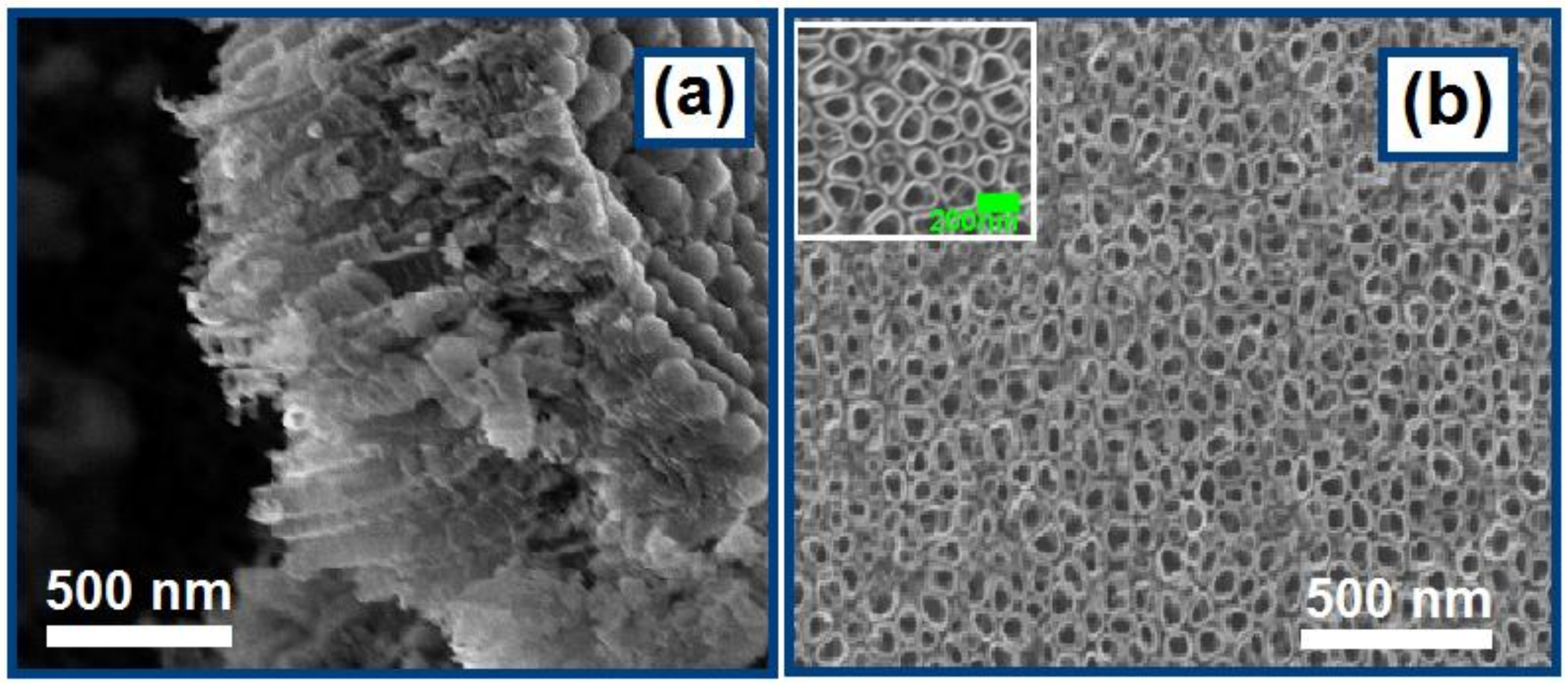
3.1. Morphological Characterization of TNTAs
3.2. Surface Functionalization of TNTAs Using ODPA and PFDPA
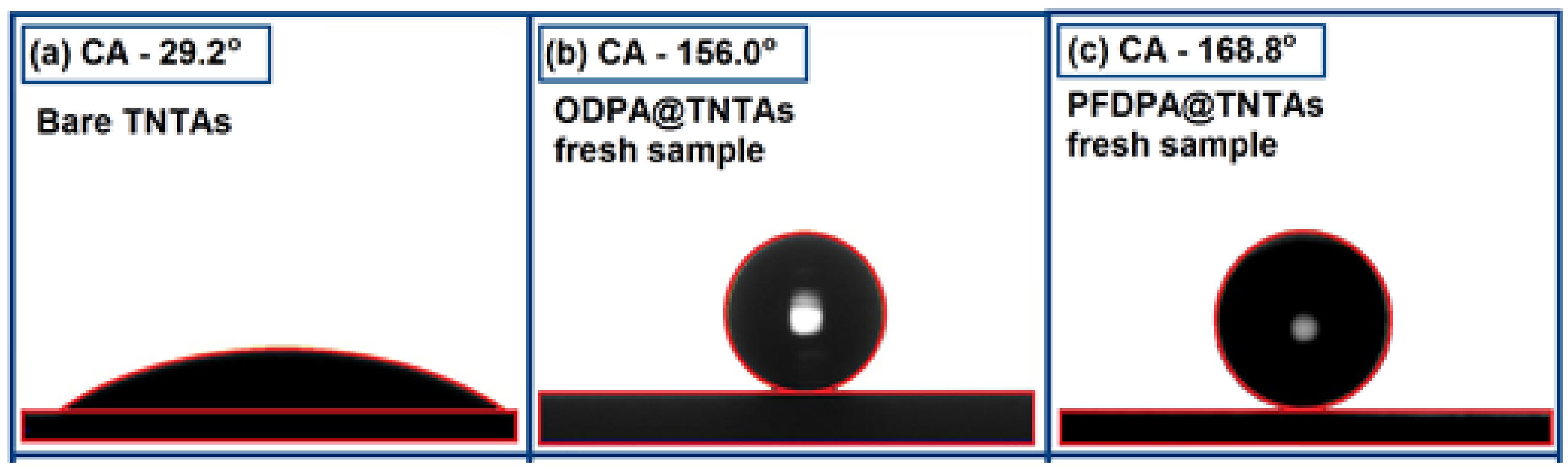
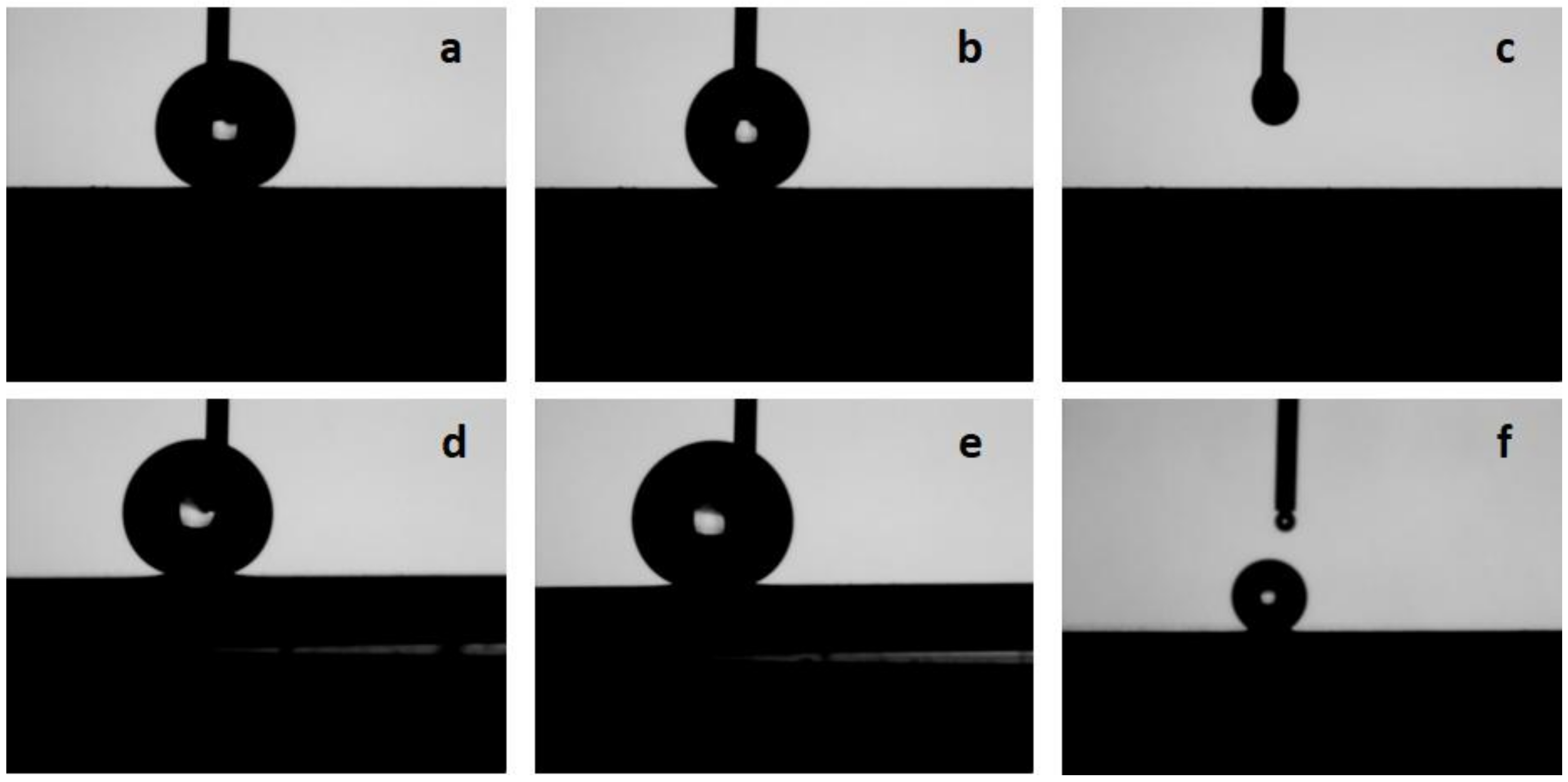
3.3. Contact Angle and Contact Angle Hysteresis
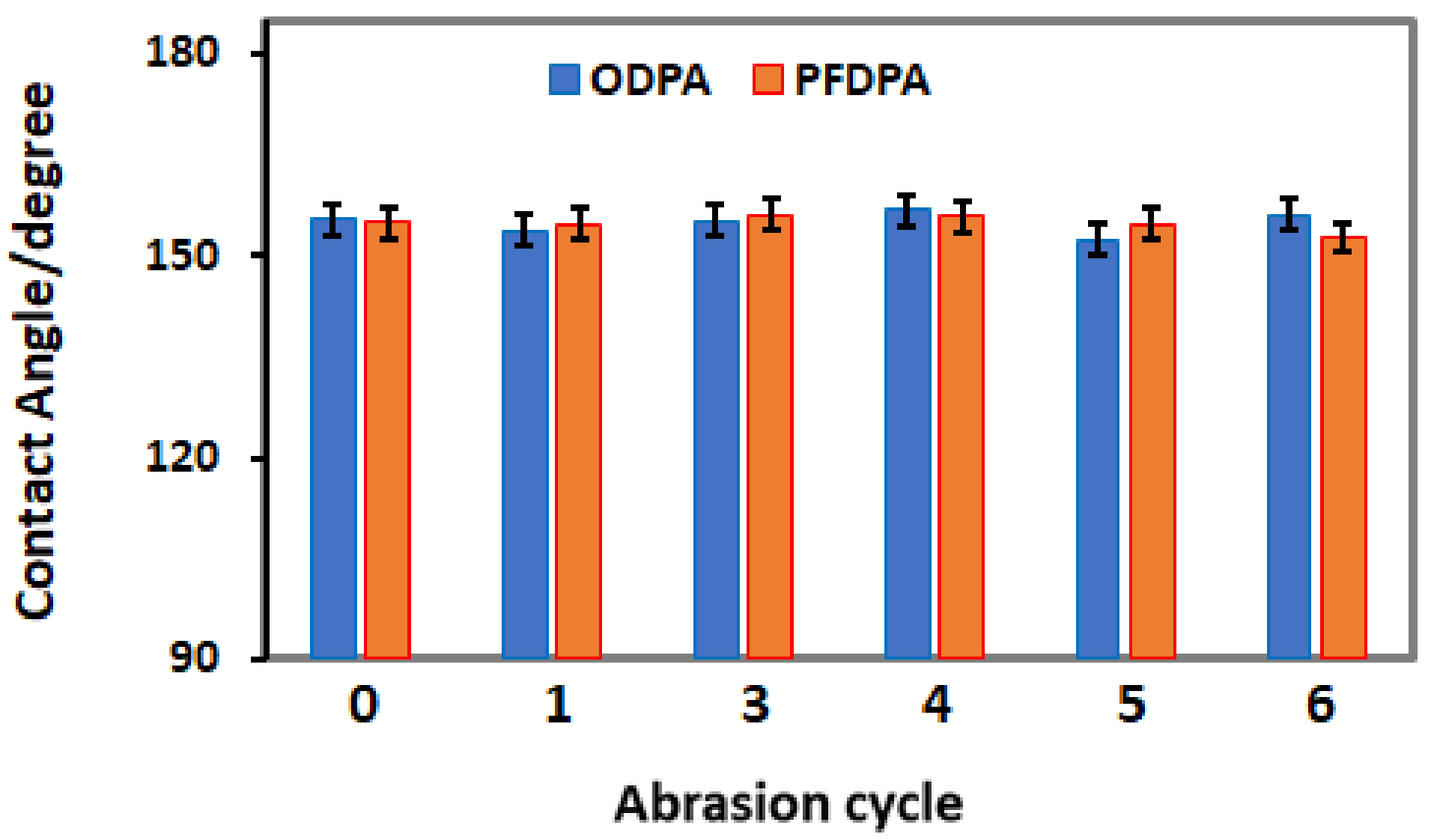
3.4. Resilience of the SAM Functionalized TNTA
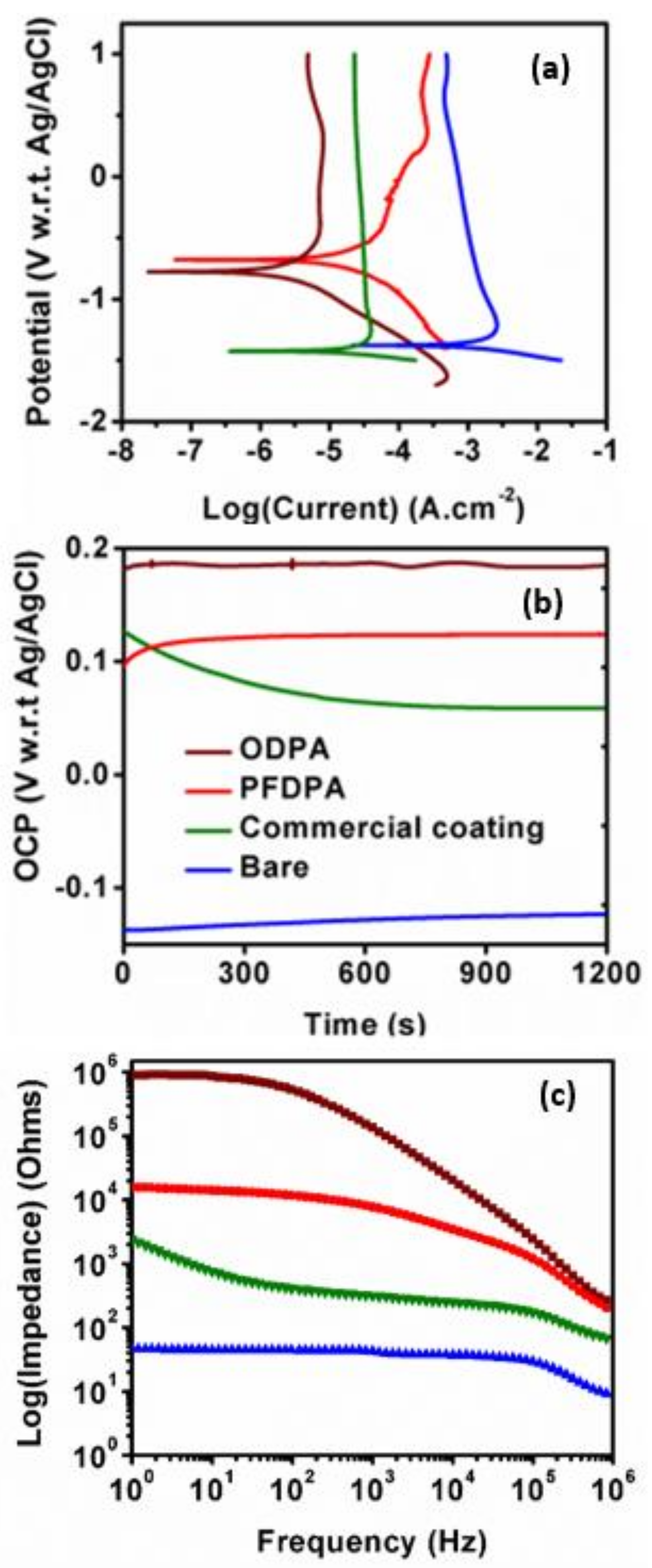
3.5. Corrosion Testing and Electrochemical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Erbil, H.Y.; Demirel, A.L.; Avcı, Y.; Mert, O. Transformation of a simple plastic into a superhydrophobic surface. Science 2003, 299, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, D.R.; Holl, M.R. Microscale bioanalytical systems. Science 2002, 297, 1197–1198. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Hashimoto, K.; Watanabe, T.; Takai, K.; Yamauchi, G.; Fujishima, A. Transparent superhydrophobic thin films with self-cleaning properties. Langmuir 2000, 16, 7044–7047. [Google Scholar] [CrossRef]
- Tian, X.; Verho, T.; Ras, R.H.A. Moving superhydrophobic surfaces toward real-world applications. Science 2016, 352, 142. [Google Scholar] [CrossRef] [PubMed]
- Falde, E.J.; Yohe, S.T.; Colson, Y.L.; Grinstaff, M.W. Superhydrophobic materials for biomedical applications. Biomaterials 2016, 104, 87–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhushan, B.; Jung, Y.C. Natural and biomimetic artificial surfaces for superhydrophobicity, self-cleaning, low adhesion, and drag reduction. Prog. Mater. Sci. 2011, 56, 1–108. [Google Scholar] [CrossRef]
- Yao, L.; He, J. Recent progress in antireflection and self-cleaning technology–from surface engineering to functional surfaces. Prog. Mater. Sci. 2014, 61, 94–143. [Google Scholar] [CrossRef]
- Lafuma, A.; Quéré, D. Superhydrophobic states. Nat. Mater. 2003, 2, 457. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Guo, Z.; Liu, W. Adhesion behaviors on superhydrophobic surfaces. Chem. Commun. 2014, 50, 3900–3913. [Google Scholar] [CrossRef] [PubMed]
- Bazin, D.; Faure, C. Superhydrophobic, highly adhesive arrays of copper hollow spheres produced by electro-colloidal lithography. Soft Matter 2017, 13, 5500–5505. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Zhang, X.; Niu, F.; He, F.; Hao, P. From initial nucleation to cassie-baxter state of condensed droplets on nanotextured superhydrophobic surfaces. Sci. Rep. 2017, 7, 42752. [Google Scholar] [CrossRef] [PubMed]
- Talley, S.J.; AndersonSchoepe, C.L.; Berger, C.J.; Leary, K.A.; Snyder, S.A.; Moore, R.B. Mechanically robust and superhydrophobic aerogels of poly (ether ether ketone). Polymer 2017, 126, 437–445. [Google Scholar] [CrossRef]
- Hönes, R.; Rühe, J. “Nickel nanoflowers” with surface-attached fluoropolymer networks by c, h insertion for the generation of metallic superhydrophobic surfaces. Langmuir 2018, 34, 5342–5351. [Google Scholar] [CrossRef] [PubMed]
- Ellinas, K.; Tserepi, A.; Gogolides, E. Durable superhydrophobic and superamphiphobic polymeric surfaces and their applications: A review. Adv. Colloid Interface Sci. 2017, 250, 132–157. [Google Scholar] [CrossRef] [PubMed]
- Khorasani, M.; Mirzadeh, H.; Kermani, Z. Wettability of porous polydimethylsiloxane surface: Morphology study. Appl. Surf. Sci. 2005, 242, 339–345. [Google Scholar] [CrossRef]
- Olde Riekerink, M.; Terlingen, J.; Engbers, G.; Feijen, J. Selective etching of semicrystalline polymers: CF4 gas plasma treatment of poly (ethylene). Langmuir 1999, 15, 4847–4856. [Google Scholar] [CrossRef]
- Shirtcliffe, N.; McHale, G.; Newton, M.; Perry, C. Wetting and wetting transitions on copper-based super-hydrophobic surfaces. Langmuir 2005, 21, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Cebeci, F.C.; Cohen, R.E.; Rubner, M.F. Stable superhydrophobic coatings from polyelectrolyte multilayers. Nano Lett. 2004, 4, 1349–1353. [Google Scholar] [CrossRef]
- Huang, J.; Lai, Y.; Wang, L.; Li, S.; Ge, M.; Zhang, K.; Fuchs, H.; Chi, L. Controllable wettability and adhesion on bioinspired multifunctional TiO2 nanostructure surfaces for liquid manipulation. J. Mater. Chem. A 2014, 2, 18531–18538. [Google Scholar] [CrossRef]
- Lauren, S. Durability of Superhydrophobic Surfaces—The Biggest Obstacle Towards Real Life Applications. 2016. Available online: https://blog.biolinscientific.com/durability-of-superhydrophobic-surfaces-the-biggest-obstacle-towards-real-life-applications (accessed on 20 March 2017).
- Song, X.; Zhai, J.; Wang, Y.; Jiang, L. Fabrication of superhydrophobic surfaces by self-assembly and their water-adhesion properties. J. Phys. Chem. B 2005, 109, 4048–4052. [Google Scholar] [CrossRef] [PubMed]
- Raman, A.; Dubey, M.; Gouzman, I.; Gawalt, E.S. Formation of self-assembled monolayers of alkylphosphonic acid on the native oxide surface of ss316l. Langmuir 2006, 22, 6469–6472. [Google Scholar] [CrossRef] [PubMed]
- Raman, A.; Gawalt, E.S. Self-assembled monolayers of alkanoic acids on the native oxide surface of ss316l by solution deposition. Langmuir 2007, 23, 2284–2288. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Kisslinger, R.; Farsinezhad, S.; Mahdi, N.; Bhatnagar, A.; Hosseini, A.; Bu, L.; Hua, W.; Wiltshire, B.D.; Eisenhawer, A.; et al. All-solution processed, scalable superhydrophobic coatings on stainless steel surfaces based on functionalized discrete titania nanotubes. Chem. Eng. J. 2018, 351, 482–489. [Google Scholar] [CrossRef]
- Kar, P.; Pandey, A.; Greer, J.J.; Shankar, K. Ultrahigh sensitivity assays for human cardiac troponin i using TiO2 nanotube arrays. Lab Chip 2012, 12, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Zarifi, M.H.; Farsinezhad, S.; Wiltshire, B.D.; Abdorrazaghi, M.; Mahdi, N.; Kar, P.; Daneshmand, M.; Shankar, K. Effect of phosphonate monolayer adsorbate on the microwave photoresponse of TiO2 nanotube membranes mounted on a planar double ring resonator. Nanotechnology 2016, 27, 375201. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpour, A.; Wiltshire, B.D.; Zhang, Y.; Farsinezhad, S.; Askar, A.M.; Kisslinger, R.; Ren, Y.; Kar, P.; Shankar, K. 100-fold improvement in carrier drift mobilities in alkanephosphonate-passivated monocrystalline TiO2 nanowire arrays. Nanotechnology 2017, 28, 144001. [Google Scholar] [CrossRef] [PubMed]
- McDermott, J.E.; McDowell, M.; Hill, I.G.; Hwang, J.; Kahn, A.; Bernasek, S.L.; Schwartz, J. Organophosphonate self-assembled monolayers for gate dielectric surface modification of pentacene-based organic thin-film transistors: A comparative study. J. Phys. Chem. A 2007, 111, 12333–12338. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, R.; Shoup, D.; Behnke, G.; Peck, C.; Agarwal, S.; Gupta, R.K.; Fagan, J.W.; Mueller, K.T.; Iuliucci, R.J.; Wang, Q. Study of perfluorophosphonic acid surface modifications on zinc oxide nanoparticles. Materials 2017, 10, 1363. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-F.; Wang, Y.-T.; Tung, P.-H.; Kuo, S.-W.; Lin, C.-H.; Sheen, Y.-C.; Chang, F.-C. Stable superhydrophobic polybenzoxazine surfaces over a wide ph range. Langmuir 2006, 22, 8289–8292. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhou, F.; Hao, J.; Liu, W. Stable biomimetic super-hydrophobic engineering materials. J. Am. Chem. Soc. 2005, 127, 15670–15671. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Lin, T.; Lin, L.; Lin, S.; Fu, F.; Wang, X.; Guo, L. Invisible security ink based on water-soluble graphitic carbon nitride quantum dots. Angew. Chem., Int. Ed. 2016, 55, 2773–2777. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Wang, Q.; Xiao, Y.; Su, C.; Chen, Q. The stability of superhydrophobic surfaces tested by high speed current scouring. Appl. Surf. Sci. 2008, 254, 2911–2916. [Google Scholar] [CrossRef]
- Extrand, C.; Moon, S.I. Repellency of the lotus leaf: Contact angles, drop retention, and sliding angles. Langmuir 2014, 30, 8791–8797. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, Z.; Men, X.; Yang, J.; Wang, K.; Xu, X.; Zhou, X.; Xue, Q. Robust superhydrophobic surfaces with mechanical durability and easy repairability. J. Mater. Chem. 2011, 21, 15793–15797. [Google Scholar] [CrossRef]
- Maitra, T.; Antonini, C.; der Mauer, M.A.; Stamatopoulos, C.; Tiwari, M.K.; Poulikakos, D. Hierarchically nanotextured surfaces maintaining superhydrophobicity under severely adverse conditions. Nanoscale 2014, 6, 8710–8719. [Google Scholar] [CrossRef] [PubMed]
- Forouzandeh, F.; Li, X.; Banham, D.W.; Feng, F.; Joseph Kakanat, A.; Ye, S.; Birss, V. Improving the corrosion resistance of proton exchange membrane fuel cell carbon supports by pentafluorophenyl surface functionalization. J. Power Sources 2018, 378, 732–741. [Google Scholar] [CrossRef]
- Li, X.; Forouzandeh, F.; Kakanat, A.J.; Feng, F.; Banham, D.W.H.; Ye, S.; Kwok, D.Y.; Birss, V. Surface characteristics of microporous and mesoporous carbons functionalized with pentafluorophenyl groups. ACS Appl. Mater. Inter. 2018, 10, 2130–2142. [Google Scholar] [CrossRef] [PubMed]
- Farsinezhad, S.; Waghmare, P.R.; Wiltshire, B.D.; Sharma, H.; Amiri, S.; Mitra, S.K.; Shankar, K. Amphiphobic surfaces from functionalized TiO2 nanotube arrays. RSC Adv. 2014, 4, 33587–33598. [Google Scholar] [CrossRef]
- Schmidt-Stein, F.; Thiemann, S.; Berger, S.; Hahn, R.; Schmuki, P. Mechanical properties of anatase and semi-metallic tio2 nanotubes. Acta Mater. 2010, 58, 6317–6323. [Google Scholar] [CrossRef]
- Grimes, C.A.; Mor, G.K. TiO2 Nanotube Arrays: Synthesis, properties, and applications; Springer Science & Business Media: New York, NY, USA, 2009. [Google Scholar]
- Mun, K.S.; Alvarez, S.D.; Choi, W.Y.; Sailor, M.J. A stable, label-free optical interferometric biosensor based on TiO2 nanotube arrays. ACS Nano 2010, 4, 2070–2076. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Park, J.; Pittrof, A.; Song, Y.Y.; von der Mark, K.; Schmuki, P. Covalent functionalization of TiO2 nanotube arrays with egf and bmp-2 for modified behavior towards mesenchymal stem cells. Integr. Biol. 2011, 3, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Punzon-Quijorna, E.; Vaquero, V.S.; Rodriguez-Lopez, S.; de la Prida, V.M.; Font, A.C.; Ruiz, J.P.G.; Hernandez-Velez, M.; Silvan, M.M. Polymerized nanoporous titania surfaces: Modification of cell adhesion by acrylic acid functionalization. Compos. Interfaces 2012, 19, 251–258. [Google Scholar] [CrossRef]
- Lee, J.K.; Choi, D.S.; Jang, I.; Choi, W.Y. Improved osseointegration of dental titanium implants by TiO2 nanotube arrays with recombinant human bone morphogenetic protein-2: A pilot in vivo study. Int. J. Nanomed. 2015, 10, 1145–1154. [Google Scholar]
- Kim, S.; Mor, G.K.; Paulose, M.; Varghese, O.K.; Shankar, K.; Grimes, C.A. Broad spectrum light harvesting in TiO2 nanotube array-hemicyanine dye-P3HT hybrid solid-state solar cells. IEEE J. Sel. Top. Quantum Electron. 2010, 16, 1573–1580. [Google Scholar] [CrossRef]
- Rather, R.A.; Singh, S.; Pal, B. A C3N4 surface passivated highly photoactive Au- TiO2 tubular nanostructure for the efficient H2 production from water under sunlight irradiation. Appl. Catal. B-Environ. 2017, 213, 9–17. [Google Scholar] [CrossRef]
- Zhu, Y.-P.; Ma, T.-Y.; Liu, Y.-L.; Ren, T.-Z.; Yuan, Z.-Y. Metal phosphonate hybrid materials: From densely layered to hierarchically nanoporous structures. Inorg. Chem. Front. 2014, 1, 360–383. [Google Scholar] [CrossRef]
- Lomoschitz, C.J.; Feichtenschlager, B.; Moszner, N.; Puchberger, M.; Müller, K.; Abele, M.; Kickelbick, G. Directing alkyl chain ordering of functional phosphorus coupling agents on ZrO2. Langmuir 2011, 27, 3534–3540. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.; Seeger, S. Superamphiphobic surfaces. Chem. Soc. Rev. 2014, 43, 2784–2798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhushan, B.; Koch, K.; Jung, Y.C. Nanostructures for superhydrophobicity and low adhesion. Soft Matter 2008, 4, 1799–1804. [Google Scholar] [CrossRef]
- Cassie, A. Contact angles. Discuss. Faraday Soc. 1948, 3, 11–16. [Google Scholar] [CrossRef]
- Kantor-Uriel, N.; Roy, P.; Saris, S.; Kiran, V.; Waldeck, D.H.; Naaman, R. Evidence for enhanced electron transfer by multiple contacts between self-assembled organic monolayers and semiconductor nanoparticles. J. Phys. Chem. C 2015, 119, 15839–15845. [Google Scholar] [CrossRef]
- Lee, W.H.; Park, J.; Kim, Y.; Kim, K.S.; Hong, B.H.; Cho, K. Control of graphene field-effect transistors by interfacial hydrophobic self-assembled monolayers. Adv. Mater. 2011, 23, 3460–3464. [Google Scholar] [CrossRef] [PubMed]
- Shimoaka, T.; Itoh, Y.; Hasegawa, T. Dynamic rearrangement of stearic acid molecules adsorbed on a gold surface induced by ambient water molecules studied by infrared spectroscopy. J. Phys. Chem. C 2012, 116, 17142–17148. [Google Scholar] [CrossRef]
- Yee, C.; Kataby, G.; Ulman, A.; Prozorov, T.; White, H.; King, A.; Rafailovich, M.; Sokolov, J.; Gedanken, A. Self-assembled monolayers of alkanesulfonic and-phosphonic acids on amorphous iron oxide nanoparticles. Langmuir 1999, 15, 7111–7115. [Google Scholar] [CrossRef]
- Saleema, N.; Sarkar, D.K.; Gallant, D.; Paynter, R.W.; Chen, X.-G. Chemical nature of superhydrophobic aluminum alloy surfaces produced via a one-step process using fluoroalkyl-silane in a base medium. ACS Appl. Mater. Inter. 2011, 3, 4775–4781. [Google Scholar] [CrossRef] [PubMed]
- Steele, A.; Davis, A.; Kim, J.; Loth, E.; Bayer, I.S. Wear independent similarity. ACS Appl. Mater. Inter. 2015, 7, 12695–12701. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.A. Principles and Prevention of Corrosion; Pearson Education: Upper Saddle River, NJ, USA, 1996. [Google Scholar]
- Bandyopadhyay, K.; Vijayamohanan, K.; Shekhawat, G.; Gupta, R.P. Impedance analysis of self-assembled naphthalene disulfide monolayer on gold using external redox probes. J. Electroanal. Chem. 1998, 447, 11–16. [Google Scholar] [CrossRef]
- Schoenfisch, M.H.; Pemberton, J.E. Air stability of alkanethiol self-assembled monolayers on silver and gold surfaces. J. Am. Chem. Soc. 1998, 120, 4502–4513. [Google Scholar] [CrossRef]
- Folkers, J.P.; Gorman, C.B.; Laibinis, P.E.; Buchholz, S.; Whitesides, G.M.; Nuzzo, R.G. Self-assembled monolayers of long-chain hydroxamic acids on the native oxide of metals. Langmuir 1995, 11, 813–824. [Google Scholar] [CrossRef]
- Mani, G.; Johnson, D.M.; Marton, D.; Dougherty, V.L.; Feldman, M.D.; Patel, D.; Ayon, A.A.; Agrawal, C.M. Stability of self-assembled monolayers on titanium and gold. Langmuir 2008, 24, 6774–6784. [Google Scholar] [CrossRef] [PubMed]
- Silverman, B.M.; Wieghaus, K.A.; Schwartz, J. Comparative properties of siloxane vs phosphonate monolayers on a key titanium alloy. Langmuir 2005, 21, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Marcinko, S.; Fadeev, A.Y. Hydrolytic stability of organic monolayers supported on TiO2 and ZrO2. Langmuir 2004, 20, 2270–2273. [Google Scholar] [CrossRef] [PubMed]
- Mor, G.K.; Varghese, O.K.; Paulose, M.; Grimes, C.A. Transparent highly ordered TiO2 nanotube arrays via anodization of titanium thin films. Adv. Funct. Mater. 2005, 15, 1291–1296. [Google Scholar] [CrossRef]
- Macak, J.M.; Tsuchiya, H.; Berger, S.; Bauer, S.; Fujimoto, S.; Schmuki, P. On wafer TiO2 nanotube-layer formation by anodization of ti-films on si. Chem. Phys. Lett. 2006, 428, 421–425. [Google Scholar] [CrossRef]
- Premchand, Y.D.; Djenizian, T.; Vacandio, F.; Knauth, P. Fabrication of self-organized TiO2 nanotubes from columnar titanium thin films sputtered on semiconductor surfaces. Electrochem. Commun. 2006, 8, 1840–1844. [Google Scholar] [CrossRef]
- Galstyan, V.; Vomiero, A.; Comini, E.; Faglia, G.; Sberveglieri, G. TiO2 nanotubular and nanoporous arrays by electrochemical anodization on different substrates. RSC Adv. 2011, 1, 1038–1044. [Google Scholar] [CrossRef]
- Galstyan, V.; Vomiero, A.; Concina, I.; Braga, A.; Brisotto, M.; Bontempi, E.; Faglia, G.; Sberveglieri, G. Vertically aligned TiO2 nanotubes on plastic substrates for flexible solar cells. Small 2011, 7, 2437–2442. [Google Scholar] [CrossRef] [PubMed]
- Weickert, J.; Palumbiny, C.; Nedelcu, M.; Bein, T.; Schmidt-Mende, L. Controlled growth of TiO2 nanotubes on conducting glass. Chem. Mater. 2011, 23, 155–162. [Google Scholar] [CrossRef]
- Farsinezhad, S.; Mohammadpour, A.; Dalrymple, A.N.; Geisinger, J.; Kar, P.; Brett, M.J.; Shankar, K. Transparent anodic TiO2 nanotube arrays on plastic substrates for disposable biosensors and flexible electronics. J. Nanosci. Nanotechnol. 2013, 13, 2885–2891. [Google Scholar] [CrossRef] [PubMed]


| βa (V.decade−1) | βc (V.decade−1) | Ecorr (V w.r.t. Ag/AgCl) | Icorr (µA.cm−2) | Rp (Ω.cm−2) | φ (%) | |
|---|---|---|---|---|---|---|
| ODPA SAM | 0.250 | −0.178 | −0.774 | 1.48 | 181676 | 99.99 |
| PFDPA SAM | 0.329 | −0.153 | −0.677 | 5.75 | 21626 | 99.72 |
| Commercial coating | 0.079 | −0.052 | −1.452 | 10.91 | 6036 | 98.16 |
| Bare | 0.481 | −0.099 | −0.774 | 729.45 | 74 | 0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hua, W.; Kar, P.; Roy, P.; Bu, L.; Shoute, L.C.T.; Kumar, P.; Shankar, K. Resistance of Superhydrophobic Surface-Functionalized TiO2 Nanotubes to Corrosion and Intense Cavitation. Nanomaterials 2018, 8, 783. https://doi.org/10.3390/nano8100783
Hua W, Kar P, Roy P, Bu L, Shoute LCT, Kumar P, Shankar K. Resistance of Superhydrophobic Surface-Functionalized TiO2 Nanotubes to Corrosion and Intense Cavitation. Nanomaterials. 2018; 8(10):783. https://doi.org/10.3390/nano8100783
Chicago/Turabian StyleHua, Weidi, Piyush Kar, Partha Roy, Lintong Bu, Lian C. T. Shoute, Pawan Kumar, and Karthik Shankar. 2018. "Resistance of Superhydrophobic Surface-Functionalized TiO2 Nanotubes to Corrosion and Intense Cavitation" Nanomaterials 8, no. 10: 783. https://doi.org/10.3390/nano8100783