Preparation of Self-supporting Bagasse Cellulose Nanofibrils Hydrogels Induced by Zinc Ions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation and Characterization of CNFs
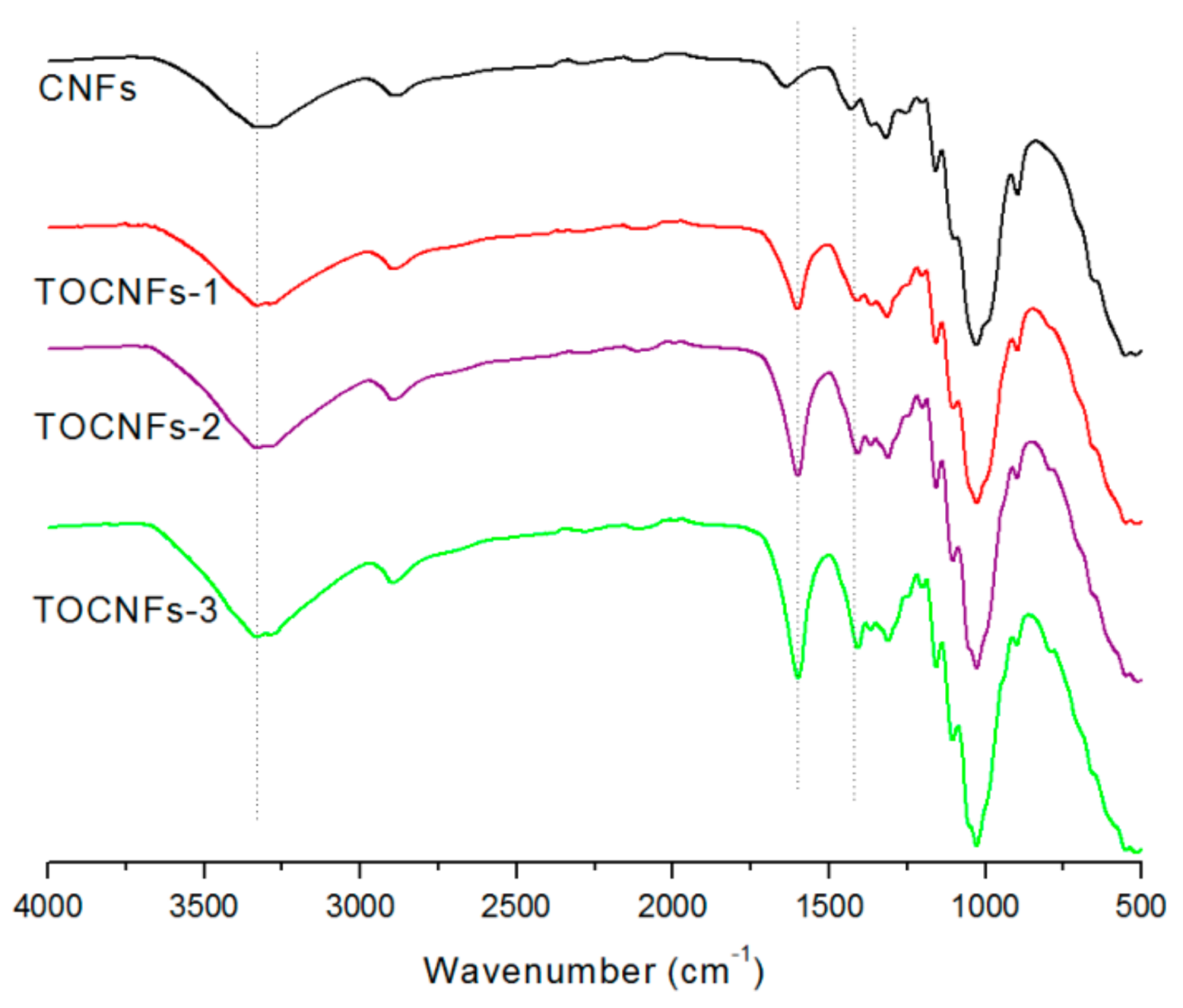
2.3. Preparation and Characterization of Carboxylated CNF
2.4. Preparation and Characterization of CNFs Hydrogels
2.5. Preparation of CNFs Hydrogels-Based CO2 Sensitive Indicator and An Application Experiment
3. Results and Discussion
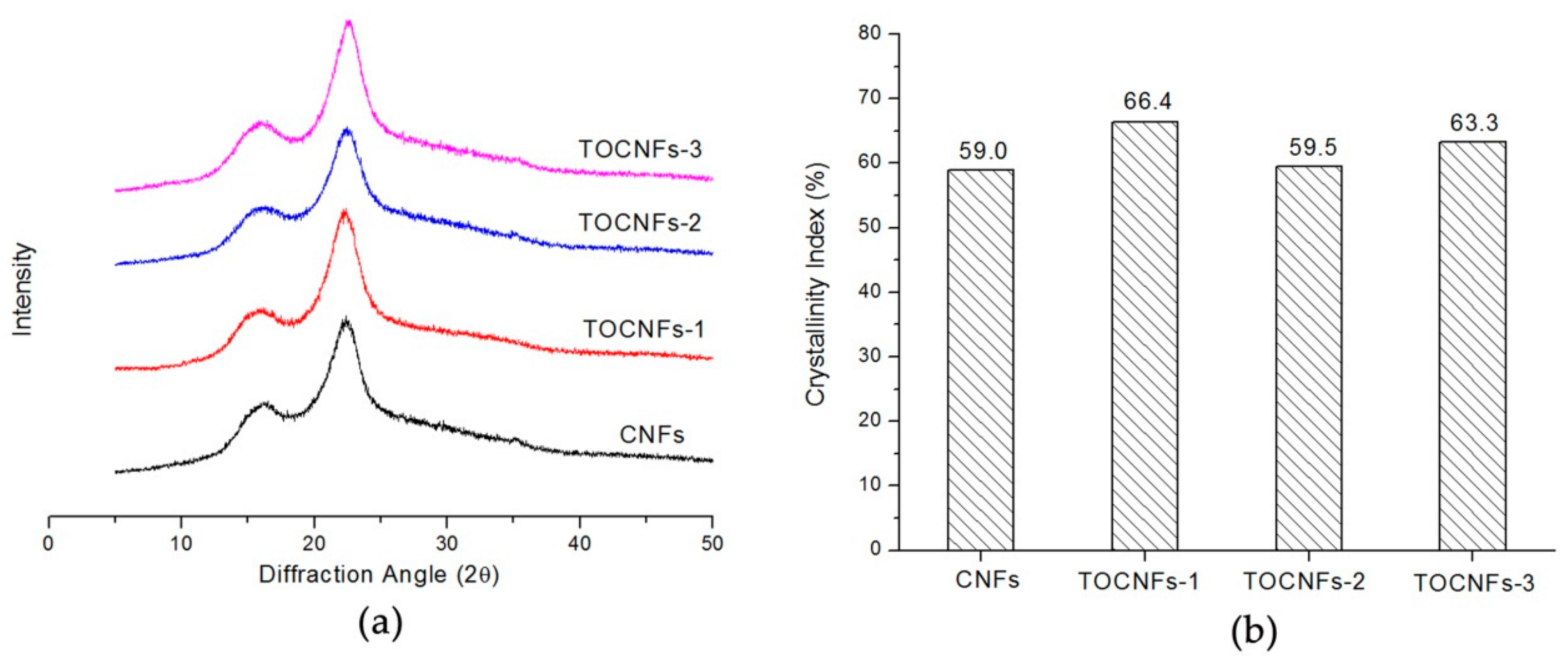
3.1. The CNFs Material
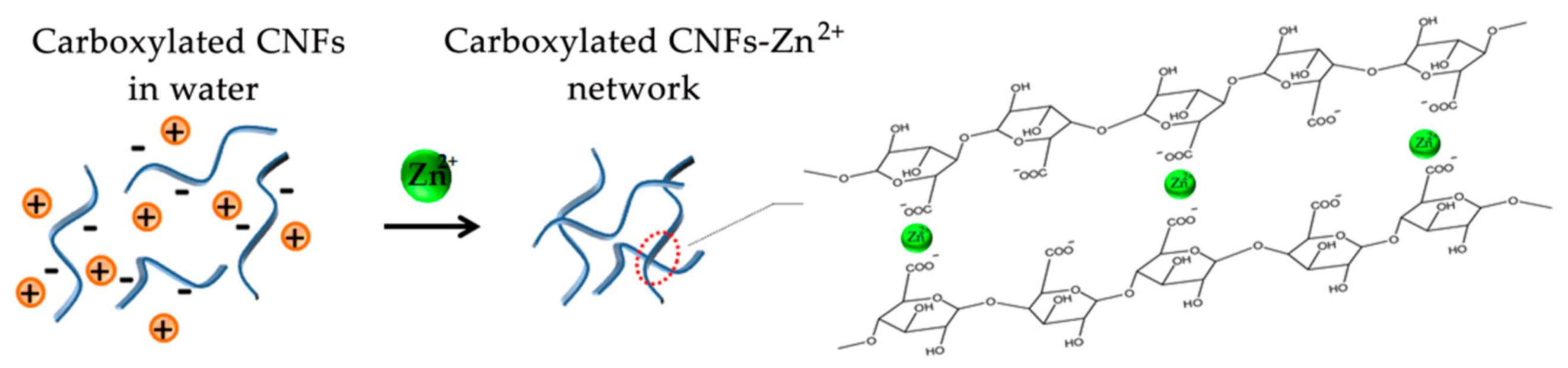
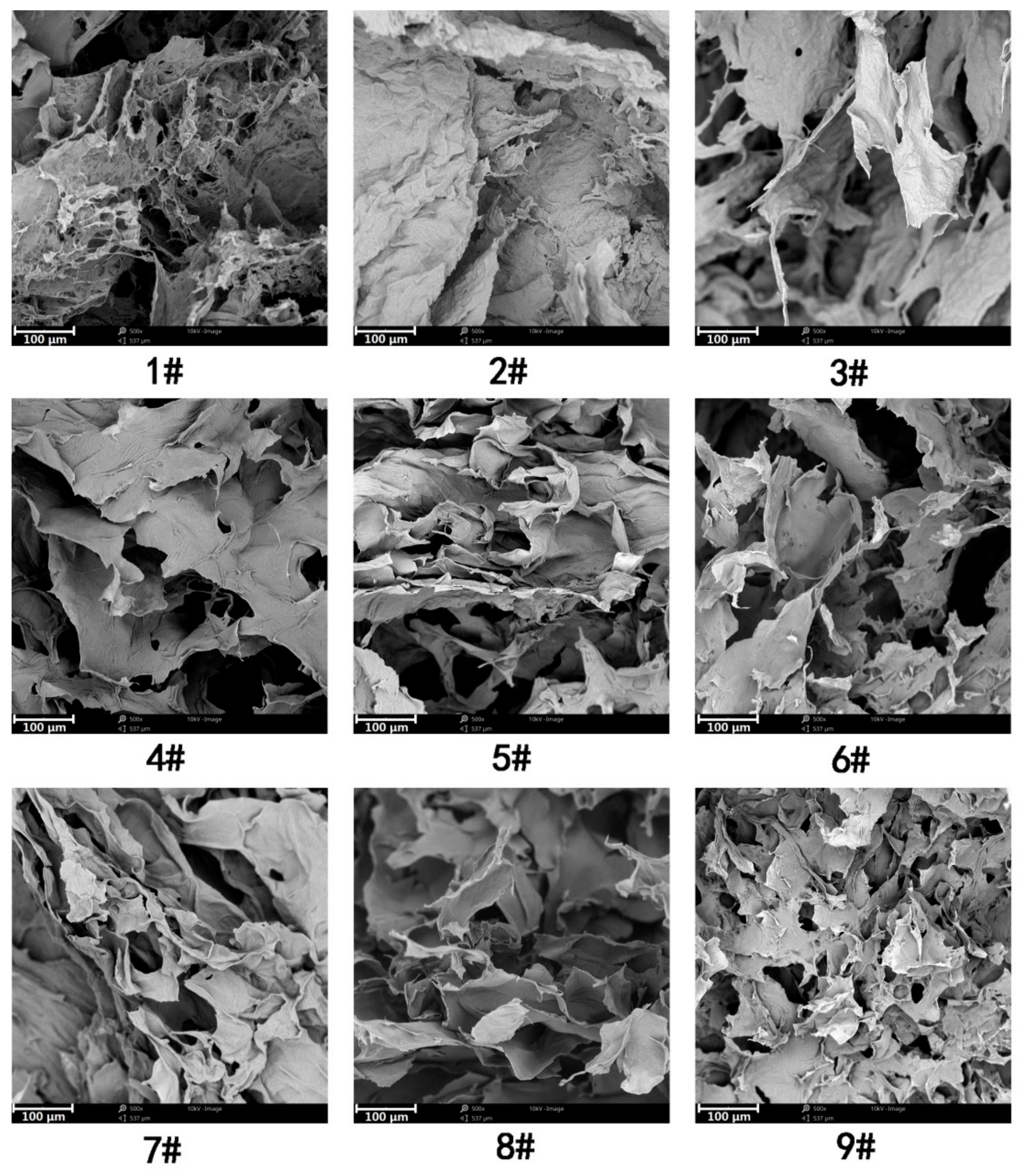
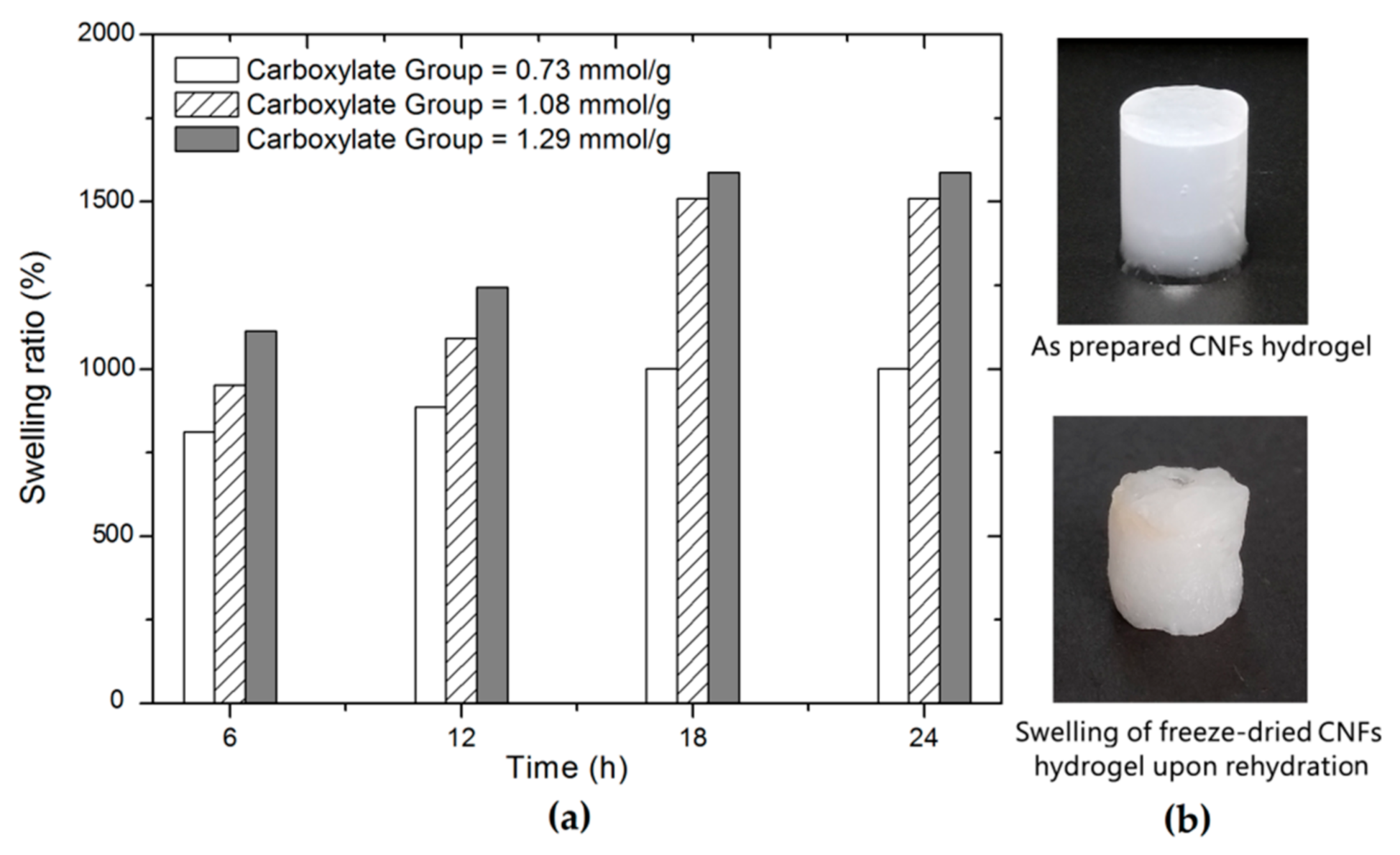
3.2. Formation of CNFs Hydrogels
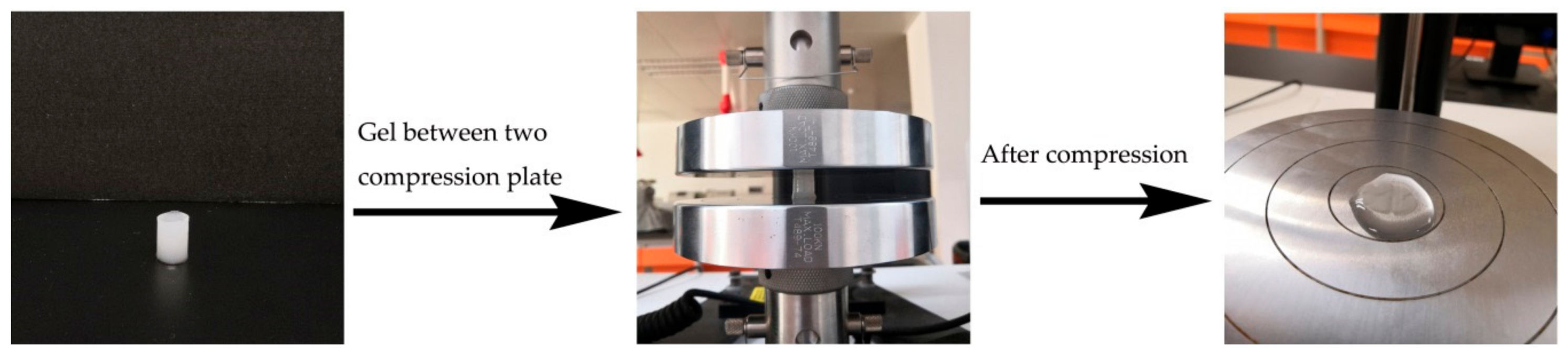
3.3. Mechanical Strength of Hydrogels
3.4. Application of Hydrogels
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lu, P.; Guo, M.; Xu, Z.; Wu, M. Application of Nanofibrillated Cellulose on BOPP/LDPE Film as Oxygen Barrier and Antimicrobial Coating Based on Cold Plasma Treatment. Coatings 2018, 8, 207. [Google Scholar] [CrossRef]
- Abitbol, T.; Rivkin, A.; Cao, Y.; Nevo, Y.; Abraham, E.; Ben-Shalom, T.; Lapidot, S.; Shoseyov, O. Nanocellulose, a tiny fiber with huge applications. Curr. Opin. Biotech. 2016, 39, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Lu, P.; Song, X.; Nie, S.; Liu, Y.; Liu, M.; Wang, Z. Enzyme-assisted mechanical production of microfibrillated cellulose from Northern Bleached Softwood Kraft pulp. Cellulose 2017, 24, 3929–3942. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Y.; Yan, D.; Zhang, C.; Nie, S. Enzyme-assisted mechanical production of cellulose nanofibrils: Thermal stability. Cellulose 2018, 29, 1–14. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Zhang, L. Cellulose-based hydrogels: Present status and application prospects. Carbohyr. Polym. 2011, 84, 40–53. [Google Scholar] [CrossRef]
- Ye, D.; Zhong, Z.; Xu, H.; Chang, C.; Yang, Z.; Wang, Y.; Ye, Q.; Zhang, L. Construction of cellulose/nanosilver sponge materials and their antibacterial activities for infected wounds healing. Cellulose 2015, 23, 749–763. [Google Scholar] [CrossRef]
- Peng, N.; Wang, Y.; Ye, Q.; Liang, L.; An, Y.; Li, Q.; Chang, C. Biocompatible cellulose-based superabsorbent hydrogels with antimicrobial activity. Carbohyr. Polym. 2016, 137, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Duan, B.; Cai, J.; Zhang, L. Superabsorbent hydrogels based on cellulose for smart swelling and controllable delivery. Eur. Polym. J. 2010, 46, 92–100. [Google Scholar] [CrossRef]
- Dong, H.; Snyder, J.F.; Tran, D.T.; Leadore, J.L. Hydrogel, aerogel and film of cellulose nanofibrils functionalized with silver nanoparticles. Carbohyr. Polym. 2013, 95, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Duan, B.; Zhang, L. Fabrication and characterization of novel macroporous cellulose–alginate hydrogels. Polymer 2009, 50, 5467–5473. [Google Scholar] [CrossRef]
- Dong, H.; Snyder, J.F.; Williams, K.S.; Andzelm, J.W. Cation-Induced Hydrogels of Cellulose Nanofibrils with Tunable Moduli. Biomacromolecules 2013, 14, 3338–3345. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Strømme, M.; Ferraz, N. Towards Tunable Protein-Carrier Wound Dressings Based on Nanocellulose Hydrogels Crosslinked with Calcium Ions. Nanomaterials 2018, 8, 550. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Mukhopadhyay, A.; Jiao, Y.; Yong, Q.; Chen, L.; Xing, Y.; Hamel, J.; Zhu, H. Ultralight, highly thermally insulating and fire resistant aerogel by encapsulating cellulose nanofibers with two-dimensional MoS2. Nanoscale 2017, 9, 11452–11462. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sui, Y.; Liu, C.; Liu, C.; Wu, M.; Li, B.; Li, Y. A physically crosslinked polydopamine/nanocellulose hydrogel as potential versatile vehicles for drug delivery and wound healing. Carbohydr. Polym. 2018, 188, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Lindh, J.; Ålander, E.; Strømme, M.; Ferraz, N. On the use of ion-crosslinked nanocellulose hydrogels for wound healing solutions: Physicochemical properties and application-oriented biocompatibility studies. Carbohydr. Polym. 2017, 174, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Heitz, K.; Strømme, M.; Welch, K.; Ferraz, N. Ion-crosslinked wood-derived nanocellulose hydrogels with tunable antibacterial properties: Candidate materials for advanced wound care applications. Carbohydr. Polym. 2018, 181, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xu, F.; Han, C.-R. Metal Ion Mediated Cellulose Nanofibrils Transient Network in Covalently Cross-linked Hydrogels: Mechanistic Insight into Morphology and Dynamics. Biomacromolecules 2017, 18, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Zander, N.E.; Dong, H.; Steele, J.; Grant, J.T. Metal Cation Cross-Linked Nanocellulose Hydrogels as Tissue Engineering Substrates. ACS Appl. Mater. Interfaces 2014, 6, 18502–18510. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Isogai, A. Ion-exchange behavior of carboxylate groups in fibrous cellulose oxidized by the TEMPO-mediated system. Carbohydr. Polym. 2005, 61, 183–190. [Google Scholar] [CrossRef]
- Saito, T.; Uematsu, T.; Kimura, S.; Enomae, T.; Isogai, A. Self-aligned integration of native cellulose nanofibrils towards producing diverse bulk materials. Soft Matter 2011, 7, 8804–8809. [Google Scholar] [CrossRef]
- Saito, T.; Isogai, A. TEMPO-mediated oxidation of native cellulose. The effect of oxidation conditions on chemical and crystal structures of the water-insoluble fractions. Biomacromolecules 2004, 5, 1983–1989. [Google Scholar] [CrossRef] [PubMed]
- Araki, J.; Wada, M.; Kuga, S.; Okano, T. Flow properties of microcrystalline cellulose suspension prepared by acid treatment of native cellulose. Colloids Surf. A 1998, 142, 75–82. [Google Scholar] [CrossRef]
- Hata, Y.; Sawada, T.; Serizawa, T. Effect of solution viscosity on the production of nanoribbon network hydrogels composed of enzymatically synthesized cellulose oligomers under macromolecular crowding conditions. Polym. J. 2017, 49, 575–581. [Google Scholar] [CrossRef]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose Nanofibers Prepared by TEMPO-Mediated Oxidation of Native Cellulose. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar] [CrossRef] [PubMed]
- Shibata, I.; Isogai, A. Depolymerization of cellouronic acid during TEMPO-mediated oxidation. Cellulose 2003, 10, 151–158. [Google Scholar] [CrossRef]
- Filipova, I.; Fridrihsone, V.; Cabulis, U.; Berzins, A. Synthesis of Nanofibrillated Cellulose by Combined Ammonium Persulphate Treatment with Ultrasound and Mechanical Processing. Nanomaterials 2018, 8, 640. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Feng, J.; Feng, J.; Jiang, Y.; Ding, F. Carbon Aerogels by Pyrolysis of TEMPO-Oxidized Cellulose. Appl. Surf. Sci. 2018, 440, 873–879. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. Recent Advances in Edible Polymer Based Hydrogels as a Sustainable Alternative to Conventional Polymers. J. Agr. Food. Chem. 2018, 66, 6940–6967. [Google Scholar] [CrossRef] [PubMed]
- Jing, M.; Fu, Y.; Fei, X.; Tian, J.; Zhi, H.; Zhang, H.; Xu, L.; Wang, X.; Wang, Y. A novel high-strength polymer hydrogel with identifiability prepared via a one-pot method. Poly. Chem. 2017, 8, 3553–3559. [Google Scholar] [CrossRef]
- Si, L.; Zheng, X.; Nie, J.; Yin, R.; Hua, Y.; Zhu, X. Silicone-based tough hydrogels with high resilience, fast self-recovery, and self-healing properties. Chem. Commun. 2016, 52, 8365–8368. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Navidbakhsh, M.; Alizadeh, M.; Razaghi, R. A comparative study on the elastic modulus of polyvinyl alcohol sponge using different stress-strain definitions. Biomed. Eng. 2014, 59, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Jen, K.P.; Majerus, J.N.; Gu, Y.P. A video technique for obtaining corrected true compressive stress. Exp. Tech. 2010, 13, 11–14. [Google Scholar] [CrossRef]
- Ting, J.H.; Shiau, S.H.; Chen, Y.J.; Pan, F.M.; Wong, H.; Pu, G.M.; Kung, C.Y. Preparation and properties of sputtered nitrogen-doped cobalt silicide film. Thin Solid Films 2004, 468, 155–160. [Google Scholar] [CrossRef]
- Kozlowska, J.; Pauter, K.; Sionkowska, A. Carrageenan-based hydrogels: Effect of sorbitol and glycerin on the stability, swelling and mechanical properties. Polym. Test. 2018, 67, 7–11. [Google Scholar] [CrossRef]
- Hossen, M.R.; Dadoo, N.; Holomakoff, D.G.; Co, A.; Gramlicha, W.M.; Mason, M.D. Wet stable and mechanically robust cellulose nanofibrils (CNF) based hydrogel. Polymer 2018, 151, 231–241. [Google Scholar] [CrossRef]
- Puligundla, P.; Jung, P.; Ko, S. Carbon dioxide sensors for intelligent food packaging applications. Food Control. 2012, 25, 328–333. [Google Scholar] [CrossRef]
- Nopwinyuwong, A.; Trevanich, S.; Suppakul, P. Development of a novel colorimetric indicator label for monitoring freshness of intermediate-moisture dessert spoilage. Talanta 2010, 81, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- Rukchon, C.; Nopwinyuwong, A.; Trevanich, S.; Jinkarn, T.; Suppakuk, P. Development of a food spoilage indicator for monitoring freshness of skinless chicken breast. Talanta 2014, 130, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, M.; Bhandari, B.; Guo, Z. Applicability of a colorimetric indicator label for monitoring freshness of fresh-cut green bell pepper. Postharvest Biol. Technol. 2018, 140, 85–92. [Google Scholar] [CrossRef]
- Mills, A.; Skinner, G.A. Water-based colourimetric optical indicators for the detection of carbon dioxide. Analyst 2010, 135, 1912–1917. [Google Scholar] [CrossRef] [PubMed]
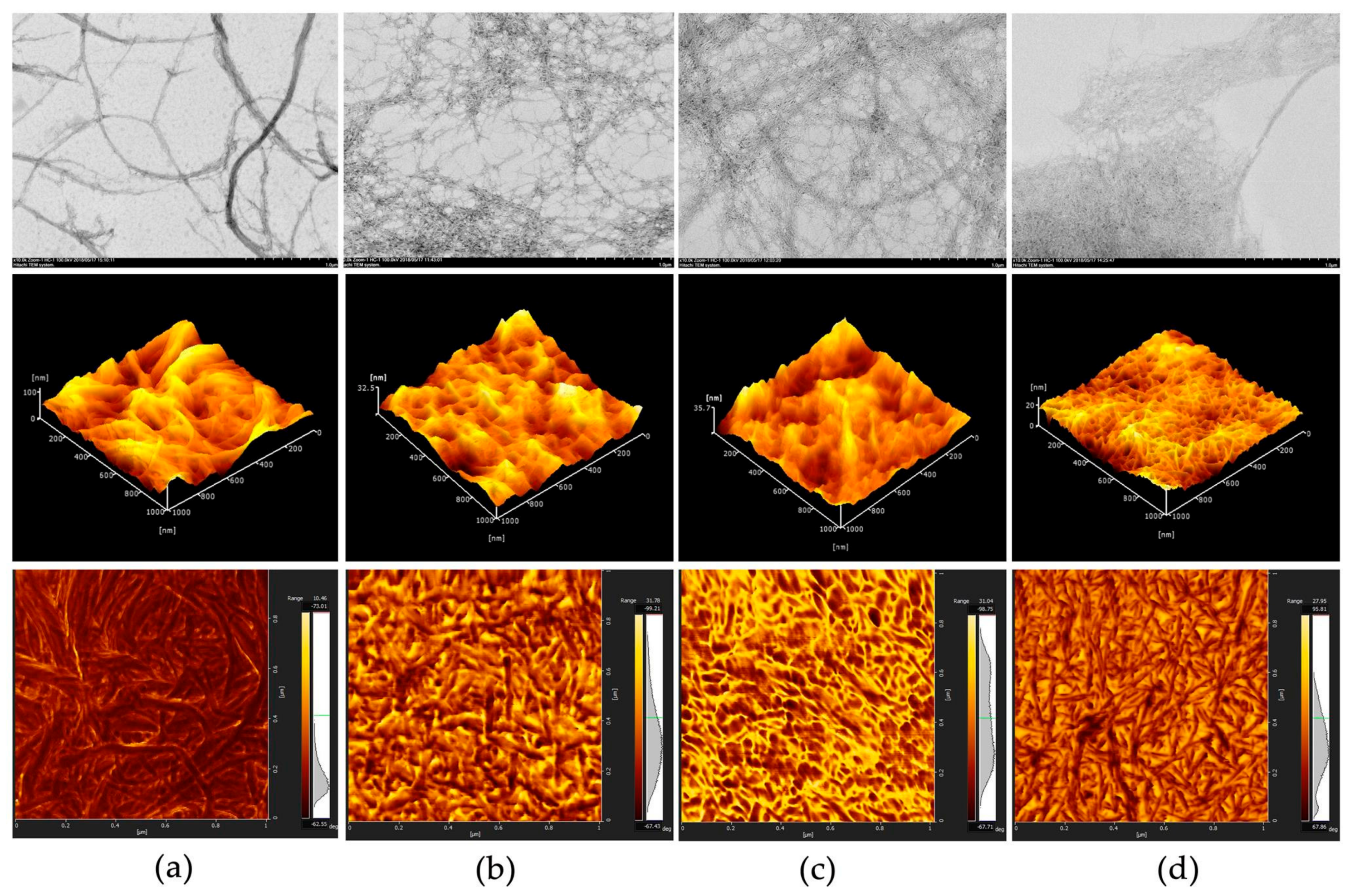

| Sample | Average Size (nm) | Zeta Potential (mv) | Carboxylate Group Content (mmol/g) |
|---|---|---|---|
| CNFs | 3705 | −20.7 | 0.12 |
| TOCNFs-1 | 3552 | −41 | 0.73 |
| TOCNFs-2 | 2673 | −43 | 1.08 |
| TOCNFs-3 | 1044 | −51.8 | 1.29 |
| Sample | TOCNFs Concentration (wt.%) | Carboxylate Group Content (mmol/g) | Zn2+ Concentration (mol/L) | Compressive Stress (kPa) |
|---|---|---|---|---|
| 1 | 1.0 | 0.73 | 0.1 | 46.63 |
| 2 | 1.0 | 1.08 | 0.2 | 76.35 |
| 3 | 1.0 | 1.29 | 0.3 | 76.61 |
| 4 | 2.0 | 0.73 | 0.2 | 156.27 |
| 5 | 2.0 | 1.08 | 0.3 | 183.46 |
| 6 | 2.0 | 1.29 | 0.1 | 170.14 |
| 7 | 3.0 | 0.73 | 0.3 | 226.73 |
| 8 | 3.0 | 1.08 | 0.1 | 253.49 |
| 9 | 3.0 | 1.29 | 0.2 | 337.16 |
| K1 1 | 66.53 | 143.21 | 156.75 | |
| K2 | 169.96 | 171.10 | 189.93 | |
| K3 | 272.46 | 194.64 | 162.27 | |
| R2 | 205.93 | 54.43 | 33.18 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, P.; Liu, R.; Liu, X.; Wu, M. Preparation of Self-supporting Bagasse Cellulose Nanofibrils Hydrogels Induced by Zinc Ions. Nanomaterials 2018, 8, 800. https://doi.org/10.3390/nano8100800
Lu P, Liu R, Liu X, Wu M. Preparation of Self-supporting Bagasse Cellulose Nanofibrils Hydrogels Induced by Zinc Ions. Nanomaterials. 2018; 8(10):800. https://doi.org/10.3390/nano8100800
Chicago/Turabian StyleLu, Peng, Ren Liu, Xin Liu, and Min Wu. 2018. "Preparation of Self-supporting Bagasse Cellulose Nanofibrils Hydrogels Induced by Zinc Ions" Nanomaterials 8, no. 10: 800. https://doi.org/10.3390/nano8100800






