New Fe2O3-Clay@C Nanocomposite Anodes for Li-Ion Batteries Obtained by Facile Hydrothermal Processes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Techniques
2.2. Materials Synthesis and Characterization
- (a)
- up to 200 °C, both hygroscopic and zeolitic water molecules are lost (theoretical mass loss of 11%):Mg8Si12O30(OH)4·(H2O)4·8H2O → Mg8Si12O30(OH)4·(H2O)4 + 8H2O
- (b)
- between 250 and 500 °C, the bound water molecules are lost, i.e., those ones which complete the coordination of the terminal Mg2+ ions at the edges. For this step, the corresponding theoretical mass loss is 5.7%.Let us note that some of the composites prepared by us are heated up to 400 °C. This means that the zeolitic water as well as two of each four bound water molecules are probably lost in them. The partial desorption of the H2O species results in the partial collapse of the tunnels [32].
- (c)
- Finally, up to 800 °C, the hydroxyl units are lost and the sepiolite structure is totally decomposed as follows:Mg8Si12O30(OH)4 → 8MgSiO3 + 4SiO2+ 2H2O
3. Results and Discussion
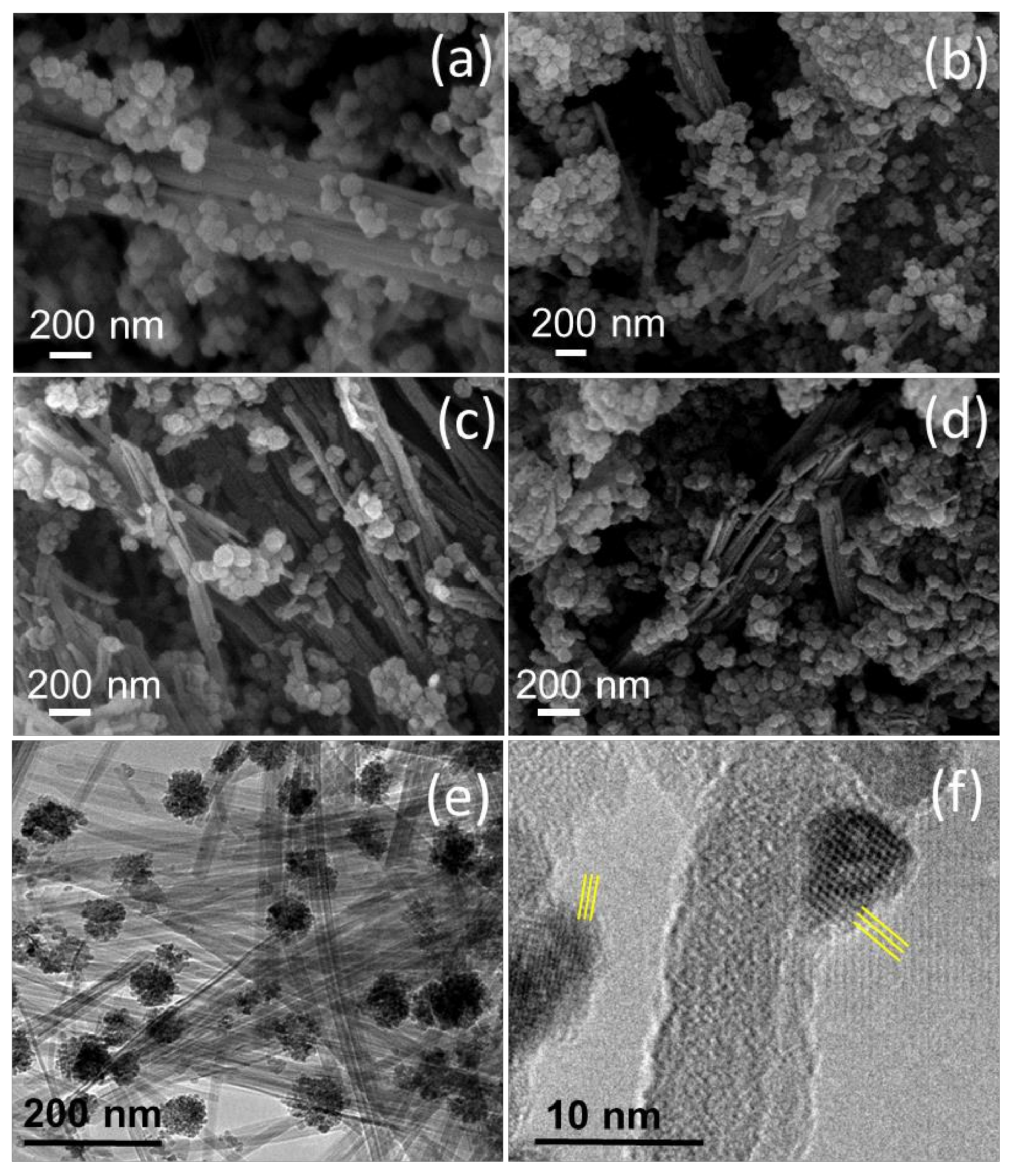
3.1. Structural and Compositional Characterization
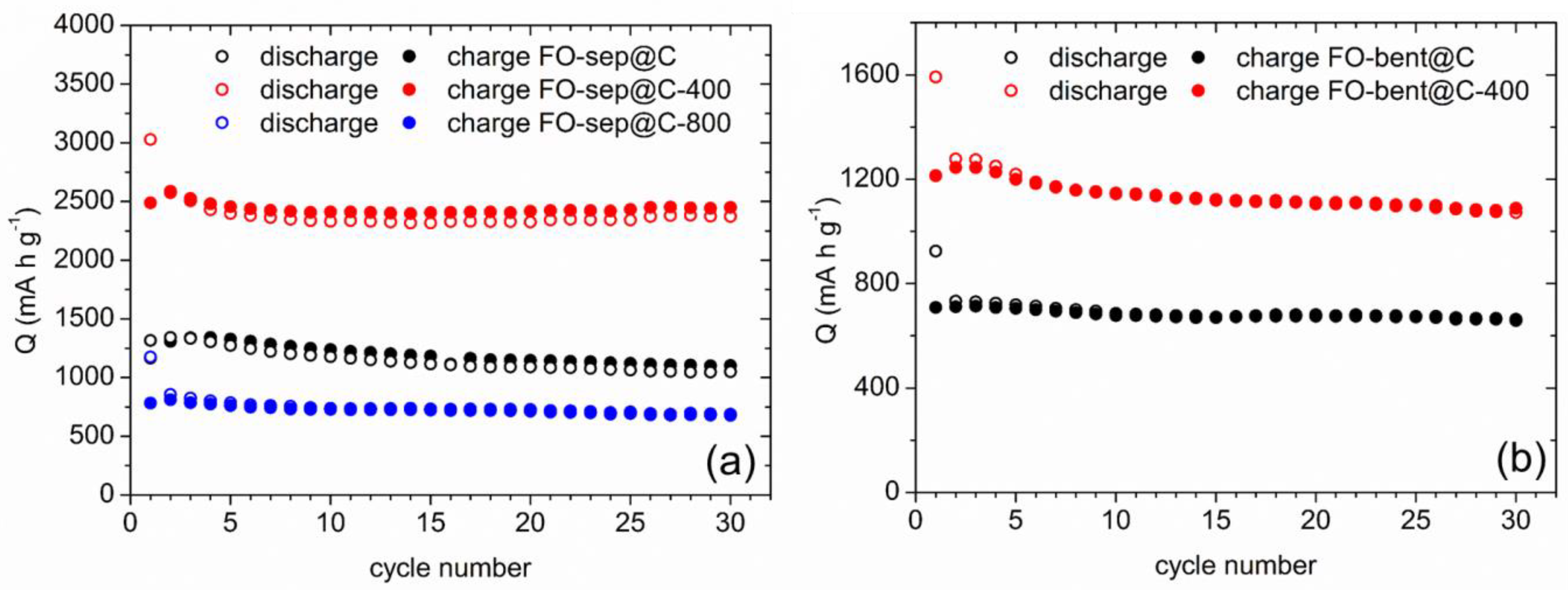
3.2. Electrochemical Behaviour
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bella, F.; Muñoz-García, A.B.; Colò, F.; Meligrana, G.; Lamberti, A.; Destro, M.; Pavone, M.; Gerbaldi, C. Combined structural, chemometric, and electrochemical investigation of vertically aligned TiO2 nanotubes for Na-ion batteries. ACS Omega 2018, 3, 8440–8450. [Google Scholar] [CrossRef]
- Zolin, L.; Nair, J.R.; Beneventi, D.; Bella, F.; Destro, M.; Jagdale, P.; Cannavaro, I.; Tagliaferro, A.; Chaussy, D.; Geobaldo, F.; et al. A simple route toward next-gen green energy storage concept by nanofibres-based self-supporting electrodes and a solid polymeric design. Carbon 2016, 107, 811–822. [Google Scholar] [CrossRef]
- Zhu, J.; Lu, Y.; Chen, C.; Ge, Y.; Jasper, S.; Leary, J.D.; Li, D.; Jiang, M.; Zhang, X. Porous one-dimensional carbon/iron oxide composite for rechargeable lithium-ion batteries with high and stable capacity. J. Alloys Compd. 2016, 672, 79–85. [Google Scholar] [CrossRef]
- Kim, T.S.; Lee, G.-H.; Lee, S.; Choi, Y.-S.; Kim, J.-C.; Song, H.J.; Kim, D.-W. Carbon-decorated iron oxide hollow granules formed using a silk fibrous template: Lithium-oxygen battery and wastewater treatment applications. NPG Asia Mater. 2017, 9, e450. [Google Scholar] [CrossRef]
- Yu, L.; Xi, S.; Wei, C.; Zhang, W.; Du, Y.; Yan, Q.; Xu, Z. Superior Lithium storage properties of β-FeOOH. Adv. Energy Mater. 2014, 5, 1401517. [Google Scholar] [CrossRef]
- Lu, X.; Yin, Y.; Zhang, L.; Huang, S.; Xi, L.; Liu, L.; Oswald, S.; Schmidt, O.G. 3D Ag/NiO-Fe2O3/Ag nanomembranes as carbon-free cathode materials for Li-O2 batteries. Energy Storage Mater. 2019, 16, 155–162. [Google Scholar] [CrossRef]
- Yin, L.; Gao, Y.J.; Jeon, I.; Yang, H.; Kim, J.-P.; Jeong, S.Y.; Cho, C.R. Rice-panicle-like γ-Fe2O3@C nanofibers as high-rate anodes for superior lithium-ion batteries. Chem. Eng. J. 2019, 356, 60–68. [Google Scholar] [CrossRef]
- Hou, K.; Wen, X.; Yan, P.; Tang, A.; Yang, H. Tin Oxide-Carbon-Coated sepiolite nanofibers with enhanced lithium-ion storage property. Nanoscale Res. Lett. 2017, 12, 215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Dong, X.; Peng, L.; Kang, W.; Li, H. The enhanced lithium-storage performance for MnO nanoparticles anchored on electrospun nitrogen-doped carbon fibers. Nanomaterials 2018, 8, 733. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Yang, Z.; Xiong, G.; Guo, R.; Liu, Z.; Luo, H. Anchoring Fe3O4 nanoparticles on three-dimensional carbon nanofibers toward flexible high-performance anodes for lithium-ion batteries. J. Power Sources 2015, 294, 414–419. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, L.; Qiao, W.; Liu, F.; Zhang, Y.; Tan, W.; Qiu, G. Facile crystal-structure-controlled synthesis of iron oxides for adsorbents and anode materials of lithium batteries. Mater. Chem. Phys. 2016, 170, 239–245. [Google Scholar] [CrossRef]
- Alonso-Domínguez, D.; Álvarez-Serrano, I.; Veiga, M.P.; López, M.L.; Urones, E.; Pico, C.; Veiga, M.L. Nanoparticulated spinel-type iron oxides obtained in supercritical water and their electrochemical performance as anodes for Li ion batteries. J. Alloys Compd. 2017, 695, 3239–3248. [Google Scholar] [CrossRef]
- Guan, X.; Liu, X.; Xu, B.; Liu, X.; Kong, Z.; Song, M.; Fu, A.; Li, Y.; Guo, P.; Li, H. Carbon wrapped Ni3S2 nanocrystals anchored on graphene sheets as anode materials for lithium-ion battery and the study on their capacity evolution. Nanomaterials 2018, 8, 760. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Tang, H.; Li, J.; Wu, F.; Wu, T.; Wang, R.; Liu, D.; Pan, M.; Xie, Z.; Qu, D. Self-assembly synthesis of a unique stable cocoon-like hematite @C nanoparticle and its application in lithium ion batteries. J. Colloid Interface Sci. 2017, 495, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, L.; Hu, R.; Ouyang, L.; Zhu, M. Facile synthesis of Fe2O3-graphite composite with stable electrochemical performance as anode material for lithium ion batteries. Electrochim. Acta 2014, 125, 421–426. [Google Scholar] [CrossRef]
- Wu, Y.; Zhan, L.; Huang, K.; Wang, H.; Yu, H.; Wang, S.; Peng, F.; Lai, C. Iron based dual-metal oxides on graphene for lithium-ion batteries anode: Effects of composition and morphology. J. Alloys Compd. 2016, 684, 47–54. [Google Scholar] [CrossRef]
- Zhong, M.; Yang, D.H.; Kong, L.J.; Shuang, W.; Zhang, Y.H.; Bu, X.H. Bimetallic metal-organic framework derived Co3O4–CoFe2O4 composites with different Fe/Co molar ratios as anode materials for lithium ion batteries. Dalton Trans. 2017, 46, 15947–15953. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Liu, S.; Ouyanga, J.; Yang, H. Sepiolite supported stearic acid composites for thermal energy storage. RSC Adv. 2016, 6, 112493–112501. [Google Scholar] [CrossRef]
- Back, C.K.; Kimb, T.J.; Choi, N.S. Activated natural porous silicate for a highly promising SiOx nanostructure finely impregnated with carbon nanofibers as a high performance anode material for lithium-ion batteries. J. Mater. Chem. A 2014, 2, 13648–13654. [Google Scholar] [CrossRef]
- Chen, T.; Wu, J.; Zhang, Q.; Su, X. Recent advancement of SiOx based anodes for lithium-ion batteries. J. Power Sources 2017, 363, 126–144. [Google Scholar] [CrossRef]
- Aranda, P.; Darder, M.; Fernández-Saavedra, R.; López-Blanco, M.; Ruiz-Hitzky, E. Relevance of polymer- and biopolymer-clay nanocomposites in electrochemical and electroanalytical applications. Thin Solid Films 2006, 495, 104–112. [Google Scholar] [CrossRef]
- Gómez-Avilés, A.; Darder, M.; Aranda, P.; Ruiz-Hitzky, E. Functionalized carbon-silicates from caramel-sepiolite nanocomposites. Angew. Chem. Int. Ed. 2007, 46, 923–925. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Avilés, A.; Darder, M.; Aranda, P.; Ruiz-Hitzky, E. Multifunctional materials based on graphene-like/sepiolite nanocomposites. Appl. Clay. Sci. 2010, 47, 203–211. [Google Scholar] [CrossRef]
- Musyoka, N.M.; Langmi, H.W. Bentonite; Mishra, A.K., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2015; Chapter 1; pp. 59–61. [Google Scholar]
- Kalaga, K.; Rodrigues, M.T.F.; Gullapalli, H.; Babu, G.; Arava, L.M.R.; Ajayan, P.M. Quasi-solid electrolytes for high temperature lithium ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 25777–25783. [Google Scholar] [CrossRef] [PubMed]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Cryst. 1969, 2, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Carvajal, J. Recent developments of the program FULLPROF, in commission on powder diffraction (IUCr). Newsletter 2001, 26, 12–19. [Google Scholar]
- Sanchis, R.; Alonso-Domínguez, D.; Dejoz, A.; Pico, M.P.; Álvarez-Serrano, I.; García, T.; López, M.L.; Solsona, B. Eco-friendly cavity-containing iron oxides prepared by mild routes as very efficient catalysts for the total oxidation of VOCs. Materials 2018, 11, 1387. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.D.; Frost, R.L.; Kloprogge, J.T.; Duong, L. Infrared spectroscopy of goethite dehydroxylation: III. FT-IR microscopy of in situ study of the thermal transformation of goethite to hematite. Spectrochim. Acta Part A 2002, 58, 967–981. [Google Scholar] [CrossRef]
- Giustetto, R.; Levy, D.; Wahyudi, O.; Ricchiardi, G.; Vitillo, J.G. Crystal structure refinement of a sepiolite/indigo Maya Blue pigment using molecular modelling and synchrotron diffraction. Eur. J. Miner. 2011, 23, 449–466. [Google Scholar] [CrossRef]
- Frost, R.L.; Ding, Z. Controlled rate thermal analysis and differential scanning calorimetry of sepiolites and palygorskites. Thermochim. Acta 2003, 397, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Post, J.E.; Bish, D.L.; Heaney, P.J. Synchrotron powder X-ray diffraction study of the structure and dehydration behavior of sepiolite. Am. Miner. 2007, 92, 91–97. [Google Scholar] [CrossRef]
- Lu, B.; Guo, H.; Li, P.; Liu, H.; Wei, Y.; Hou, D. Comparison study on transformation of iron oxyhydroxides: Based on theoretical and experimental data. J. Solid State Chem. 2011, 184, 2139–2144. [Google Scholar] [CrossRef]
- Barrón, V.; Torrent, J.; De Grave, E. Hydromaghemite, an intermediate in the hydrothermal transformation of 2-line ferrihydrite into hematite. Am. Mineral. 2003, 88, 1679–1688. [Google Scholar] [CrossRef]
- Liu, H.; Guo, H.; Li, P.; Wei, Y. Transformation from δ-FeOOH to hematite in the presence of trace Fe (II). J. Phys. Chem. Sol. 2009, 70, 186–191. [Google Scholar] [CrossRef]
- Cudennec, Y.; Lecerf, A. The transformation of ferrihydrite into goethite or hematite, revisited. J. Solid State Chem. 2006, 179, 716–722. [Google Scholar] [CrossRef]
- Liu, H.; Wexler, D.; Wang, G. One-pot facile synthesis of iron oxide nanowires as high capacity anode materials for lithium ion batteries. J. Alloys Compd. 2009, 487, L24–L27. [Google Scholar] [CrossRef]
- Krajewski, M.; Lee, P.H.; Wu, S.H.; Brzozka, K.; Malolepszy, A.; Stobinski, L.; Tokarczyk, M.; Kowalski, G.; Wasik, D. Nanocomposite composed of multiwall carbon nanotubes covered by hematite nanoparticles as anode material for Li-ion batteries. Electrochim. Acta 2017, 228, 82–90. [Google Scholar] [CrossRef]
- Maiti, D.; Aravindan, V.; Madhavi, S.; Devi, P.S. Electrochemical performance of hematite nanoparticles derived from spherical maghemite and elongated goethite particles. J. Power Sources 2015, 276, 291–298. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses; John Wiley & Sons: New York, NY, USA, 2003. [Google Scholar]
- An, S.J.; Li, J.; Daniel, C.; Mohanty, D.; Nagpure, S.; Wood, D.L., III. The state of understanding of the lithium-ion-battery graphite solid electrolyte interphase (SEI) and its relationship to formation cycling. Carbon 2016, 105, 52–76. [Google Scholar] [CrossRef]
- Xu, C.; Zeng, Y.; Rui, X.; Zhu, J.; Tan, H.; Guerrero, A.; Toribio, J.; García-Belmonte, G.; Yan, Q. Amorphous iron oxyhydroxide nanosheets: Synthesis, Li storage, and conversion reaction kinetics. J. Phys. Chem. C 2013, 117, 17462–17469. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Guo, J.; McDonald, M.J.; Li, S.; Xu, B.; Yang, Y. Synthesis and characterization of urchin-like Mn0.33Co0.67C2O4 for Li-ion batteries: Role of SEI layers for enhanced electrochemical properties. Electrochim. Acta 2015, 163, 93–101. [Google Scholar] [CrossRef]
- Cavanagh, A.S.; Lee, Y.; Yoon, B.; George, S. Atomic layer deposition of LiOH and Li2CO3 using lithium t-butoxide as the lithium source. ECS Trans. 2010, 33, 223–229. [Google Scholar]
- Zheng, X.; Li, J. A review of research on hematite as anode material for lithium-ion batteries. Ionics 2014, 20, 1651–1663. [Google Scholar] [CrossRef]
- Yu, L.H.; Wei, C.; Yan, Q.Y.; Xu, Z.J. Controlled synthesis of high-performance β-FeOOH anodes for lithium-ion batteries and their size effects. Nano Energy 2015, 13, 397–404. [Google Scholar] [CrossRef]
- Wang, J.; Li, L.; Wong, C.L.; Sun, L.; Shen, Z.; Madhavi, S. Controlled synthesis of α-FeOOH nanorods and their transformation to mesoporous α-Fe2O3, Fe3O4@C nanorods as anodes for lithium ion batteries. RSC Adv. 2013, 3, 15316–15326. [Google Scholar] [CrossRef]
- Barik, R.; Pandey, B.; Anand, S.; Mohapatra, M. A facile, single-step synthesis of flowery shaped, pure/lithium-doped 3D iron oxides. J. Mater. Chem. A 2014, 2, 12380–12389. [Google Scholar] [CrossRef]
- Qin, M.; Li, Y.; Lv, X.-J. Preparation of Ce- and La-Doped Li4Ti5O12. Nanosheets and their electrochemical performance in Li half cell and Li4Ti5O12/LiFePO4 full cell batteries. Nanomaterials 2017, 7, 150. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Domínguez, D.; López, M.L.; García-Quintana, L.; Álvarez-Serrano, I.; Pico, C.; Veiga, M.L. Lithium-ion full cell battery with spinel-type nanostructured electrodes. Nano-Struct. Nano-Objects 2017, 11, 88–93. [Google Scholar] [CrossRef]
- Varzi, A.; Bresser, D.; von Zamory, J.; Müller, F.; Passerini, S. ZnFe2O4-C/LiFePO4-CNT: A novel high-power lithium-ion battery with excellent cycling performance. Adv. Energy Mater. 2014, 4, 1400054. [Google Scholar] [CrossRef] [PubMed]
- Sandi, G.; Carrado, K.A.; Winans, R.E.; Johnson, C.S.; Csencsits, R. Carbons for lithium battery applications prepared using sepiolite as inorganic template. J. Electrochem. Soc. 1999, 146, 3644–3648. [Google Scholar] [CrossRef]
- Esteban-Cubillo, A.; Tulliani, J.M.; Pecharromán, C.; Moya, J.S. Iron-oxide nanoparticles supported on sepiolite as a novel humidity sensor. J. Eur. Ceram. Soc. 2007, 27, 1983–1989. [Google Scholar] [CrossRef]
- Chanéac, C.; Tronc, E.; Jolivet, J.P. Magnetic iron oxide–silica nanocomposites. Synthesis and characterization. J. Mater. Chem. 1996, 6, 1905–1911. [Google Scholar] [CrossRef]









| Notation | Clay | T(°C) 1 |
|---|---|---|
| FO | - | - |
| FO-400 | - | 400 |
| FO-800 | - | 800 |
| Sep@C | sepiolite | - |
| FO-Sep@C | sepiolite | - |
| FO-Sep@C-400 | sepiolite | 400 |
| FO-Sep@C-800 | sepiolite | 800 |
| FO-Ben@C | bentonite | - |
| FO-Ben@C-400 | bentonite | 400 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alonso-Domínguez, D.; Pico, M.P.; Álvarez-Serrano, I.; López, M.L. New Fe2O3-Clay@C Nanocomposite Anodes for Li-Ion Batteries Obtained by Facile Hydrothermal Processes. Nanomaterials 2018, 8, 808. https://doi.org/10.3390/nano8100808
Alonso-Domínguez D, Pico MP, Álvarez-Serrano I, López ML. New Fe2O3-Clay@C Nanocomposite Anodes for Li-Ion Batteries Obtained by Facile Hydrothermal Processes. Nanomaterials. 2018; 8(10):808. https://doi.org/10.3390/nano8100808
Chicago/Turabian StyleAlonso-Domínguez, Daniel, María Pilar Pico, Inmaculada Álvarez-Serrano, and María Luisa López. 2018. "New Fe2O3-Clay@C Nanocomposite Anodes for Li-Ion Batteries Obtained by Facile Hydrothermal Processes" Nanomaterials 8, no. 10: 808. https://doi.org/10.3390/nano8100808
APA StyleAlonso-Domínguez, D., Pico, M. P., Álvarez-Serrano, I., & López, M. L. (2018). New Fe2O3-Clay@C Nanocomposite Anodes for Li-Ion Batteries Obtained by Facile Hydrothermal Processes. Nanomaterials, 8(10), 808. https://doi.org/10.3390/nano8100808