Orthogonal Functionalization of Nanodiamond Particles after Laser Modification and Treatment with Aromatic Amine Derivatives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Functionalization of Nanodiamond Powder with Olefin Bonds on the Surface
2.1.1. Attachment of Benzoic Acid Derivative to the Surface of the Nanodiamond Powder (1)
2.1.2. Synthesis of Aminonaphtalene Derivative Linked to the Surface of the Nanodiamond Powder (2)
2.1.3. Coupling of 6-Aminohexanoic Acid Methyl Ester to Benzoic Acid Derivative on Nanodiamond Powder Surface (3)
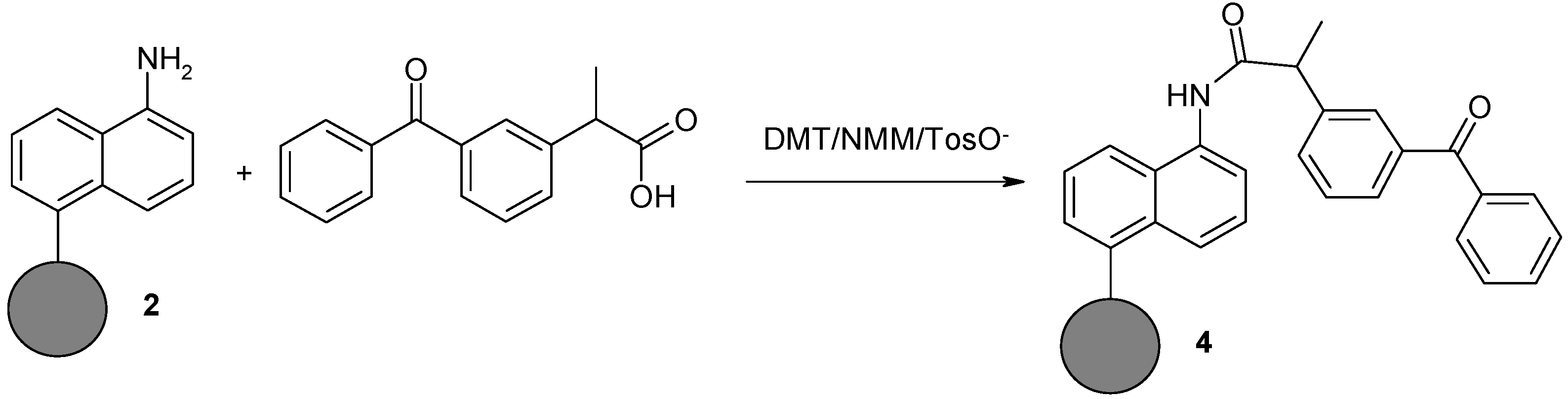
2.1.4. Synthesis of Naphthylamine Derivative with Ketoprofen Attached to the Nanodiamond Powder Surface (4)
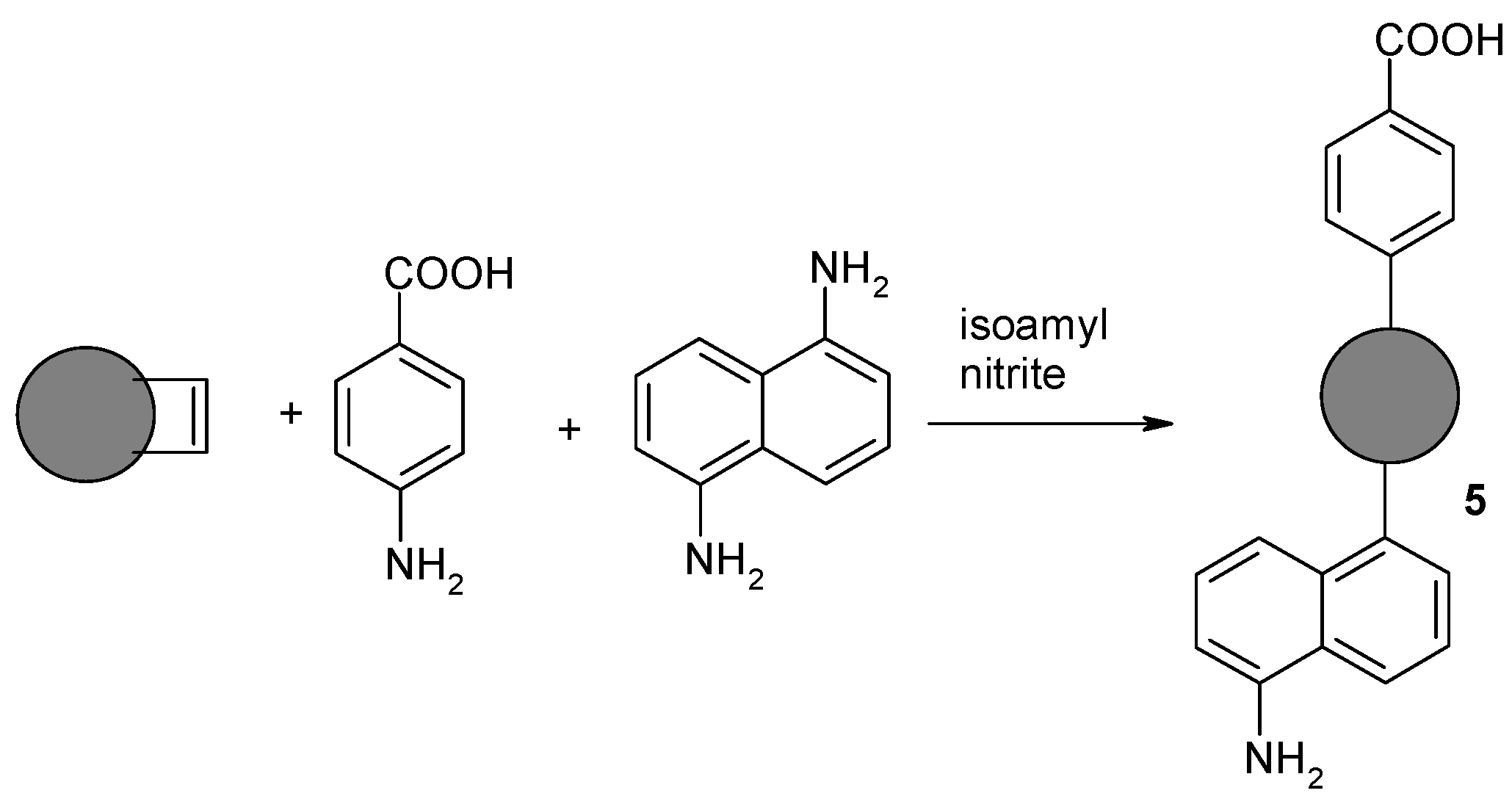
2.1.5. Synthesis of Nanodiamond Derivative (5) Containing Both Benzoic Acid Residue and Naphthylamine on the Surface
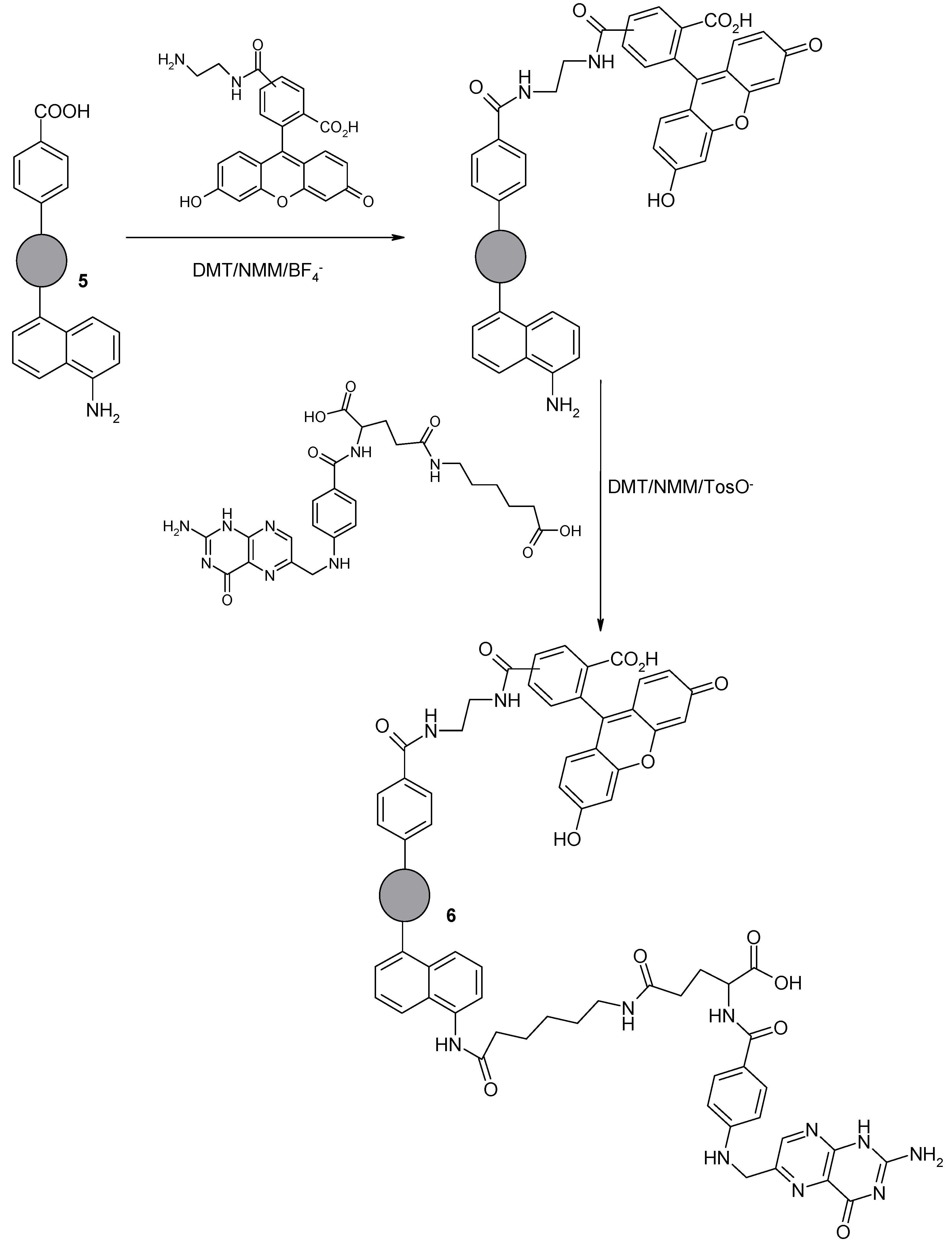
2.1.6. Synthesis of Nanodiamond Derivative (6) Containing Folic Acid Residue and 5(6)-Carboxyfluorescein Orthogonally Attached to the Surface via Two Different, Amphiphilic Linkers
2.2. Method for Determining the Content of Functional Groups on the Surface of a Modified Nanodiamond Powder
2.2.1. Determination of the Amount of Carboxyl Groups
2.2.2. Determination of the Amount of Amine Groups
3. Results and Discussion
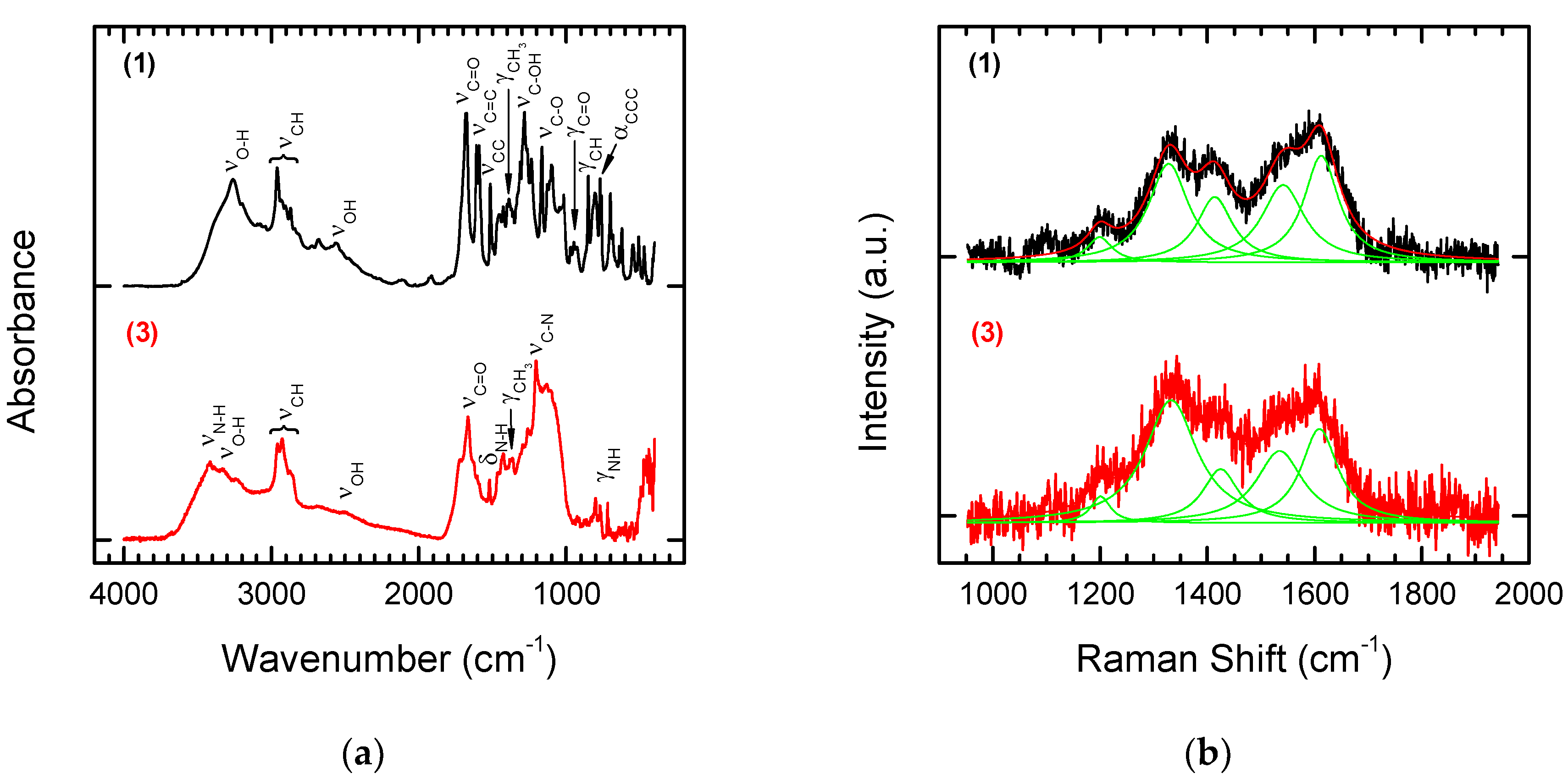
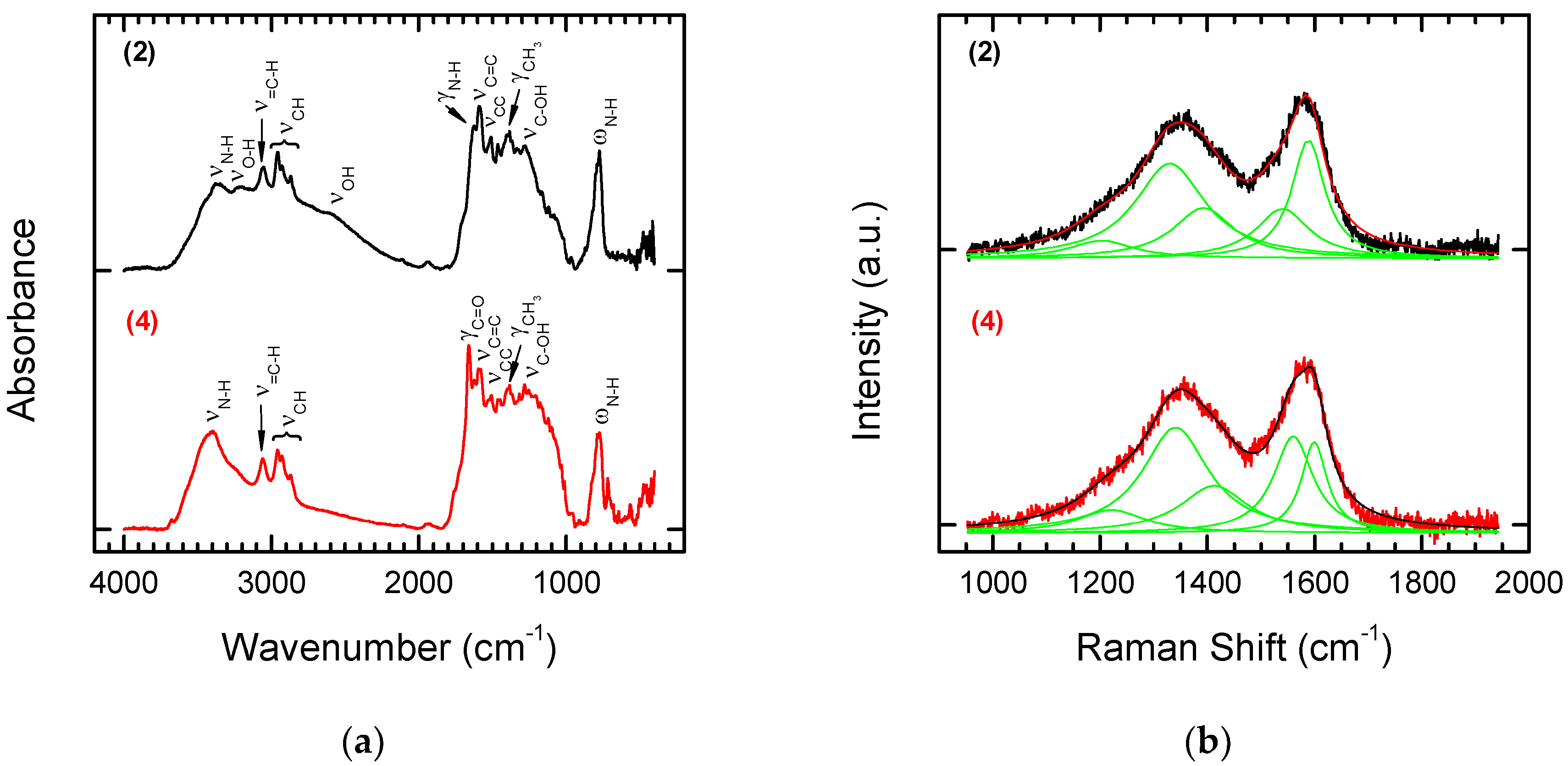
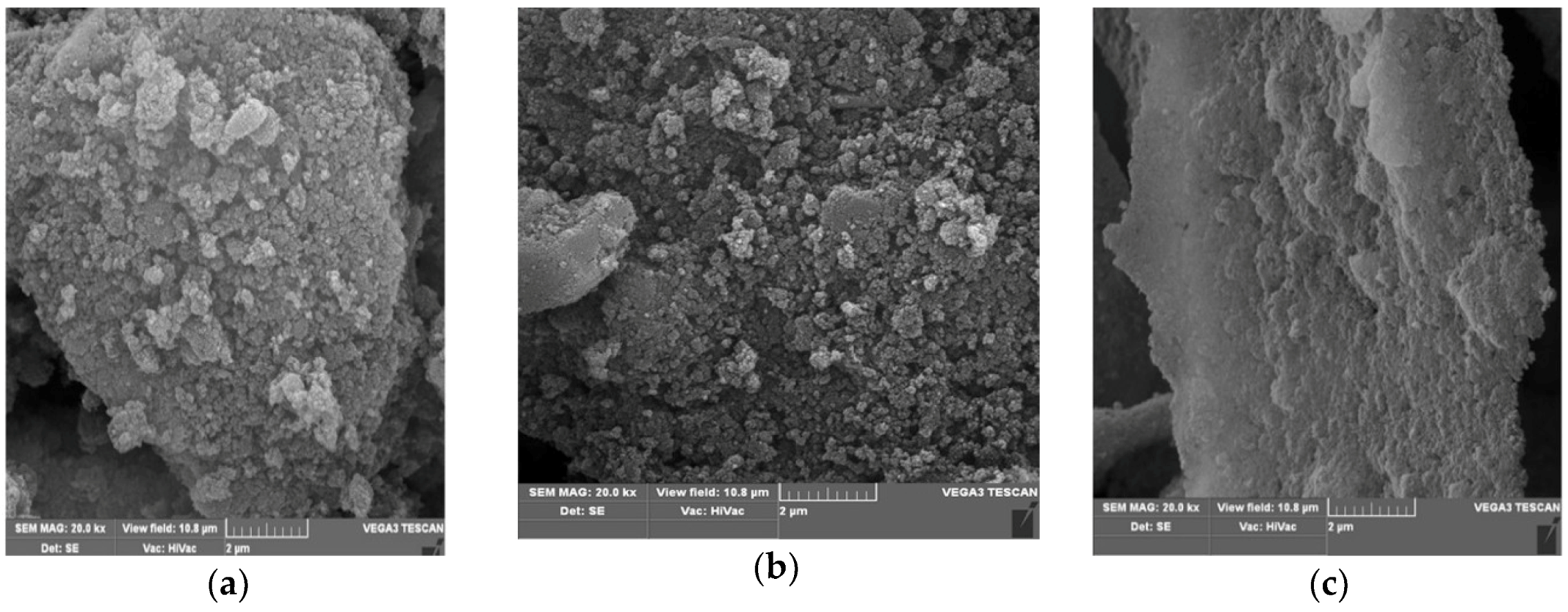
3.1. Laser Treatment—Introduction of sp2 Carbon onto the NDP Surface
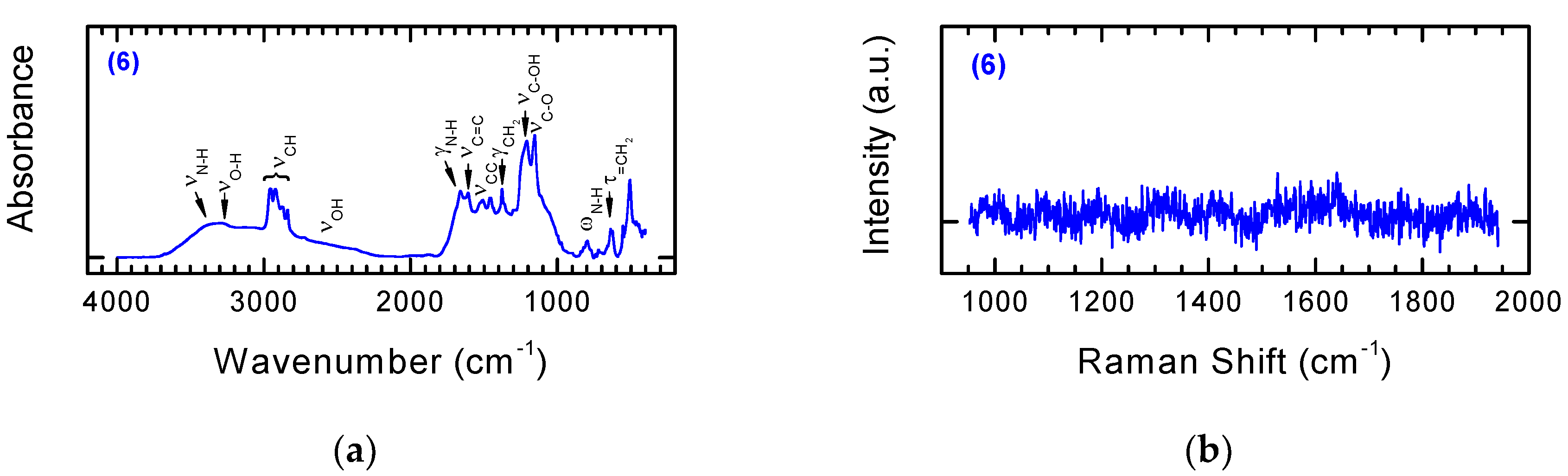
3.2. Chemical Functionalization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Danilenko, V.V. On the history of the discovery of nanodiamond synthesis. Phys. Solid State 2004, 46, 595–599. [Google Scholar] [CrossRef]
- Passeri, D.; Rinaldi, F.; Ingallina, C.; Carafa, M.; Rossi, M.; Terranova, M.L.; Marianecci, C. Biomedical Applications of Nanodiamonds: An Overview. J. Nanosci. Nanotechnol. 2015, 15, 972–988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fresta, C.G.; Chakraborty, A.; Wijesinghe, M.B.; Amorini, A.M.; Lazzarino, G.; Lazzarino, G.; Tavazzi, B.; Lunte, S.M.; Caraci, F.; Dhar, P.; et al. Non-toxic engineered carbon nanodiamond concentrations induce oxidative/nitrosative stress, imbalance of energy metabolism, and mitochondrial dysfunction in microglial and alveolar basal epithelial cells. Cell Death Disease 2018, 9, 245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turcheniuk, K.; Mochalin, V.M. Biomedical Applications of Nanodiamond. Nanotechnology 2017, 28, 252001. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.G.; Prim, R.E.; Kim, K.H.; Kang, E.; Park, K.; Jeong, S.H. Combinatorial nanodiamond in pharmaceutical and biomedical applications. Int. J. Pharm. 2016, 514, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Rosenholm, J.M.; Vlasov, I.I.; Burikov, S.A.; Dolenko, T.A.; Shenderova, O.A. Nanodiamond-Based Composite Structures for Biomedical Imaging and Drug Delivery. J. Nanosci. Nanotechnol. 2015, 15, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.P.L.; Shaikh, A.Q.; Reitzig, M.; Kovalenko, D.A.; Michael, J.; Beutner, R.; Cuniberti, G.; Scharnweber, D.; Opitz, J. Detonation nanodiamonds biofunctionalization and immobilization to titanium alloy surfaces as first steps towards medical application. Beilstein J. Org. Chem. 2014, 10, 2765–2773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazi, S. A Review Article on Nanodiamonds Discussing Their Properties and Applications. Int. J. Pharm. Sci. Invent. 2014, 3, 40–45. [Google Scholar]
- Paci, J.T.; Man, H.B.; Saha, B.; Ho, D.; Schatz, G.C. Understanding the Surfaces of Nanodiamonds. J. Phys. Chem. C 2013, 117, 17256–17267. [Google Scholar] [CrossRef]
- Lai, L.; Barnard, A.S. Functionalized Nanodiamonds for Biological and Medical Applications. J. Nanosci. Nanotechnol. 2015, 15, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Williams, O.A. Nanocrystalline diamond. Diamond Relat. Mater. 2011, 20, 621–640. [Google Scholar] [CrossRef]
- Shcherbakova, I.; Mitra, S.; Beer, R.H.; Brenowitz, M. Fast Fenton footprinting: A laboratory-based method for the time-resolved analysis of DNA, RNA and proteins. Nucleic Acids Res. 2006, 34, e48. [Google Scholar] [CrossRef] [PubMed]
- Jee, A.-Y.; Lee, M. Surface functionalization and physicochemical characterization of diamond nanoparticles. Curr. Appl. Phys. 2009, 9, e144–e147. [Google Scholar] [CrossRef]
- Shenderova, O.; Panich, A.M.; Moseenkov, S.; Hens, S.C.; Kuznetsov, V.; Vieth, A.M. Hydroxylated Detonation Nanodiamond: FTIR, XPS, and NMR Studies. J. Phys. Chem. C 2011, 115, 19005–19011. [Google Scholar] [CrossRef]
- Krueger, A.; Lang, D. Functionality is Key: Recent Progress in the Surface Modification of Nanodiamond. Adv. Funct. Mater. 2012, 22, 890–906. [Google Scholar] [CrossRef]
- Barras, A.; Szunerits, S.; Marcon, L.; Monfilliette-Dupont, N.; Boukherroub, R. Functionalization of Diamond Nanoparticles Using “Click” Chemistry. Langmuir 2010, 26, 13168–13172. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Ozawa, M.; Krueger, A. A General Procedure to Functionalize Agglomerating Nanoparticles Demonstrated on Nanodiamond. ACS Nano 2009, 3, 2288–2296. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, K.; Snyder, T.M.; Liu, D.R.J. DNA-templated functional group transformations enable sequence-programmed synthesis using small-molecule reagents. J. Am. Chem. Soc. 2005, 127, 1660–1661. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, M.; Gattass, R.R.; Mazur, E. Femtosecond laser-induced formation of nanometer-width grooves on synthetic single-crystal diamond surfaces. J. Appl. Phys. 2009, 105, 053102. [Google Scholar] [CrossRef]
- Komlenok, M.S.; Kononenko, V.V.; Ralchenko, V.G.; Pimenov, S.M.; Konov, V.I. Laser Induced Nanoablation of Diamond Materials. Phys. Procedia 2011, 12, 37–45. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, C.-S.; Shang, S.; Liu, D.; Perrie, W.; Dearden, G.; Watkins, K. A review of ultrafast laser materials micromachining. Opt. Laser Technol. 2013, 46, 88–102. [Google Scholar] [CrossRef]
- Li, X.Y.; Wang, H.Q.; Shimizu, Y.; Pyatenko, A.; Kawaguchi, K.; Koshizaki, N. Preparation of carbon quantum dots with tunable photoluminescence by rapid laser passivation in ordinary organic solvents. Chem. Commun. 2011, 47, 932–934. [Google Scholar] [CrossRef] [PubMed]
- Dudek, M.; Rosowski, A.; Koperkiewicz, A.; Grobelny, J.; Wach, R.; Sharp, M.; French, P.; Janasz, L.; Kozanecki, M. Carbon nanoparticles fabricated by infrared laser ablation of graphite and polycrystalline diamond targets. Phys. Status Solidi A 2017, 214, 1600318. [Google Scholar] [CrossRef]
- Palosz, B.; Pantea, C.; Grzanka, E.; Shelmakh, S.; Proffen, T.; Zerda, T.W.; Palosz, W. Investigation of relaxation of nanodiamond surface in real and reciprocal spaces. Diam. Relat. Mater. 2006, 15, 1813–1817. [Google Scholar] [CrossRef]
- Holt, K.B. Diamond at the nanoscale: Applications of diamond nanoparticles from cellular biomarkers to quantum computing. Philos. Trans. R. Soc. A 2007, 365, 2845–2861. [Google Scholar] [CrossRef] [PubMed]
- Krueger, A. The structure and reactivity of nanoscale diamond. J. Mater. Chem. 2008, 18, 1485–1492. [Google Scholar] [CrossRef]
- Dillon, R.O.; Wollam, J.A.; Katkanant, V. Use of Raman scattering to investigate disorder and crystallite formation in as-deposited and annealed carbon films. Phys. Rev. B 1984, 29, 3482–3489. [Google Scholar] [CrossRef]
- Tsai, H.; Bogy, D.B. Characterization of diamondlike carbon films and their application as overcoats on thin-film media for magnetic recording. J. Vac. Sci. Technol. A 1987, 5, 3287–3313. [Google Scholar] [CrossRef]
- Schwan, J.; Ulrich, S.; Batori, V.; Ehrhardt, H.; Silva, S.R.P. Raman spectroscopy on amorphous carbon films. J. Appl. Phys. 1996, 80, 440–447. [Google Scholar] [CrossRef] [Green Version]
- Mochalin, V.; Osswald, S.; Gogotsi, Y. Contribution of Functional Groups to the Raman Spectrum of Nanodiamond Powders. Chem. Mater. 2009, 21, 273–279. [Google Scholar] [CrossRef]
- Korepanov, V.I.; Hamaguchi, H.; Osawa, E.; Ermolenkov, V.; Lednev, I.K.; Etzold, B.J.M.; Levinson, O.; Zousman, B.; Epperla, C.P.; Chang, H.-C. Carbon structure in nanodiamonds elucidated from Raman spectroscopy. Carbon 2017, 121, 322–329. [Google Scholar] [CrossRef]
- Meinhardt, T.; Lang, D.; Dill, H.; Krueger, A. Pushing the Functionality of Diamond Nanoparticles to New Horizons: Orthogonally Functionalized Nanodiamond Using Click Chemistry. Adv. Funct. Mater. 2011, 21, 494–500. [Google Scholar] [CrossRef]
- Poonthiyil, V.; Lindhorst, T.K.; Golovko, V.B.; Fairbanks, A.J. Recent applications of click chemistry for the functionalization of gold nanoparticles and their conversion to glyco-gold nanoparticles. Beilstein J. Org. Chem. 2018, 14, 11–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, M.S.; Chowdhury, S.; Koley, S. Advances of azide-alkyne cycloaddition-click chemistry over the recent decade. Tetrahedron 2016, 72, 5257–5283. [Google Scholar] [CrossRef]
- Kolesinska, B.; Rozniakowski, K.K.; Fraczyk, J.; Relich, I.; Papini, A.M.; Kaminski, Z.J. The Effect of Counterion and Tertiary Amine on the Efficiency of N-Triazinylammonium Sulfonates in Solution and Solid-Phase Peptide Synthesis. Eur. J. Org. Chem. 2015, 2, 401–408. [Google Scholar] [CrossRef]
- Fraczyk, J.; Walczak, M.; Szymanski, L.; Kolacinski, Z.; Wrzosek, H.; Majsterek, I.; Przybylowska-Sygut, K.; Kaminski, Z.J. Carbon nanotubes functionalized with folic acid attached via biomimetic peptide linker. Nanomedicine 2017, 12, 2161–2182. [Google Scholar] [CrossRef] [PubMed]
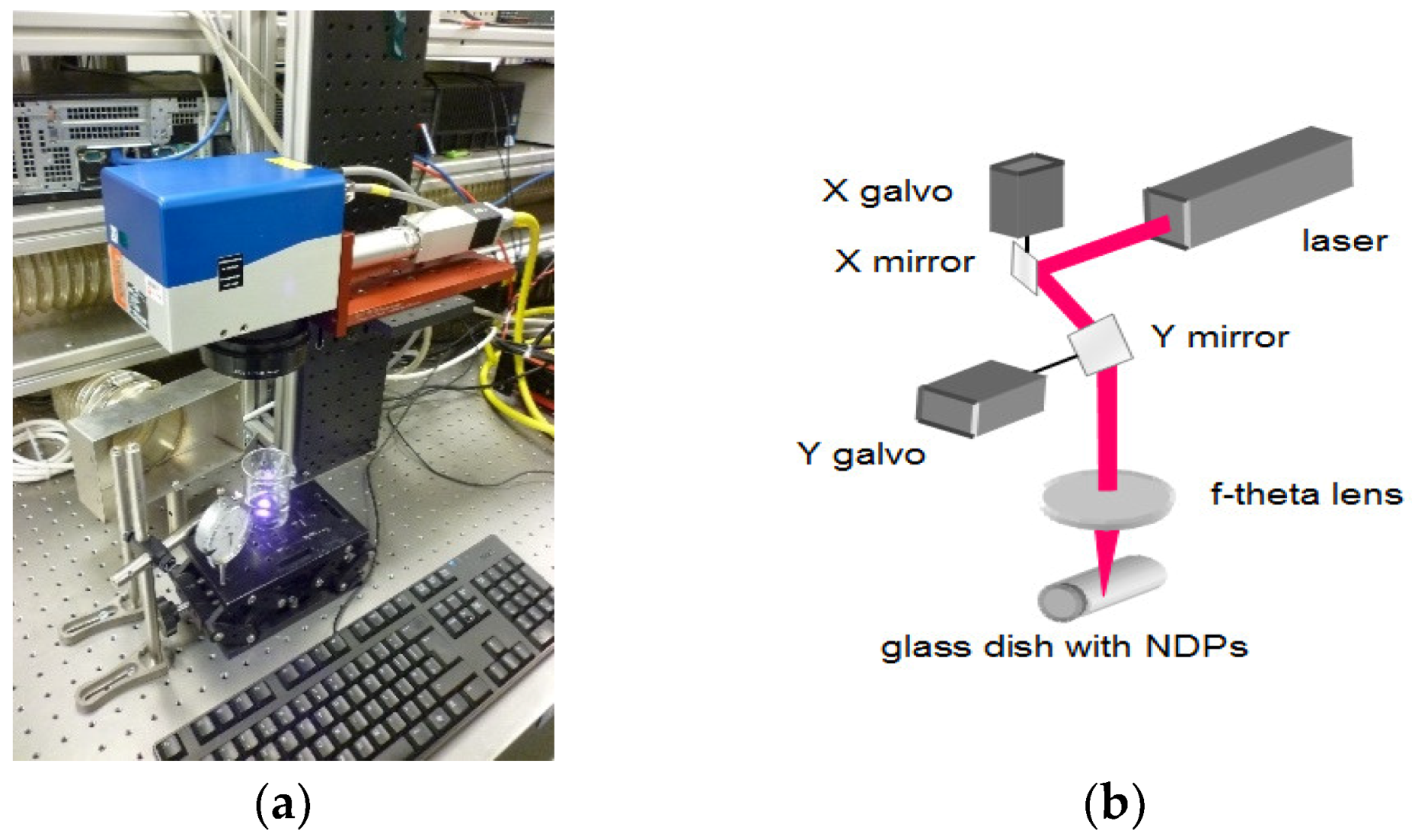
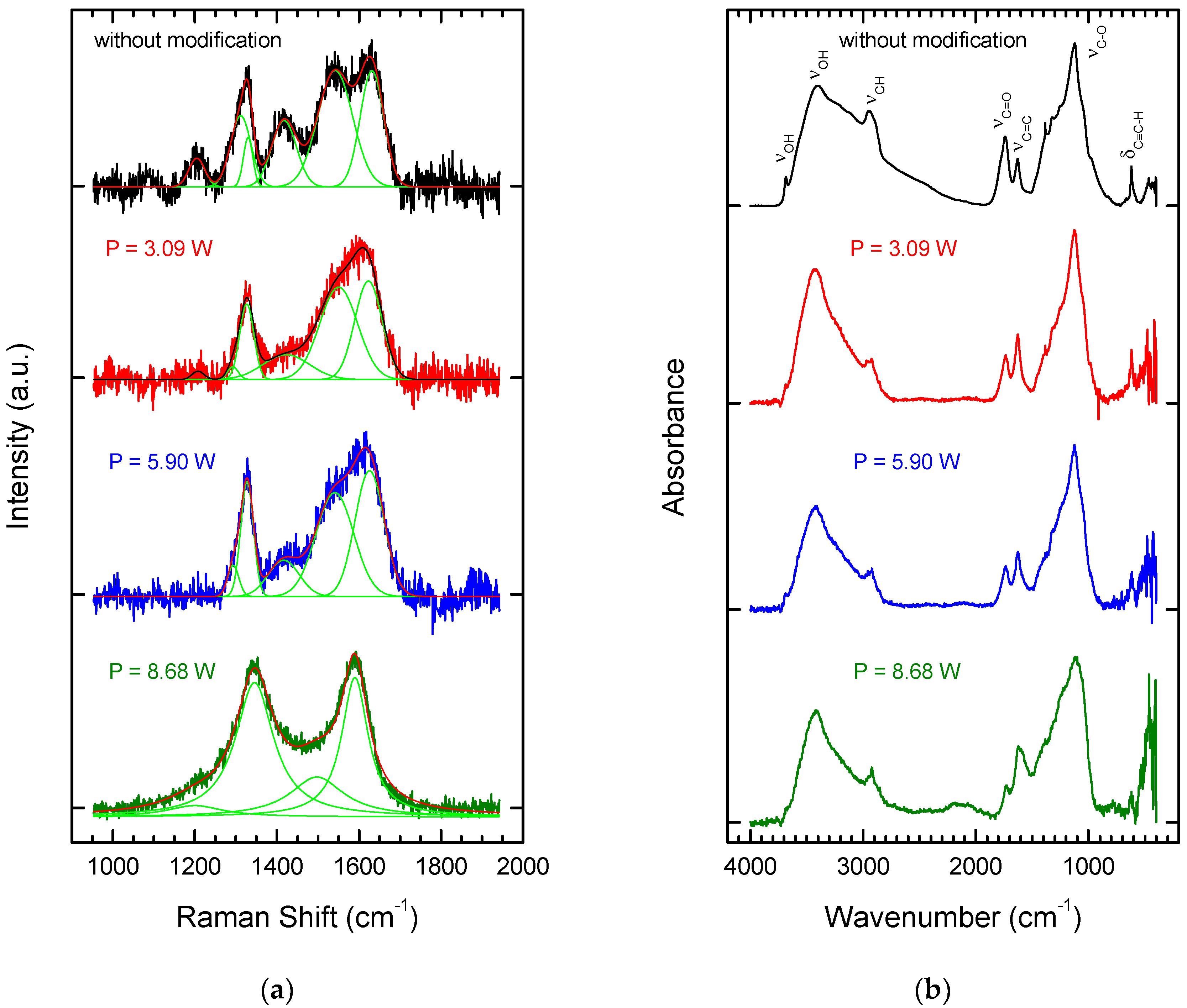

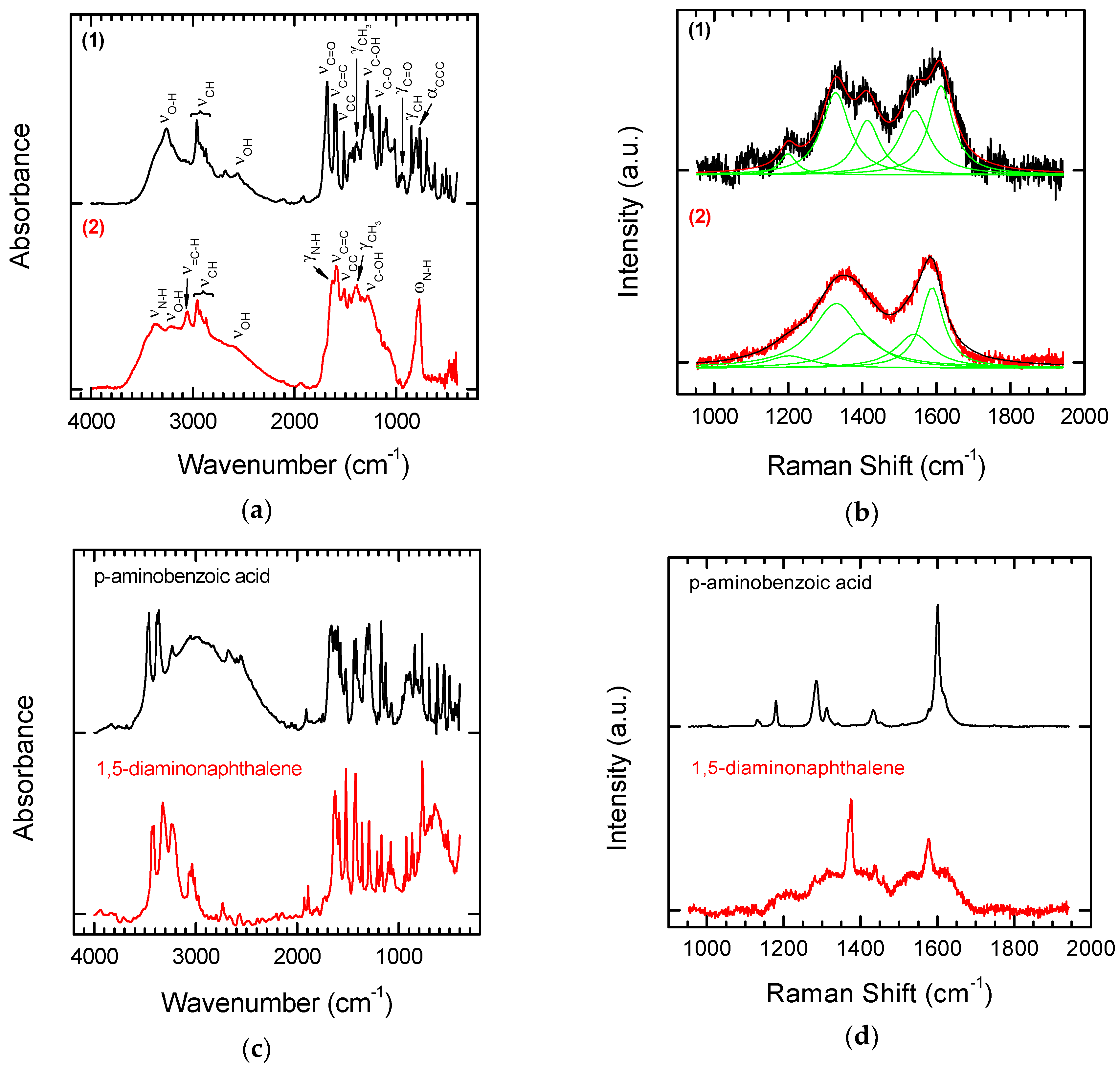


| Parameters | Values |
|---|---|
| Wavelength | 1064 nm |
| Pulse frequency | 25 kHz |
| Pulse duration | 200 ns |
| Average power | 3.09, 5.90 and 8.68 W |
| Spot size | 30 µm |
| Work area | 14 × 6 mm |
| Hatching distance between parallel lines (lines along the long side of work area) | 30 µm |
| Scanning speed | 600 mm/s |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fraczyk, J.; Rosowski, A.; Kolesinska, B.; Koperkiewcz, A.; Sobczyk-Guzenda, A.; Kaminski, Z.J.; Dudek, M. Orthogonal Functionalization of Nanodiamond Particles after Laser Modification and Treatment with Aromatic Amine Derivatives. Nanomaterials 2018, 8, 908. https://doi.org/10.3390/nano8110908
Fraczyk J, Rosowski A, Kolesinska B, Koperkiewcz A, Sobczyk-Guzenda A, Kaminski ZJ, Dudek M. Orthogonal Functionalization of Nanodiamond Particles after Laser Modification and Treatment with Aromatic Amine Derivatives. Nanomaterials. 2018; 8(11):908. https://doi.org/10.3390/nano8110908
Chicago/Turabian StyleFraczyk, Justyna, Adam Rosowski, Beata Kolesinska, Anna Koperkiewcz, Anna Sobczyk-Guzenda, Zbigniew J. Kaminski, and Mariusz Dudek. 2018. "Orthogonal Functionalization of Nanodiamond Particles after Laser Modification and Treatment with Aromatic Amine Derivatives" Nanomaterials 8, no. 11: 908. https://doi.org/10.3390/nano8110908






