Ag2CO3 Decorating BiOCOOH Microspheres with Enhanced Full-Spectrum Photocatalytic Activity for the Degradation of Toxic Pollutants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of Catalysts
2.3. Characterization
2.4. Photocatalytic Performance Tests
3. Results and Discussion
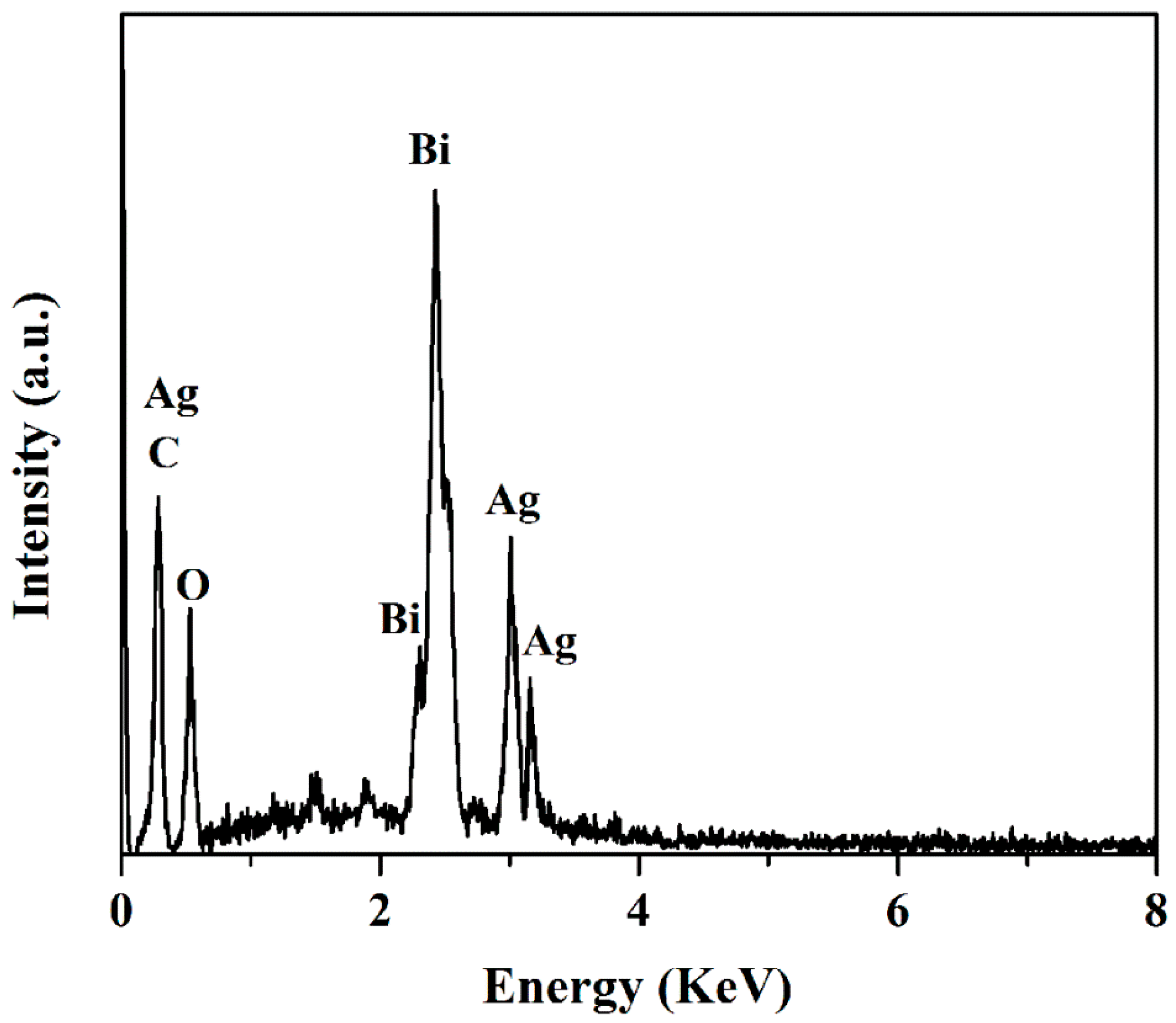
3.1. Characterization
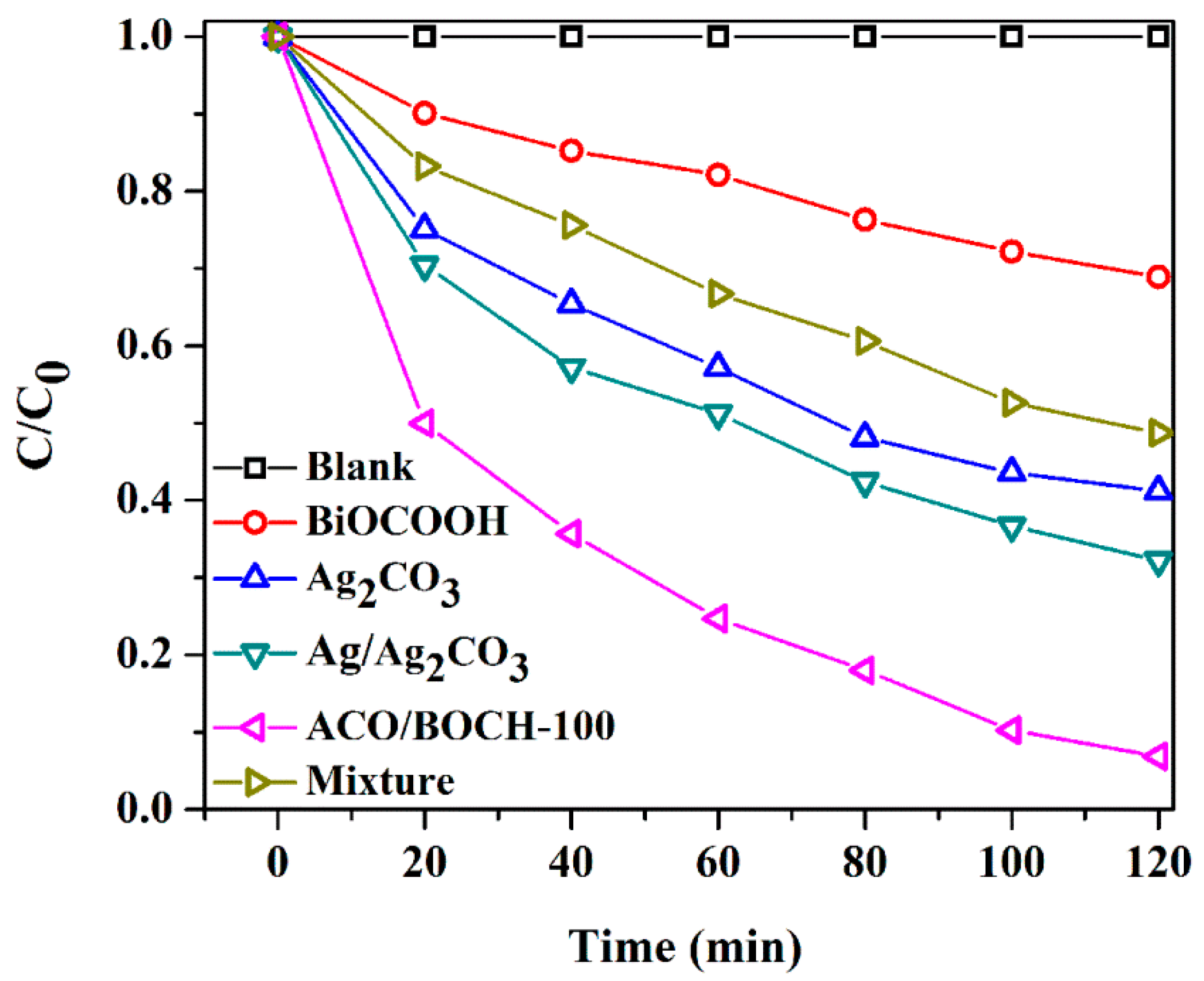
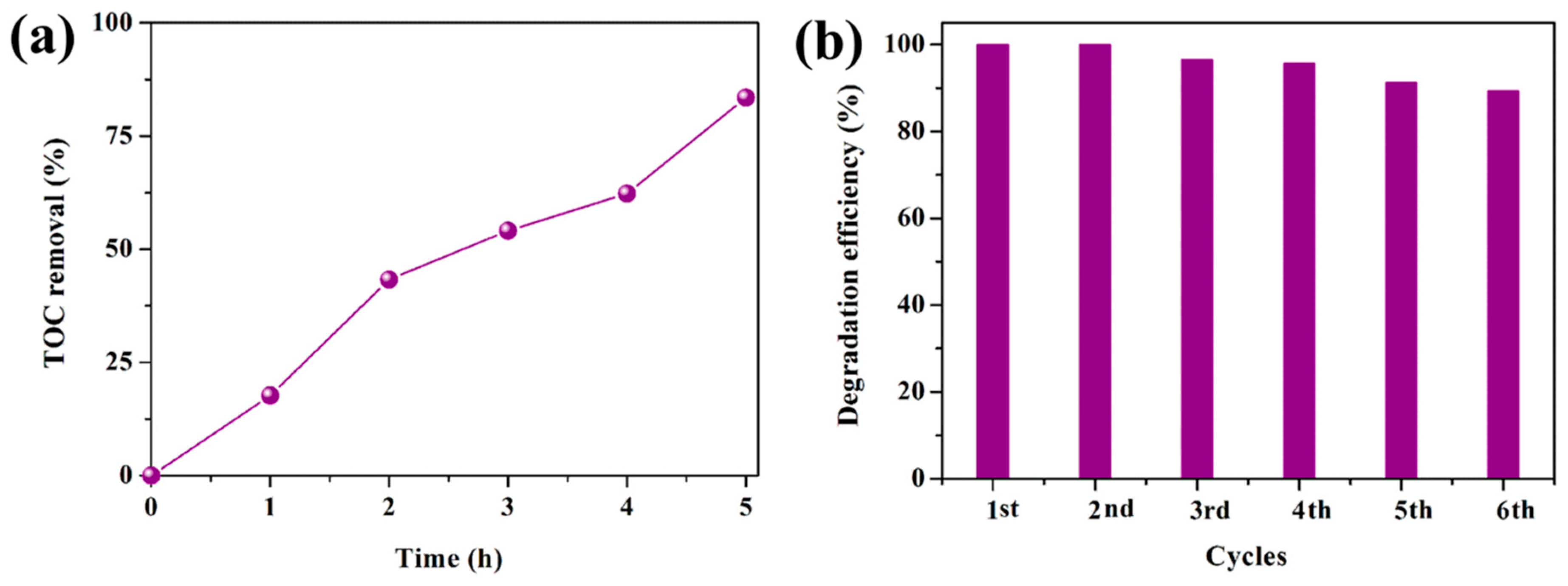
3.2. Photocatalytic Performance
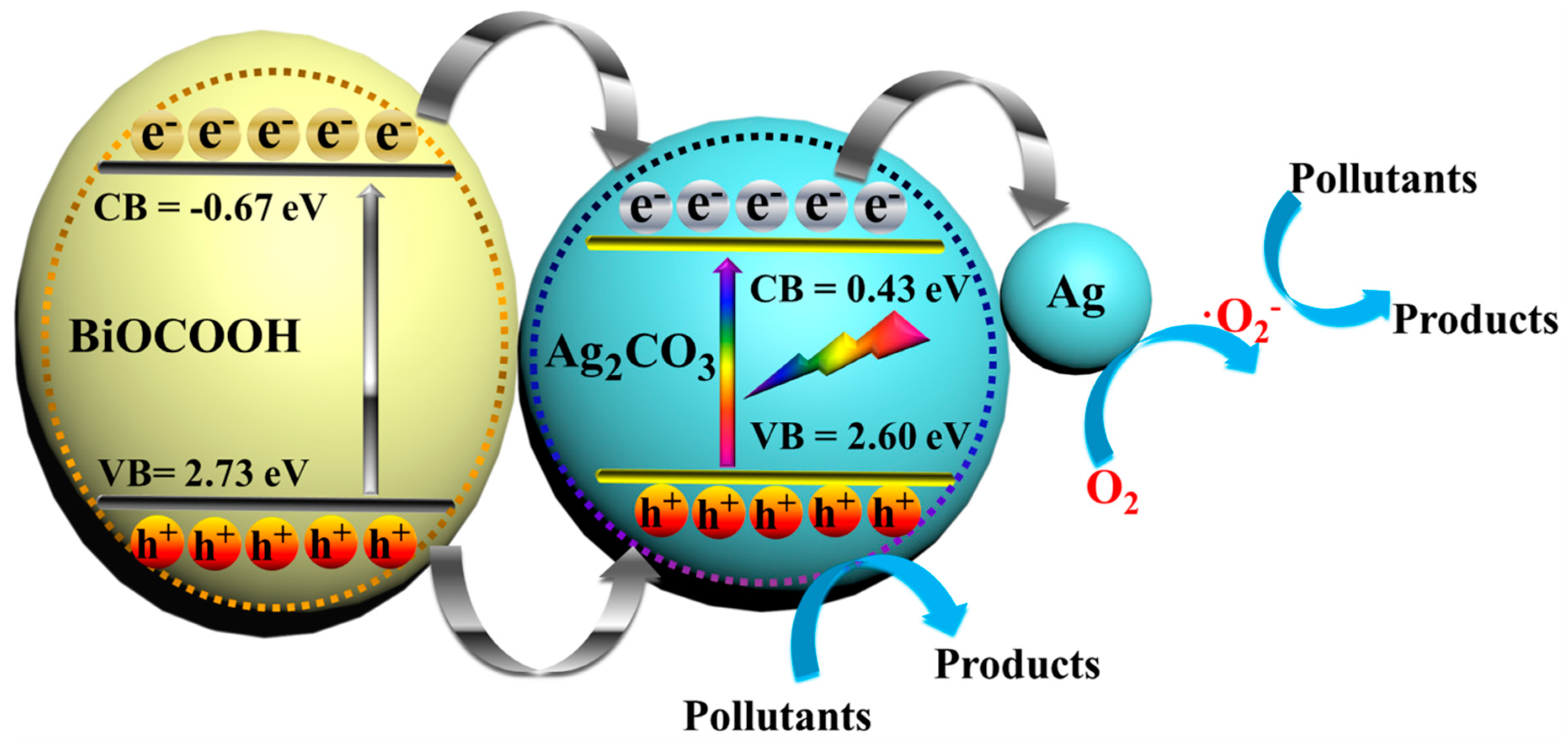
3.3. Photocatalytic Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, S.S.; Wang, D.W. Photocatalysis: Basic principles, diverse forms of implementations and emerging scientifc opportunities. Adv. Energy Mater. 2017, 7, 1700841. [Google Scholar] [CrossRef]
- Li, S.; Shen, X.; Liu, J.; Zhang, L. Synthesis of Ta3N5/Bi2MoO6 core-shell fiber-shaped heterojunctions as efficient and easily recyclable photocatalysts. Environ. Sci. Nano 2017, 4, 1155–1167. [Google Scholar] [CrossRef]
- Li, S.; Hu, S.; Xu, K.B.; Jiang, W.; Liu, J.; Wang, Z. A novel heterostructure of BiOI nanosheets anchored onto MWCNTs with excellent visible-light photocatalytic activity. Nanomaterials 2017, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, C.; Lai, C.; Zeng, G.; Huang, D.; Cheng, M.; Wang, J.; Chen, F.; Zhou, C.; Xiong, W. BiOX (X = Cl, Br, I) photocatalytic nanomaterials: Applications for fuels and environmental management. Adv. Colloid Interface 2018, 254, 76–93. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hu, S.; Jiang, W.; Liu, Y.; Zhou, Y.; Liu, Y.; Mo, L. Hierarchical architectures of bismuth molybdate nanosheets onto nickel titanate nanofibers: Facile synthesis and efficient photocatalytic removal of tetracycline hydrochloride. J. Colloid Interface Sci. 2018, 521, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Huang, Y.; Zhang, Y.; Cao, J.-j.; Li, H.; Bian, C.; Lee, S.C. Oxygen vacancy engineering of Bi2O3/Bi2O2CO3 heterojunctions: Implications of the interfacial charge transfer, NO adsorption and removal. Appl. Catal. B 2018, 231, 357–367. [Google Scholar] [CrossRef]
- Zhang, J.L.; Ma, Z.J. Flower-like Ag2MoO4/Bi2MoO6 heterojunctions with enhanced photocatalytic activity under visible light irradiation. Taiwan Inst. Chem. Eng. 2017, 71, 156–164. [Google Scholar] [CrossRef]
- Li, S.; Jiang, W.; Hu, S.; Liu, Y.; Liu, Y.; Xu, K.; Liu, J. Hierarchical heterostructure of Bi2MoO6 micro-flowers decorated with Ag2CO3 nanoparticles for efficient visible-light-driven photocatalytic removal of toxic pollutants. Beilstein J. Nanotechnol. 2018, 9, 2297–2305. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; An, Y.; Liu, Y.; Qin, X.; Zhang, X.; Dai, Y.; Wang, Z.; Wang, P.; Whangbo, M.-H.; Huang, B. Enhancing the photocatalytic activity of BiOX (X = Cl, Br, I), (BiO)2CO3 and Bi2O3 by modifying their surfaces with polar organic anions, 4-substituted thiophenolates. J. Mater. Chem. A 2017, 5, 14406–14414. [Google Scholar] [CrossRef]
- Ganose, A.M.; Cuff, M.; Butler, K.T.; Scanlon, D.O. Interplay of orbital and relativistic effects in bismuth oxyhalides: BiOF, BiOCl, BiOBr, and BiOl. Chem. Mater. 2016, 28, 1980–1984. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Niu, C.; Wen, X.; Yang, S.; Guo, H.; Zeng, G.-M. Construction of 2D heterojunction system with enhanced photocatalytic performance: Plasmonic Bi and reduced graphene oxide co-modified Bi5O7I with high-speed charge transfer channels. J. Hazard Mater. 2018, 361, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.Y.; Cheng, G.; Lu, Z.; Tang, J.L.; Yu, X.L.; Chen, R. BiOCOOH hierarchical nanostructures: Shape-controlled solvothermal synthesis and photocatalytic degradation performances. CrystEngComm 2011, 13, 2381–2390. [Google Scholar] [CrossRef]
- Duan, F.; Zheng, Y.; Liu, L.; Chen, M.Q.; Xie, Y. Synthesis and photocatalytic behaviour of 3D flowerlike bismuth oxide formate architectures. Mater. Lett. 2010, 64, 1566–1569. [Google Scholar] [CrossRef]
- Ye, R.; Zhao, J.; Wickemeyer, B.B.; Toste, F.D.; Somorjai, G.A. Foundations and strategies of the construction of hybrid catalysts for optimized performances. Nat. Catal. 2018, 1, 318–325. [Google Scholar] [CrossRef]
- Wu, M.J.; Wu, J.Z.; Zhang, J.; Chen, H.; Zhou, J.Z.; Qian, G.R.; Xu, Z.P.; Du, Z.; Rao, Q.L. A review on fabricating heterostructures from layered double hydroxides for enhanced photocatalytic activities. Catal. Sci. Technol. 2018, 8, 1207–1228. [Google Scholar] [CrossRef]
- Aguirre, M.E.; Zhou, R.; Eugene, A.J.; Guzman, M.I.; Grela, M.A. Cu2O/TiO2 heterostructures for CO2 reduction through a direct Z-scheme: Protecting Cu2O from photocorrosion. Appl. Catal. B 2017, 217, 485–493. [Google Scholar] [CrossRef]
- Li, S.; Jiang, W.; Xu, K.; Hu, S.; Liu, Y.; Zhou, Y.; Liu, J. Synthesis of flower-like AgI/BiOCOOH p-n heterojunctions with enhanced visible-light photocatalytic performance for the removal of toxic pollutants. Front. Chem. 2018, 6, 518. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.; Long, Y.; Mo, L.; Zhang, H.; Liu, J. Facile synthesis of Bi2MoO6 microspheres decorated by CdS nanoparticles with efficient photocatalytic removal of levfloxacin antibiotic. Catalysts 2018, 8, 477. [Google Scholar] [CrossRef]
- Li, S.J.; Xu, K.B.; Hu, S.W.; Jiang, W.; Zhang, J.L.; Liu, J.S.; Zhang, L.S. Synthesis of flower-like Ag2O/BiOCOOH p-n heterojunction with enhanced visible light photocatalytic activity. Appl. Surf. Sci. 2017, 397, 95–103. [Google Scholar] [CrossRef]
- Hu, S.W.; Li, S.J.; Xu, K.B.; Jiang, W.; Zhang, J.L.; Liu, J.S. MWCNTs/BiOCOOH composites with improved sunlight photocatalytic activity. Mater. Lett. 2017, 191, 157–160. [Google Scholar] [CrossRef]
- Xia, S.-H.; Dong, C.; Wei, X.-W.; Wang, J.; Wu, K.-L.; Hu, Y.; Ye, Y. Reduced graphene oxide modified flower-like BiOCOOH architectures with enhanced photocatalytic activity. Mater. Lett. 2015, 156, 36–38. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, X.; Zhang, H.; Cheng, Q.; Cheng, X. Construction of BiOCOOH/g-C3N4 composite photocatalyst and its enhanced visible light photocatalytic degradation of amido black 10B. Sep. Purif. Technol. 2019, 210, 125–134. [Google Scholar] [CrossRef]
- Chai, B.; Wang, X. Enhanced visible light photocatalytic activity of BiOI/BiOCOOH composites synthesized via ion exchange strategy. RSC Adv. 2015, 5, 7589–7596. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, Q.; Su, Y.; Shen, L.; Wang, F.; Liu, H.; Liu, Y.; Cai, Z.; Lv, W.; Liu, G. Accelerated photocatalytic degradation of diclofenac by a novel CQDs/BiOCOOH hybrid material under visible-light irradiation: Dechloridation, detoxicity, and a new superoxide radical model study. Chem. Eng. J. 2018, 332, 737–748. [Google Scholar] [CrossRef]
- Liu, Y.; Kong, J.J.; Yuan, J.L.; Zhao, W.; Zhu, X.; Sun, C.; Xie, J.M. Enhanced photocatalytic activity over flower-like sphere Ag/Ag2CO3/BiVO4 plasmonic heterojunction photocatalyst for tetracycline degradation. Chem. Eng. J. 2018, 331, 242–254. [Google Scholar] [CrossRef]
- Li, T.T.; Hu, X.L.; Liu, C.C.; Tang, C.M.; Wang, X.K.; Luo, S.L. Efficient photocatalytic degradation of organic dyes and reaction mechanism with Ag2CO3/Bi2O2CO3 photocatalyst under visible light irradiation. J. Mol. Catal. A Chem. 2016, 425, 124–135. [Google Scholar] [CrossRef]
- Yao, X.; Liu, X. One-pot synthesis of ternary Ag2CO3/Ag/AgCl photocatalyst in natural geothermal water with enhanced photocatalytic activity under visible light irradiation. J. Hazard Mater. 2014, 280, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Su, Y.; Qi, X.; Han, X. A facile method to prepare novel Ag2O/Ag2CO3 three-dimensional hollow hierarchical structures and their water purification function. ACS Sustain. Chem. Eng. 2017, 5, 6148–6158. [Google Scholar] [CrossRef]
- Yu, C.; Wei, L.; Zhou, W.; Dionysiou, D.D.; Zhu, L.; Shu, Q.; Liu, H. A visible-light-driven core-shell like Ag2S@Ag2CO3 composite photocatalyst with high performance in pollutants degradation. Chemosphere 2016, 157, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Ma, Z. Ag-Ag2CO3/Bi2MoO6 composites with enhanced visible-light-driven catalytic activity. J. Taiwan Inst. Chem. Eng. 2018, 71, 156–164. [Google Scholar] [CrossRef]
- Fang, S.S.; Ding, C.Y.; Liang, Q.; Li, Z.Y.; Xu, S.; Peng, Y.Y.; Lu, D.Y. In-situ precipitation synthesis of novel BiOCl/Ag2CO3 hybrids with highly efficient visible-light-driven photocatalytic activity. J. Alloy. Compd. 2016, 684, 230–236. [Google Scholar] [CrossRef]
- Yuan, X.Z.; Jiang, L.B.; Chen, X.H.; Leng, L.J.; Wang, H.; Wu, Z.B.; Xiong, T.; Liang, J.; Zeng, G.M. Highly efficient visible-light-induced photoactivity of Z-scheme Ag2CO3/Ag/WO3 photocatalysts for organic pollutant degradation. Environ. Sci. Nano 2017, 4, 2175–2185. [Google Scholar] [CrossRef]
- Pirhashemi, M.; Habibi-Yangjeh, A. Photosensitization of ZnO by AgBr and Ag2CO3: Nanocomposites with tandem n-n heterojunctions and highly enhanced visible-light photocatalytic activity. J. Colloid Interface Sci. 2016, 474, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Guzman, M.I. Photocatalytic Reduction of Fumarate to Succinate on ZnS Mineral Surfaces. J. Phys. Chem. C 2016, 120, 7349–7357. [Google Scholar] [CrossRef]
- Zhou, R.; Guzman, M.I. CO2 Reduction under Periodic Illumination of ZnS. J. Phys. Chem. C 2014, 118, 11649–11656. [Google Scholar] [CrossRef]
- Ma, H.; Zhao, Y.; Souvanhthong, B.; Zhao, J. Time-dependent synthesis of BiO2−x/Bi composites with efficient visible-light induced photocatalytic activity. J. Colloid Interface Sci. 2018, 531, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hu, S.; Jiang, W.; Zhou, Y.; Liu, J.; Wang, Z. Facile synthesis of cerium oxide nanoparticles decorated flower-like bismuth molybdate for enhanced photocatalytic activity toward organic pollutant degradation. J. Colloid Interface Sci. 2018, 530, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hu, S.; Jiang, W.; Liu, Y.; Liu, Y.; Zhou, Y.; Mo, L.; Liu, J. Ag3VO4 nanoparticles decorated Bi2O2CO3 micro-flowers: An efficient visible-light-driven photocatalyst for the removal of toxic contaminants. Front. Chem. 2018, 6, 255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, J.; Jiang, W.J.; Yao, W.Q.; Yang, H.P.; Zhou, Y.F. Self-assembled perylene diimide based supramolecular heterojunction with Bi2WO6 for efficient visible-light-driven photocatalysis. Appl. Catal. B 2018, 232, 175–181. [Google Scholar] [CrossRef]
- Li, S.; Hu, S.; Zhang, J.; Jiang, W.; Liu, J. Facile synthesis of Fe2O3 nanoparticles anchored on Bi2MoO6 microflowers with improved visible light photocatalytic activity. J. Colloid Interface Sci. 2017, 497, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Yu, C.; Liu, R.; Dionysiou, D.D.; Yang, K.; Wang, C.; Liu, H. Novel fluorinated Bi2MoO6 nanocrystals for efficient photocatalytic removal of water organic pollutants under different light source illumination. Appl. Catal. B 2017, 209, 1–11. [Google Scholar]
- Wang, Y.; Wang, H.; Xu, A.; Song, Z. Facile synthesis of Ag3PO4 modified with GQDs composites with enhanced visible-light photocatalytic activity. J. Mater. Sci. Mater. Electron. 2018, 29, 16691–16701. [Google Scholar] [CrossRef]
- Wang, F.F.; Li, Q.; Xu, D.S. Recent Progress in Semiconductor-Based Nanocomposite Photocatalysts for Solar-to-Chemical Energy Conversion. Adv. Energy Mater. 2017, 7, 1700529. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.B.; Che, H.N.; Huang, K.; Liu, C.B.; Shi, W.D. Fabrication of a ternary plasmonic photocatalyst CQDs/Ag/Ag2O to harness charge flow for photocatalytic elimination of pollutants. Appl. Catal. B 2016, 192, 134–144. [Google Scholar] [CrossRef]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Mo, L.; Liu, Y.; Zhang, H.; Ge, Y.; Zhou, Y. Ag2CO3 Decorating BiOCOOH Microspheres with Enhanced Full-Spectrum Photocatalytic Activity for the Degradation of Toxic Pollutants. Nanomaterials 2018, 8, 914. https://doi.org/10.3390/nano8110914
Li S, Mo L, Liu Y, Zhang H, Ge Y, Zhou Y. Ag2CO3 Decorating BiOCOOH Microspheres with Enhanced Full-Spectrum Photocatalytic Activity for the Degradation of Toxic Pollutants. Nanomaterials. 2018; 8(11):914. https://doi.org/10.3390/nano8110914
Chicago/Turabian StyleLi, Shijie, Liuye Mo, Yanping Liu, Huiqiu Zhang, Yaming Ge, and Yingtang Zhou. 2018. "Ag2CO3 Decorating BiOCOOH Microspheres with Enhanced Full-Spectrum Photocatalytic Activity for the Degradation of Toxic Pollutants" Nanomaterials 8, no. 11: 914. https://doi.org/10.3390/nano8110914



