Growth and Self-Assembly of Silicon–Silicon Carbide Nanoparticles into Hybrid Worm-Like Nanostructures at the Silicon Wafer Surface
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the CSNW
2.3. Characterization
3. Results and Discussion
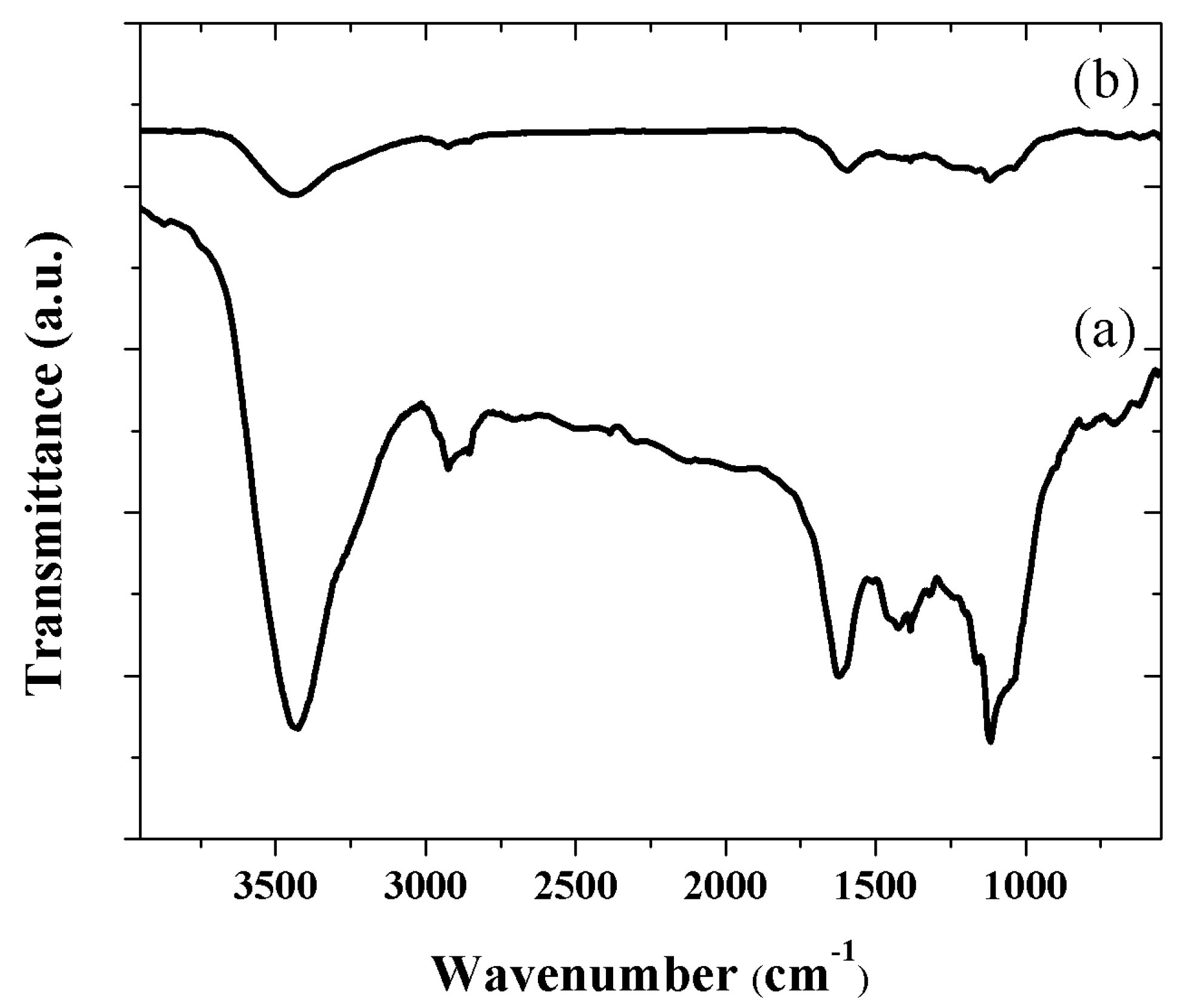
3.1. Raman and FT-IR Characterization
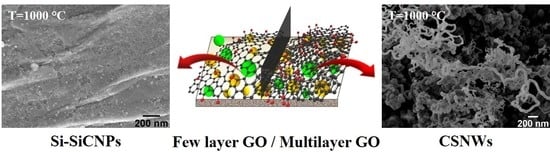
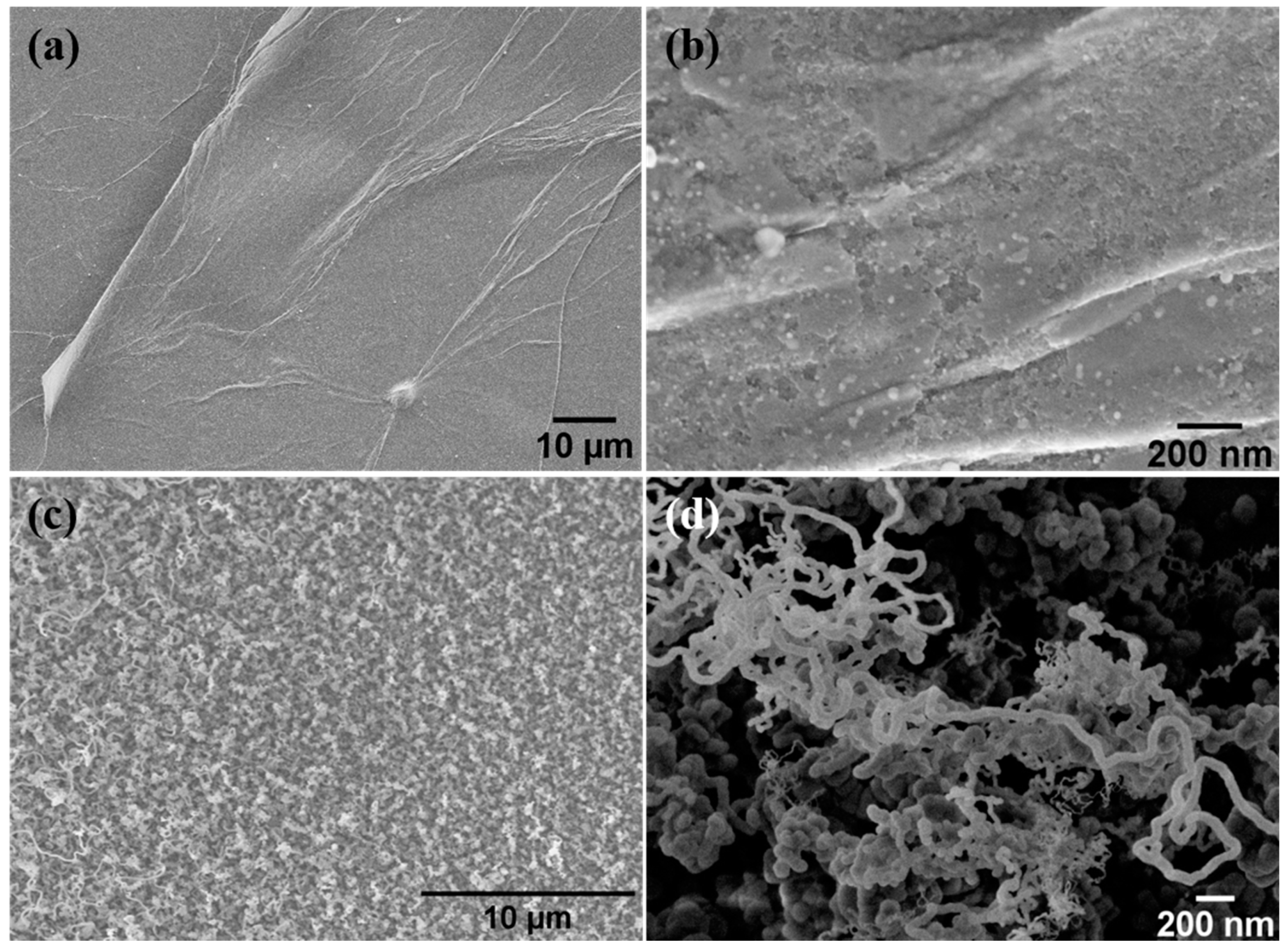
3.2. Morphology and Structure
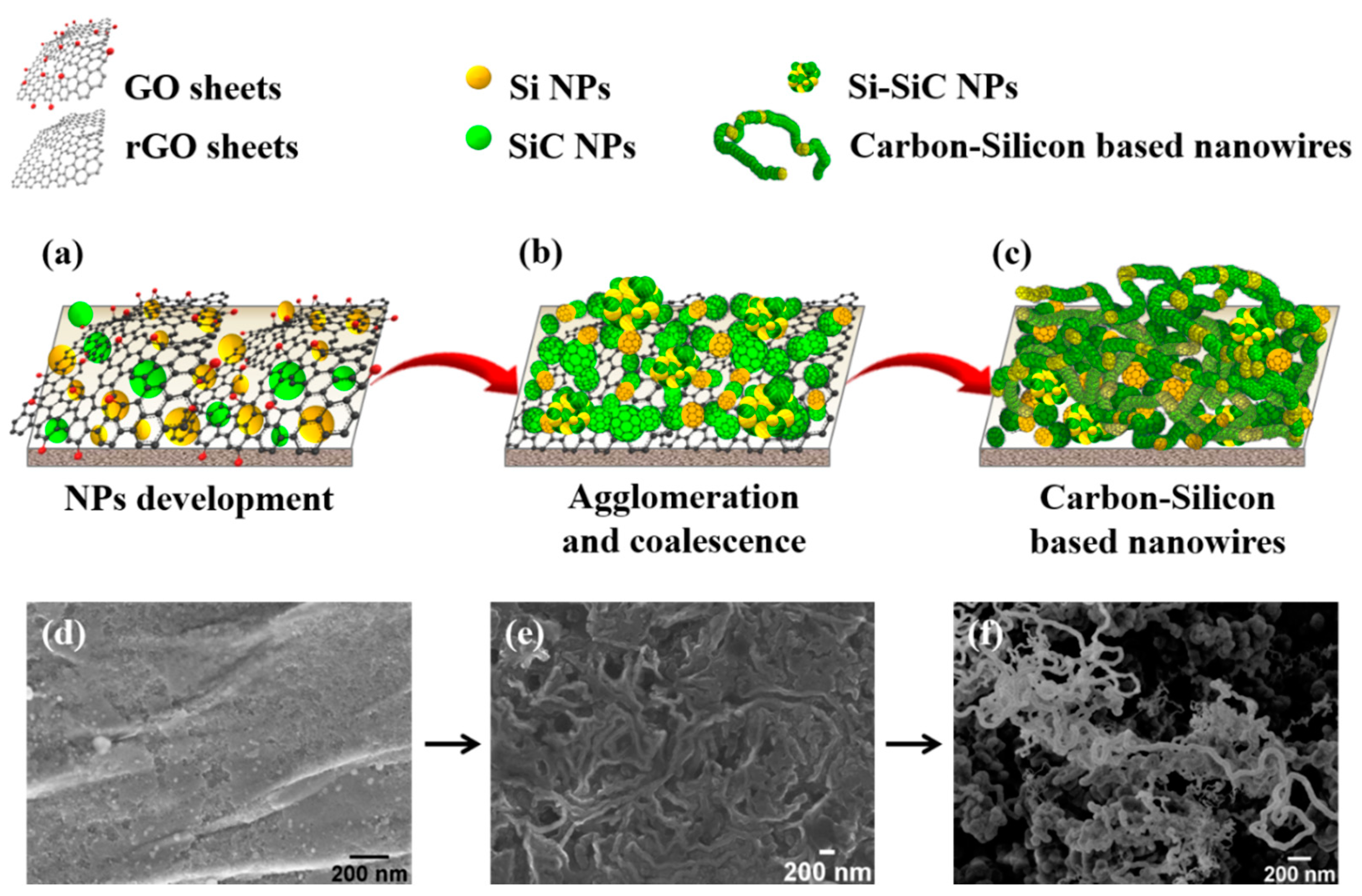
3.3. Mechanism Proposed for the CSNW Formation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ghosh, B.; Sarma, S.; Pontsho, M.; Ray, S.C. Tuning of magnetic behaviour in nitrogenated graphene oxide functionalized with iron oxide. Diam. Relat. Mater. 2018, 89, 35–42. [Google Scholar] [CrossRef]
- Velasco-Soto, M.A.; Pérez-García, S.A.; Alvarez-Quintana, J.; Cao, Y.; Nyborg, L.; Licea-Jiménez, L. Selective band gap manipulation of graphene oxide by its reduction with mild reagents. Carbon 2015, 93, 967–973. [Google Scholar] [CrossRef]
- Wang, R.; Lu, K.-Q.; Tang, Z.-R.; Xu, Y.-J. Recent progress in carbon quantum dots: Synthesis, properties and applications in photocatalysis. J. Mater. Chem. A 2017, 5, 3717–3734. [Google Scholar] [CrossRef]
- Lucien, R.; Sunjie, Y.; Samuel, C.T.M.; Kevin, C.; Coletta, P.L.; Stephen, D.E. Morphological control of seedlessly-synthesized gold nanorods using binary surfactants. Nanotechnology 2018, 29, 135601. [Google Scholar] [Green Version]
- Zhu, K.; Ju, Y.; Xu, J.; Yang, Z.; Gao, S.; Hou, Y. Magnetic Nanomaterials: Chemical Design, Synthesis, and Potential Applications. Acc. Chem. Res. 2018, 51, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Hableel, G.; Zhao, E.R.; Jokerst, J.V. Multifunctional nanomedicine with silica: Role of silica in nanoparticles for theranostic, imaging, and drug monitoring. J. Colloid Interface Sci. 2018, 521, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-S.; Zhou, G.; Yin, L.-C.; Ren, W.; Li, F.; Cheng, H.-M. Graphene/metal oxide composite electrode materials for energy storage. Nano Energy 2012, 1, 107–131. [Google Scholar] [CrossRef]
- Thomas, V.J.; Ramaswamy, S. Application of Graphene and Graphene Compounds for Environmental Remediation. Sci. Adv. Mater. 2016, 8, 477–500. [Google Scholar] [CrossRef]
- Bai, S.; Shen, X. Graphene-inorganic nanocomposites. RSC Adv. 2012, 2, 64–98. [Google Scholar] [CrossRef]
- Huang, J.; Li, Y.; Jia, X.; Song, H. Preparation and tribological properties of core–shell Fe3O4@C microspheres. Tribol. Int. 2019, 129, 427–435. [Google Scholar] [CrossRef]
- Hou, L.; Zheng, H.; Cui, R.; Jiang, Y.; Li, Q.; Jiang, X.; Gao, J.; Gao, F. Silicon carbon nanohybrids with expandable space: A high-performance lithium battery anodes. Microporous Mesoporous Mater. 2019, 275, 42–49. [Google Scholar] [CrossRef]
- Hong, W.G.; Kim, B.H.; Lee, S.M.; Yu, H.Y.; Yun, Y.J.; Jun, Y.; Lee, J.B.; Kim, H.J. Agent-free synthesis of graphene oxide/transition metal oxide composites and its application for hydrogen storage. Int. J. Hydrogen Energy 2012, 37, 7594–7599. [Google Scholar] [CrossRef]
- Sarkar, S.; Howli, P.; Das, B.; Das, N.S.; Samanta, M.; Das, G.C.; Chattopadhyay, K.K. Novel Quaternary Chalcogenide/Reduced Graphene Oxide-Based Asymmetric Supercapacitor with High Energy Density. ACS Appl. Mater. Interfaces 2017, 9, 22652–22664. [Google Scholar] [CrossRef] [PubMed]
- Piao, S.H.; Choi, H.J. Smart Graphene Oxide Based Composite Materials and their Electric and Magnetic Stimuli-response. Procedia Eng. 2017, 171, 64–70. [Google Scholar] [CrossRef]
- Sun, S.; Zhou, A.; Gu, D.; Hu, X.; Li, Z.; Chen, L.; Ma, C.; Dong, L.; Yin, Y.; Chang, X. A general strategy for the synthesis of reduced graphene oxide-based composites. Ceram. Int. 2015, 41, 7661–7668. [Google Scholar] [CrossRef]
- Ge, M.; Rong, J.; Fang, X.; Zhang, A.; Lu, Y.; Zhou, C. Scalable preparation of porous silicon nanoparticles and their application for lithium-ion battery anodes. Nano Res. 2013, 6, 174–181. [Google Scholar] [CrossRef]
- Miyano, M.; Endo, S.; Takenouchi, H.; Nakamura, S.; Iwabuti, Y.; Shiino, O.; Nakanishi, T.; Hasegawa, Y. Novel Synthesis and Effective Surface Protection of Air-Stable Luminescent Silicon Nanoparticles. J. Phys. Chem. C 2014, 118, 19778–19784. [Google Scholar] [CrossRef]
- Lal, S.; Caseley, E.A.; Hall, R.M.; Tipper, J.L. Biological Impact of Silicon Nitride for Orthopaedic Applications: Role of Particle Size, Surface Composition and Donor Variation. Sci. Rep. 2018, 8, 9109. [Google Scholar] [CrossRef] [PubMed]
- Ege, D.; Kamali, A.R.; Boccaccini, A.R. Graphene Oxide/Polymer-Based Biomaterials. Adv. Eng. Mater. 2017, 19, 1700627. [Google Scholar] [CrossRef] [Green Version]
- O’Farrell, N.; Houlton, A.; Horrocks, B.R. Silicon nanoparticles: Applications in cell biology and medicine. Int. J. Nanomed. 2006, 1, 451–472. [Google Scholar] [CrossRef]
- Su, S.; He, Y.; Zhang, M.; Yang, K.; Song, S.; Zhang, X.; Fan, C.; Lee, S.-T. High-sensitivity pesticide detection via silicon nanowires-supported acetylcholinesterase-based electrochemical sensors. Appl. Phys. Lett. 2008, 93, 023113. [Google Scholar] [CrossRef]
- Arunmetha, S.; Vinoth, M.; Srither, S.R.; Karthik, A.; Sridharpanday, M.; Suriyaprabha, R.; Manivasakan, P.; Rajendran, V. Study on Production of Silicon Nanoparticles from Quartz Sand for Hybrid Solar Cell Applications. J. Electron. Mater. 2018, 47, 493–502. [Google Scholar] [CrossRef]
- Zywietz, U.; Evlyukhin, A.B.; Reinhardt, C.; Chichkov, B.N. Laser printing of silicon nanoparticles with resonant optical electric and magnetic responses. Nat. Commun. 2014, 5, 3402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garín, M.; Fenollosa, R.; Alcubilla, R.; Shi, L.; Marsal, L.F.; Meseguer, F. All-silicon spherical-Mie-resonator photodiode with spectral response in the infrared region. Nat. Commun. 2014, 5, 3440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shavel, A.; Guerrini, L.; Alvarez-Puebla, R.A. Colloidal synthesis of silicon nanoparticles in molten salts. Nanoscale 2017, 9, 8157–8163. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Barrios, E.; Zhai, L. Bulk Polymer-Derived Ceramic Composites of Graphene Oxide. ACS Omega 2018, 3, 4006–4016. [Google Scholar] [CrossRef]
- Jeong, M.-G.; Du, H.L.; Islam, M.; Lee, J.K.; Sun, Y.-K.; Jung, H.-G. Self-Rearrangement of Silicon Nanoparticles Embedded in Micro-Carbon Sphere Framework for High-Energy and Long-Life Lithium-Ion Batteries. Nano Lett. 2017, 17, 5600–5606. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, Y.; Shen, Z.; Chen, R.; He, X.; Zhang, X.; Li, Y.; Wu, K. Hollow core–shell structured silicon@carbon nanoparticles embed in carbon nanofibers as binder-free anodes for lithium-ion batteries. J. Power Sources 2017, 342, 467–475. [Google Scholar] [CrossRef]
- Huang, R.; Fan, X.; Shen, W.; Zhu, J. Carbon-coated silicon nanowire array films for high-performance lithium-ion battery anodes. Appl. Phys. Lett. 2009, 95, 133119. [Google Scholar] [CrossRef]
- Wang, J.; Fan, F.; Liu, Y.; Jungjohann, K.L.; Lee, S.W.; Mao, S.X.; Liu, X.; Zhu, T. Structural Evolution and Pulverization of Tin Nanoparticles during Lithiation-Delithiation Cycling. J. Electrochem. Soc. 2014, 161, F3019–F3024. [Google Scholar] [CrossRef]
- Gribov, B.G.; Zinov’ev, K.V.; Kalashnik, O.N.; Gerasimenko, N.N.; Smirnov, D.I.; Sukhanov, V.N.; Kononov, N.N.; Dorofeev, S.G. Production of Silicon Nanoparticles for Use in Solar Cells. Semiconductors 2017, 51, 1675–1680. [Google Scholar] [CrossRef]
- Zyuzin, M.V.; Baranov, D.G.; Escudero, A.; Chakraborty, I.; Tsypkin, A.; Ushakova, E.V.; Kraus, F.; Parak, W.J.; Makarov, S.V. Photoluminescence quenching of dye molecules near a resonant silicon nanoparticle. Sci. Rep. 2018, 8, 6107. [Google Scholar] [CrossRef] [PubMed]
- Hardman, R. A Toxicologic Review of Quantum Dots: Toxicity Depends on Physicochemical and Environmental Factors. Environ. Health Perspect. 2006, 114, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Amaya, R.; Matsumoto, Y.; Flores-Conde, A.; Pérez-Guzmán, M.A.; Ortega-López, M. In situ formation of rGO quantum dots during GO reduction via interaction with citric acid in aqueous medium. Mater. Res. Express 2016, 3, 105601. [Google Scholar] [CrossRef]
- Ortega-Amaya, R.; Matsumoto, Y.; Pérez-Guzmán, M.A.; Ortega-López, M. In situ synthesis of Cu2O and Cu nanoparticles during the thermal reduction of copper foil-supported graphene oxide. J. Nanopart. Res. 2015, 17, 397. [Google Scholar] [CrossRef]
- Sobon, G.; Sotor, J.; Jagiello, J.; Kozinski, R.; Zdrojek, M.; Holdynski, M.; Paletko, P.; Boguslawski, J.; Lipinska, L.; Abramski, K.M. Graphene Oxide vs. Reduced Graphene Oxide as saturable absorbers for Er-doped passively mode-locked fiber laser. Opt. Express 2012, 20, 19463–19473. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yan, L. Preparation of graphene by a low-temperature thermal reduction at atmosphere pressure. Nanoscale 2010, 2, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Shen, X.; Li, M.; Zhou, H.; Zhu, G.; Chen, K. Synthesis of reduced graphene oxide/CeO2 nanocomposites and their photocatalytic properties. Nanotechnology 2013, 24, 115603. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, H.-L.; Zhang, Q.; Peng, J.; Li, J.; Zhai, M.; Yu, Z.-Z. Facile synthesis of well-dispersed graphene by [gamma]-ray induced reduction of graphene oxide. J. Mater. Chem. 2012, 22, 13064–13069. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Chan Lee, S.; Some, S.; Wook Kim, S.; Jun Kim, S.; Seo, J.; Lee, J.; Lee, T.; Ahn, J.-H.; Choi, H.-J.; Chan Jun, S. Efficient Direct Reduction of Graphene Oxide by Silicon Substrate. Sci. Rep. 2015, 5, 12306. [Google Scholar] [CrossRef] [PubMed]
- Kole, A.; Chaudhuri, P. Growth of silicon quantum dots by oxidation of the silicon nanocrystals embedded within silicon carbide matrix. AIP Adv. 2014, 4, 107106. [Google Scholar] [CrossRef] [Green Version]
- Parul, S.; Anguita, J.V.; Stolojan, V.; Henley, S.J.; Silva, S.R.P. The growth of silica and silica-clad nanowires using a solid-state reaction mechanism on Ti, Ni and SiO2 layers. Nanotechnology 2010, 21, 295603. [Google Scholar]
- Meier, C.; Lüttjohann, S.; Kravets, V.G.; Nienhaus, H.; Lorke, A.; Wiggers, H. Raman properties of silicon nanoparticles. Phys. E Low-Dimens. Syst. Nanostruct. 2006, 32, 155–158. [Google Scholar] [CrossRef]
- Dong, J.; Liu, W.; Li, H.; Su, X.; Tang, X.; Uher, C. In situ synthesis and thermoelectric properties of PbTe-graphene nanocomposites by utilizing a facile and novel wet chemical method. J. Mater. Chem. A 2013, 1, 12503–12511. [Google Scholar] [CrossRef]
- Shen, J.; Yan, B.; Shi, M.; Ma, H.; Li, N.; Ye, M. One step hydrothermal synthesis of TiO2-reduced graphene oxide sheets. J. Mater. Chem. 2011, 21, 3415–3421. [Google Scholar] [CrossRef]
- Necmi, S.; Tülay, S.; Şeyda, H.; Yasemin, Ç. Annealing effects on the properties of copper oxide thin films prepared by chemical deposition. Semicond. Sci. Technol. 2005, 20, 398. [Google Scholar]
- Durand, F.; Duby, J.C. Carbon solubility in solid and liquid silicon—A review with reference to eutectic equilibrium. J. Phase Equilib. 1999, 20, 61. [Google Scholar] [CrossRef]
- Barman, B.K.; Nanda, K.K. Ultrafast-Versatile-Domestic-Microwave-Oven Based Graphene Oxide Reactor for the Synthesis of Highly Efficient Graphene Based Hybrid Electrocatalysts. ACS Sustain. Chem. Eng. 2018, 6, 4037–4045. [Google Scholar] [CrossRef]
- Bae, J.J.; Yoon, J.H.; Jeong, S.; Moon, B.H.; Han, J.T.; Jeong, H.J.; Lee, G.-W.; Hwang, H.R.; Lee, Y.H.; Jeong, S.Y.; et al. Sensitive photo-thermal response of graphene oxide for mid-infrared detection. Nanoscale 2015, 7, 15695–15700. [Google Scholar] [CrossRef] [PubMed]
- Araki, K.; Takeda, R.; Sudo, H.; Izunome, K.; Zhao, X. Dependence of Atomic-Scale Si(110) Surface Roughness on Hydrogen Introduction Temperature after High-Temperature Ar Annealing. J. Surf. Eng. Mater. Adv. Technol. 2014, 4, 249–256. [Google Scholar] [CrossRef]
- Shive, L.W.; Gilmore, B.L. Impact of Thermal Processing on Silicon Wafer Surface Roughness. ECS Trans. 2008, 16, 401–405. [Google Scholar]
- Shi, Z.; Shao, S.; Wang, Y. Improved the Surface Roughness of Silicon Nanophotonic Devices by Thermal Oxidation Method. J. Phys. Conf. Ser. 2011, 276, 012087. [Google Scholar] [CrossRef] [Green Version]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez-Guzman, M.A.; Ortega-Amaya, R.; Matsumoto, Y.; Espinoza-Rivas, A.M.; Morales-Corona, J.; Santoyo-Salazar, J.; Ortega-Lopez, M. Growth and Self-Assembly of Silicon–Silicon Carbide Nanoparticles into Hybrid Worm-Like Nanostructures at the Silicon Wafer Surface. Nanomaterials 2018, 8, 954. https://doi.org/10.3390/nano8110954
Perez-Guzman MA, Ortega-Amaya R, Matsumoto Y, Espinoza-Rivas AM, Morales-Corona J, Santoyo-Salazar J, Ortega-Lopez M. Growth and Self-Assembly of Silicon–Silicon Carbide Nanoparticles into Hybrid Worm-Like Nanostructures at the Silicon Wafer Surface. Nanomaterials. 2018; 8(11):954. https://doi.org/10.3390/nano8110954
Chicago/Turabian StylePerez-Guzman, Manuel Alejandro, Rebeca Ortega-Amaya, Yasuhiro Matsumoto, Andres Mauricio Espinoza-Rivas, Juan Morales-Corona, Jaime Santoyo-Salazar, and Mauricio Ortega-Lopez. 2018. "Growth and Self-Assembly of Silicon–Silicon Carbide Nanoparticles into Hybrid Worm-Like Nanostructures at the Silicon Wafer Surface" Nanomaterials 8, no. 11: 954. https://doi.org/10.3390/nano8110954