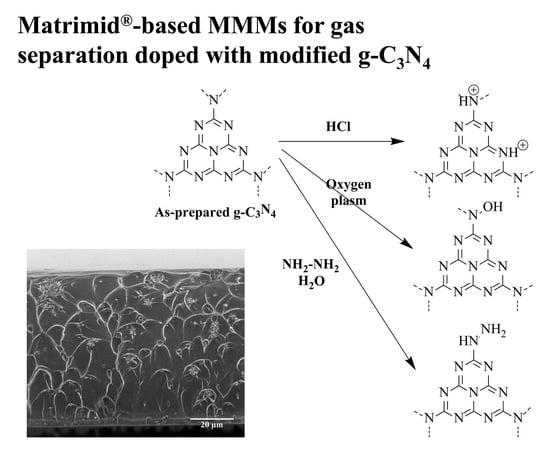
Effects of Protonation, Hydroxylamination, and Hydrazination of g-C3N4 on the Performance of Matrimid®/g-C3N4 Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of Graphitic Carbon Nitride (g-C3N4)
2.2.2. Synthesis of Protonated g-C3N4
2.2.3. Modifications of g-C3N4 with Oxygen Plasma and Hydrazine Monohydrate
2.2.4. Preparation of the Matrimid/g-C3N4 Mixed Matrix Membranes
2.2.5. Characterization Methods
2.2.6. Gas Separation Performance Measurement
3. Results and Discussion
3.1. Vibrational Characterization
3.1.1. ATR-FTIR Spectra of the Modified g-C3N4 Fillers
3.1.2. ATR-FTIR Spectra of Matrimid/g-C3N4 MMMs
3.2. SEM Analysis
3.3. Studies on Thermal Stability of the Matrimid/g-C3N4 Membranes
3.4. CO2/CH4 and O2/N2 Gas Separation Performance of Matrimid/g-C3N4 MMMs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Drioli, E.; Romano, M. Progress and new perspectives on integrated membrane operations for sustainable industrial growth. Ind. Eng. Chem. Res. 2001, 40, 1277–1300. [Google Scholar] [CrossRef]
- Tuinier, M.J.; van Sint Annaland, M.; Kramer, G.J.; Kuipers, J.A.M. Cryogenic CO2 capture using dynamically operated packed beds. Chem. Eng. Sci. 2010, 65, 114–119. [Google Scholar] [CrossRef]
- Brunetti, A.; Sun, Y.; Caravella, A.; Drioli, E.; Barbieri, G. Process intensification for greenhouse gas separation from biogas: More efficient process schemes based on membrane-integrated systems. Int. J. Greenh. Gas Control 2015, 35, 18–29. [Google Scholar] [CrossRef]
- Dorosti, F.; Omidkhah, M.; Abedini, R. Fabrication and characterization of Matrimid/MIL-53 mixed matrix membrane for CO2/CH4 separation. Chem. Eng. Res. Des. 2014, 92, 2439–2448. [Google Scholar] [CrossRef]
- Burdyny, T.; Struchtrup, H. Hybrid membrane/cryogenic separation of oxygen from air for use in the oxy-fuel process. Energy 2010, 35, 1884–1897. [Google Scholar] [CrossRef]
- Basu, S.; Khan, A.L.; Cano-Odena, A.; Liu, C.; Vankelecom, I.F.J. Membrane-based technologies for biogas separations. Chem. Soc. Rev. 2010, 39, 750–768. [Google Scholar] [CrossRef]
- Zhang, Y.; Sunarso, J.; Liu, S.; Wang, R. Current status and development of membranes for CO2/CH4 separation: A review. Int. J. Greenh. Gas Control 2013, 12, 84–107. [Google Scholar] [CrossRef]
- Carreon, M.A.; Li, S.; Falconer, J.L.; Noble, R.D. Alumina-supported SAPO-34 membranes for CO2/CH4 separation. J. Am. Chem. Soc. 2008, 130, 5412–5413. [Google Scholar] [CrossRef]
- Carreon, M.A.; Li, S.; Falconer, J.L.; Noble, R.D. SAPO-34 seeds and membranes prepared using multiple structure directing agents. Adv. Mater. 2008, 20, 729–732. [Google Scholar] [CrossRef]
- Li, S.; Carreon, M.A.; Zhang, Y.; Funke, H.H.; Noble, R.D.; Falconer, J.L. Scale-up of SAPO-34 membranes for CO2/CH4 separation. J. Membr. Sci. 2010, 352, 7–13. [Google Scholar] [CrossRef]
- Li, S. SAPO-34 membranes for CO2/CH4 separation. J. Membr. Sci. 2004, 241, 121–135. [Google Scholar] [CrossRef]
- Falconer, J.L.; Carreon, M.A.; Li, S.; Noble, R.D. Synthesis of Zeolites and Zeolite Membranes Using Multiple Structure Directing Agents. U.S. Patent 8,302,782 B2, 6 November 2012. [Google Scholar]
- Aziz, F.; Ismail, A.F. Preparation and characterization of cross-linked Matrimid® membranes using para-phenylenediamine for O2/N2 separation. Sep. Purif. Technol. 2010, 73, 421–428. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Martin-Gil, V.; Ahmad, M.Z.; Fíla, V. Matrimid® 5218 in preparation of membranes for gas separation: Current state-of-the-art. Chem. Eng. Commun. 2017, 205, 161–196. [Google Scholar] [CrossRef]
- Zhu, J.; Hou, J.; Uliana, A.; Zhang, Y.; Tian, M.; Van der Bruggen, B. The rapid emergence of two-dimensional nanomaterials for high-performance separation membranes. J. Mater. Chem. A 2018, 6, 3773–3792. [Google Scholar] [CrossRef]
- Mamba, G.; Mishra, A.K. Graphitic carbon nitride (g-C3N4) nanocomposites: A new and exciting generation of visible light driven photocatalysts for environmental pollution remediation. Appl. Catal. B 2016, 198, 347–377. [Google Scholar] [CrossRef]
- Ding, F.; Yang, D.; Tong, Z.; Nan, Y.; Wang, Y.; Zou, X.; Jiang, Z. Graphitic carbon nitride-based nanocomposites as visible-light driven photocatalysts for environmental purification. Environ. Sci. Nano 2017, 4, 1455–1469. [Google Scholar] [CrossRef]
- De Silva, S.W.; Du, A.; Senadeera, W.; Gu, Y. Strained graphitic carbon nitride for hydrogen purification. J. Membr. Sci. 2017, 528, 201–205. [Google Scholar] [CrossRef]
- Ji, Y.; Dong, H.; Lin, H.; Zhang, L.; Hou, T.; Li, Y. Heptazine-based graphitic carbon nitride as an effective hydrogen purification membrane. RSC Adv. 2016, 6, 52377–52383. [Google Scholar] [CrossRef]
- Li, F.; Qu, Y.; Zhao, M. Efficient helium separation of graphitic carbon nitride membrane. Carbon 2015, 95, 51–57. [Google Scholar] [CrossRef]
- Qu, Y.; Li, F.; Zhao, M. Theoretical design of highly efficient CO2/N2 separation membranes based on electric quadrupole distinction. J. Phys. Chem. C 2017, 121, 17925–17931. [Google Scholar] [CrossRef]
- Hou, J.; Wei, Y.; Zhou, S.; Wang, Y.; Wang, H. Highly efficient H2/CO2 separation via an ultrathin metal-organic framework membrane. Chem. Eng. Sci. 2018, 182, 180–188. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, S.; Wang, Y.; Ma, X.; Cao, K.; Peng, D.; Wu, X.; Wu, H.; Jiang, Z. Enhanced gas separation performance of mixed matrix membranes from graphitic carbon nitride nanosheets and polymers of intrinsic microporosity. J. Membr. Sci. 2016, 514, 15–24. [Google Scholar] [CrossRef]
- Wang, Y.; Ou, R.; Wang, H.; Xu, T. Graphene oxide modified graphitic carbon nitride as a modifier for thin film composite forward osmosis membrane. J. Membr. Sci. 2015, 475, 281–289. [Google Scholar] [CrossRef]
- Chen, J.; Li, Z.; Wang, C.; Wu, H.; Liu, G. Synthesis and characterization of g-C3N4 nanosheet modified polyamide nanofiltration membranes with good permeation and antifouling properties. RSC Adv. 2016, 6, 112148–112157. [Google Scholar] [CrossRef]
- Gao, X.; Li, Y.; Yang, X.; Shang, Y.; Wang, Y.; Gao, B.; Wang, Z. Highly permeable and antifouling reverse osmosis membranes with acidified graphitic carbon nitride nanosheets as nanofillers. J. Mater. Chem. A 2017, 5, 19875–19883. [Google Scholar] [CrossRef]
- Cao, K.; Jiang, Z.; Zhang, X.; Zhang, Y.; Zhao, J.; Xing, R.; Yang, S.; Gao, C.; Pan, F. Highly water-selective hybrid membrane by incorporating g-C3N4 nanosheets into polymer matrix. J. Membr. Sci. 2015, 490, 72–83. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.; Zhou, S.; Xue, A.; Zhang, Y.; Zhao, Y.; Zhong, J.; Zhang, Q. Graphitic carbon nitride nanosheets embedded in poly(vinyl alcohol) nanocomposite membranes for ethanol dehydration via pervaporation. Sep. Purif. Technol. 2017, 188, 24–37. [Google Scholar] [CrossRef]
- Ding, H.; Pan, F.; Mulalic, E.; Gomaa, H.; Li, W.; Yang, H.; Wu, H.; Jiang, Z.; Wang, B.; Cao, X.; et al. Enhanced desulfurization performance and stability of Pebax membrane by incorporating Cu+ and Fe2+ ions co-impregnated carbon nitride. J. Membr. Sci. 2017, 526, 94–105. [Google Scholar] [CrossRef]
- Niu, P.; Zhang, L.; Liu, G.; Cheng, H.-M. Graphene-Like Carbon Nitride Nanosheets for Improved Photocatalytic Activities. Adv. Funct. Mater. 2012, 22, 4763–4770. [Google Scholar] [CrossRef]
- Dante, R.C.; Martín-Ramos, P.; Sánchez-Arévalo, F.M.; Huerta, L.; Bizarro, M.; Navas-Gracia, L.M.; Martín-Gil, J. Synthesis of crumpled nanosheets of polymeric carbon nitride from melamine cyanurate. J. Solid State Chem. 2013, 201, 153–163. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Chai, S.-P.; Yong, S.-T.; Mohamed, A.R. Surface charge modification via protonation of graphitic carbon nitride (g-C3N4) for electrostatic self-assembly construction of 2D/2D reduced graphene oxide (rGO)/g-C3N4 nanostructures toward enhanced photocatalytic reduction of carbon dioxide to methane. Nano Energy 2015, 13, 757–770. [Google Scholar] [CrossRef]
- Park, O.-K.; Young Kim, W.; Min Kim, S.; You, N.-H.; Jeong, Y.; Su Lee, H.; Ku, B.-C. Effect of oxygen plasma treatment on the mechanical properties of carbon nanotube fibers. Mater. Lett. 2015, 156, 17–20. [Google Scholar] [CrossRef]
- Bu, X.; Li, J.; Yang, S.; Sun, J.; Deng, Y.; Yang, Y.; Wang, G.; Peng, Z.; He, P.; Wang, X.; et al. Surface modification of C3N4 through oxygen-plasma treatment: A simple way toward excellent hydrophilicity. ACS Appl. Mater. Interfaces 2016, 8, 31419–31425. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lin, B.; Wang, H.; Yang, Y.; Zhu, H.; Yu, W.; Basset, J.-M. Surface modification of g-C3N4 by hydrazine: Simple way for noble-metal free hydrogen evolution catalysts. Chem. Eng. J. 2016, 286, 339–346. [Google Scholar] [CrossRef]
- Recio, R.; Lozano, Á.E.; Prádanos, P.; Marcos, Á.; Tejerina, F.; Hernández, A. Effect of fractional free volume and Tg on gas separation through membranes made with different glassy polymers. J. Appl. Polym. Sci. 2008, 107, 1039–1046. [Google Scholar] [CrossRef]
- Dong, F.; Zhao, Z.; Xiong, T.; Ni, Z.; Zhang, W.; Sun, Y.; Ho, W.-K. In situ construction of g-C3N4/g-C3N4 metal-free heterojunction for enhanced visible-light photocatalysis. ACS Appl. Mater. Interfaces 2013, 5, 11392–11401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mori, T.; Niu, L.; Ye, J. Non-covalent doping of graphitic carbon nitride polymer with graphene: Controlled electronic structure and enhanced optoelectronic conversion. Energy Environ. Sci. 2011, 4, 4517. [Google Scholar] [CrossRef]
- Ebadi Amooghin, A.; Omidkhah, M.; Kargari, A. The effects of aminosilane grafting on NaY zeolite–Matrimid®5218 mixed matrix membranes for CO2/CH4 separation. J. Membr. Sci. 2015, 490, 364–379. [Google Scholar] [CrossRef]
- Venna, S.R.; Lartey, M.; Li, T.; Spore, A.; Kumar, S.; Nulwala, H.B.; Luebke, D.R.; Rosi, N.L.; Albenze, E. Fabrication of MMMs with improved gas separation properties using externally-functionalized MOF particles. J. Mater. Chem. A 2015, 3, 5014–5022. [Google Scholar] [CrossRef]
- Zornoza, B.; Casado, C.; Navajas, A. Advances in hydrogen separation and purification with membrane technology. In Renewable Hydrogen Technologies; Gandía, L.M., Arzamendi, G., Diéguez, P.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 245–268. [Google Scholar]
- Shao, L.; Liu, L.; Cheng, S.-X.; Huang, Y.-D.; Ma, J. Comparison of diamino cross-linking in different polyimide solutions and membranes by precipitation observation and gas transport. J. Membr. Sci. 2008, 312, 174–185. [Google Scholar] [CrossRef]
- Loloei, M.; Omidkhah, M.; Moghadassi, A.; Amooghin, A.E. Preparation and characterization of Matrimid® 5218 based binary and ternary mixed matrix membranes for CO2 separation. Int. J. Greenh. Gas Control 2015, 39, 225–235. [Google Scholar] [CrossRef]
- Rahmani, M.; Kazemi, A.; Talebnia, F.; Abbasszadeh Gamali, P. Fabrication and characterization of brominated Matrimid® 5218 membranes for CO2/CH4 separation: Application of response surface methodology (RSM). e-Polymers 2016, 16, 481–492. [Google Scholar] [CrossRef]
- Ebadi Amooghin, A.; Omidkhah, M.; Sanaeepur, H.; Kargari, A. Preparation and characterization of Ag+ ion-exchanged zeolite-Matrimid®5218 mixed matrix membrane for CO2/CH4 separation. J. Energy Chem. 2016, 25, 450–462. [Google Scholar] [CrossRef]
- Sterescu, D.M.; Stamatialis, D.F.; Wessling, M. Boltorn-modified polyimide gas separation membranes. J. Membr. Sci. 2008, 310, 512–521. [Google Scholar] [CrossRef]
- Clausi, D.T.; Koros, W.J. Formation of defect-free polyimide hollow fiber membranes for gas separations. J. Membr. Sci. 2000, 167, 79–89. [Google Scholar] [CrossRef]
- Tejero, R.; Lozano, Á.E.; Álvarez, C.; de Abajo, J. Synthesis, characterization, and evaluation of novel polyhydantoins as gas separation membranes. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 4052–4060. [Google Scholar] [CrossRef]
- Yong, W.F.; Li, F.Y.; Chung, T.-S.; Tong, Y.W. Highly permeable chemically modified PIM-1/Matrimid membranes for green hydrogen purification. J. Mater. Chem. A 2013, 1, 13914–13925. [Google Scholar] [CrossRef]
- Xiao, Y.; Chung, T.; Guan, H.; Guiver, M. Synthesis, cross-linking and carbonization of co-polyimides containing internal acetylene units for gas separation. J. Membr. Sci. 2007, 302, 254–264. [Google Scholar] [CrossRef] [Green Version]






| Pristine Matrimid | g-C3N4 | Protonated g-C3N4 | Oxygen Plasma-Treated g-C3N4 | Hydrazinated g-C3N4 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Weight Loss (%) | Midpoint (°C) | Weight Loss (%) | Midpoint (°C) | Weight Loss (%) | Midpoint (°C) | Weight Loss (%) | Midpoint (°C) | Weight Loss (%) | Midpoint (°C) |
| 2.8 | 287 | 97.6 | 700 | 4.2 | 29.4 | 2.70 | 284 | 2.2 | 280 |
| 23.4 | 516 | 23.3 | 518 | 24.8 | 518 | 25.9 | 517 | ||
| 14.9 | 609 | 15.9 | 611 | 17.0 | 611 | 17.1 | 611 | ||
| MMM | Permeability (Barrer) | Selectivity | Ref. | |||||
|---|---|---|---|---|---|---|---|---|
| He | N2 | O2 | CH4 | CO2 | O2/N2 | CO2/CH4 | ||
| Matrimid (control) | 22.00 | 0.27 | 1.90 | 0.24 | 8.70 | 7.0 | 36.3 | [48] |
| Matrimid/protonated g-C3N4 0.5 wt % | 23.53 | 0.26 | 1.70 | 0.15 | 7.69 | 6.6 | 49.6 | This study |
| Matrimid/protonated g-C3N4 2 wt % | 20.17 | 0.20 | 1.44 | 0.15 | 6.45 | 7.1 | 42.2 | This study |
| Matrimid/hydroxylaminated g-C3N4 0.5 wt % | 24.12 | 0.19 | 1.71 | 0.21 | 7.86 | 8.9 | 37.9 | This study |
| Matrimid/hydroxylaminated g-C3N4 2 wt % | 23.63 | 0.23 | 1.69 | 0.13 | 7.15 | 7.2 | 55.2 | This study |
| Matrimid/hydrazinated g-C3N4 0.5 wt % | 20.60 | 0.20 | 1.44 | 0.17 | 6.56 | 7.1 | 39.0 | This study |
| Matrimid/hydrazinated g-C3N4 2 wt % | 18.65 | 0.20 | 1.36 | 0.15 | 6.19 | 6.8 | 40.4 | This study |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soto-Herranz, M.; Sánchez-Báscones, M.; Hérnandez-Giménez, A.; Calvo-Díez, J.I.; Martín-Gil, J.; Martín-Ramos, P. Effects of Protonation, Hydroxylamination, and Hydrazination of g-C3N4 on the Performance of Matrimid®/g-C3N4 Membranes. Nanomaterials 2018, 8, 1010. https://doi.org/10.3390/nano8121010
Soto-Herranz M, Sánchez-Báscones M, Hérnandez-Giménez A, Calvo-Díez JI, Martín-Gil J, Martín-Ramos P. Effects of Protonation, Hydroxylamination, and Hydrazination of g-C3N4 on the Performance of Matrimid®/g-C3N4 Membranes. Nanomaterials. 2018; 8(12):1010. https://doi.org/10.3390/nano8121010
Chicago/Turabian StyleSoto-Herranz, María, Mercedes Sánchez-Báscones, Antonio Hérnandez-Giménez, José I. Calvo-Díez, Jesús Martín-Gil, and Pablo Martín-Ramos. 2018. "Effects of Protonation, Hydroxylamination, and Hydrazination of g-C3N4 on the Performance of Matrimid®/g-C3N4 Membranes" Nanomaterials 8, no. 12: 1010. https://doi.org/10.3390/nano8121010