Strontium Oxide Deposited onto a Load-Bearable and Porous Titanium Matrix as Dynamic and High-Surface-Contact-Area Catalysis for Transesterification
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of P-Ti and Srx-P-Ti_55
2.2. Quality Assessment of P-Ti_55 and Srx-P-Ti_55
2.3. Scratch Test and Catalytic Evaluation of the Coated SrO
2.4. Surface Analysis on the Thin Disc Srx-P-Ti_55
3. Results and Discussion
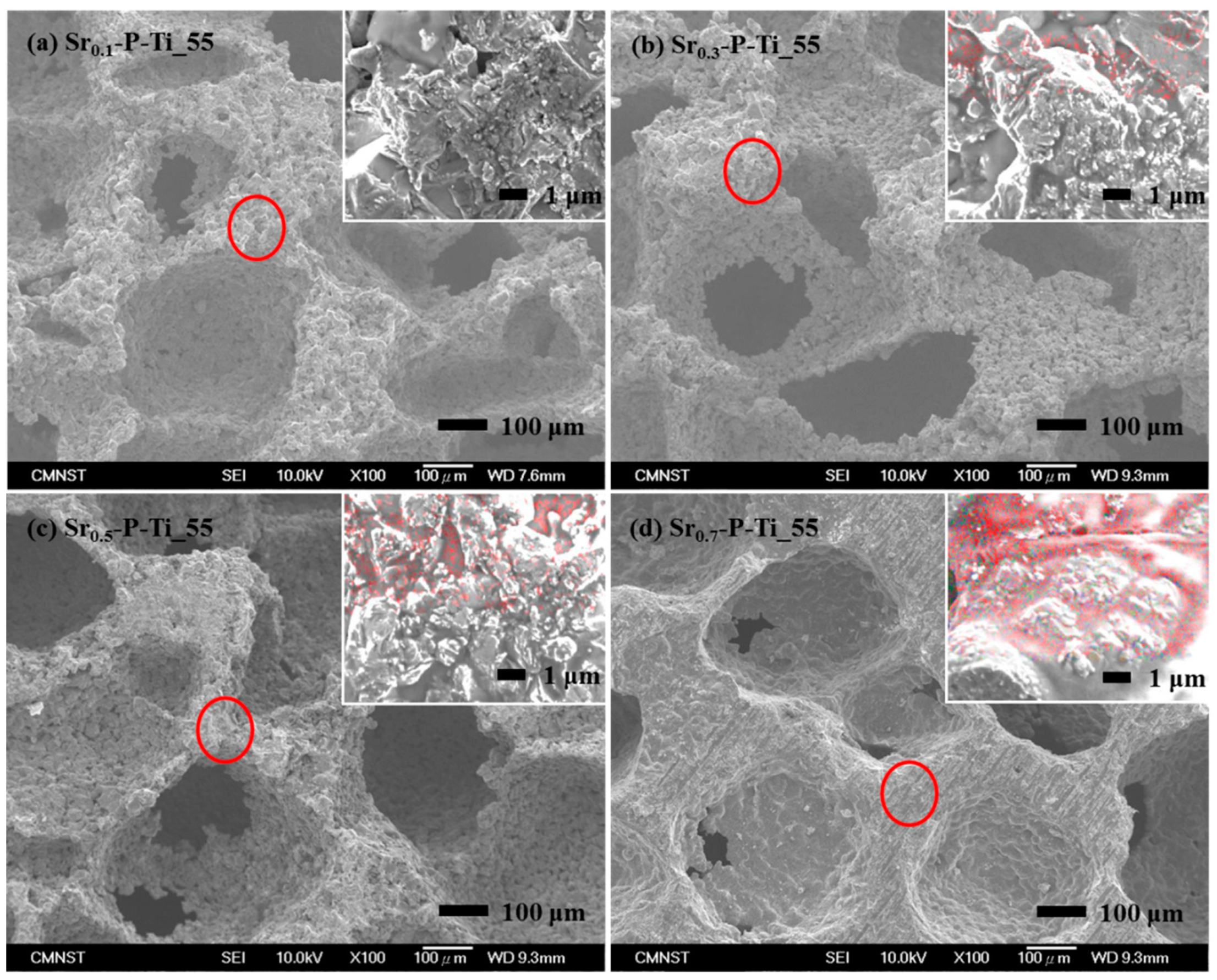
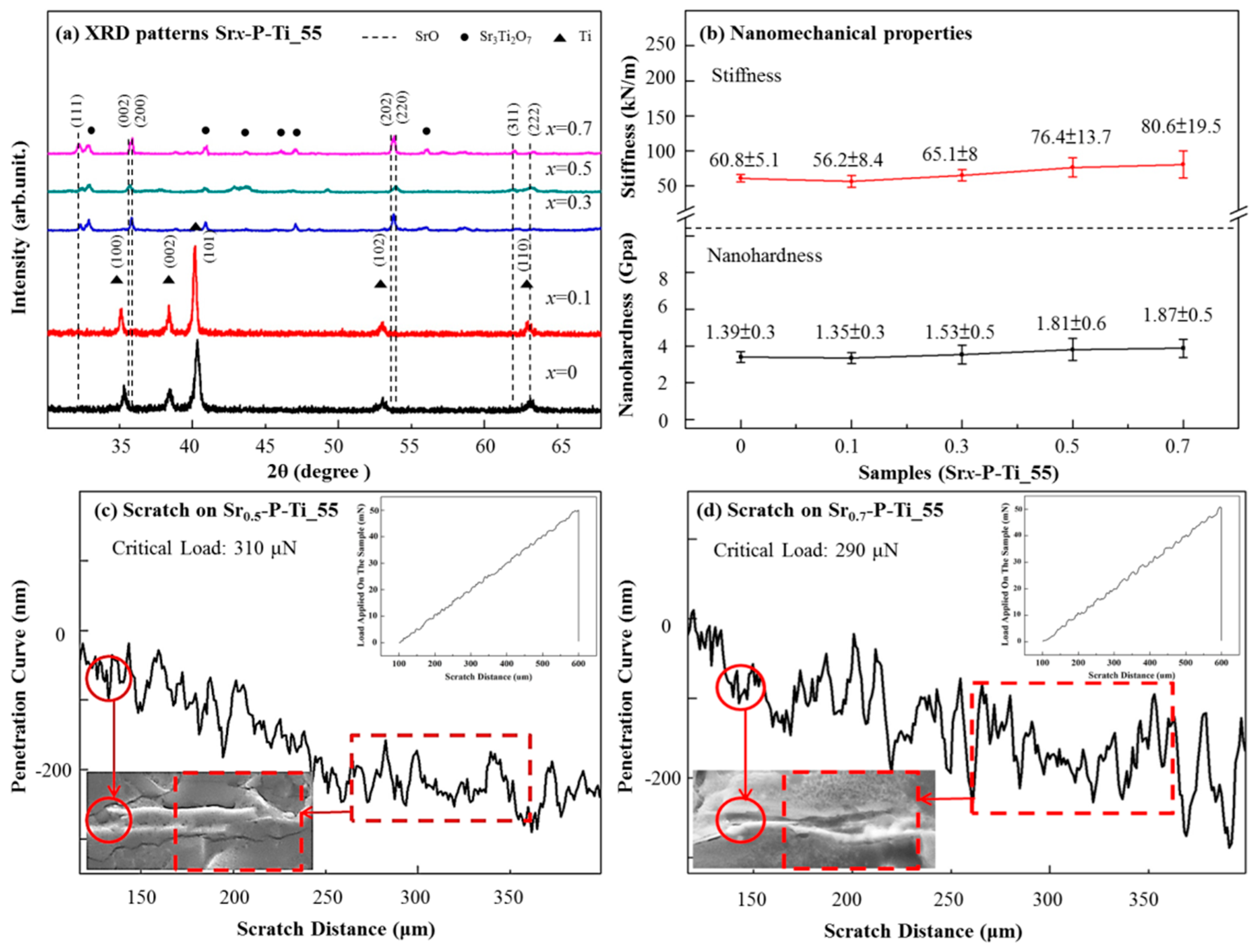
3.1. Physical Properties of the Cross-Sectioned Surface of Srx-P-Ti_55
3.2. Mechanical Properties of the Cross-Sectioned Surfaces of Srx-P-Ti_55
3.3. Chemical Analysis on the x = 0.5* and x = 0.7*
3.4. XPS Characterization on the Surfaces of the Discs x = 0.5* and x = 0.7*
3.5. Chemical Kinetics for the Transesterification Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Schulze, E.D.; Valentini, R.; Sanz, M.J. The long way from Kyoto to Marrakesh: Implications of the Kyoto Protocol negotiations for global ecology. Glob. Chang. Biol. 2002, 8, 505–518. [Google Scholar] [CrossRef]
- Adeniyi, O.M.; Azimov, U.; Burluka, A. Algae biofuel: Current status and future applications. Renew. Sustain. Energy Rev. 2018, 90, 316–335. [Google Scholar] [CrossRef]
- Mantovani, A.C.G.; Chendynski, L.T.; Salviato, A.; Borsato, D.; Santana, V.T.; Di Mauro, E. Monitoring free radicals formation in the biodiesel oxidation reaction via electronic paramagnetic resonance. Fuel 2018, 224, 255–260. [Google Scholar] [CrossRef]
- Nayebzadeh, H.; Haghighi, M.; Saghatoleslami, N.; Tabasizadeh, M.; Yousefi, S. Fabrication of carbonated alumina doped by calcium oxide via microwave combustion method used as nanocatalyst in biodiesel production: Influence of carbon source type. Energy Convers. Manag. 2018, 171, 566–575. [Google Scholar] [CrossRef]
- Yilmaz, N.; Atmanli, A.; Vigil, F.M. Quaternary blends of diesel, biodiesel, higher alcohols and vegetable oil in a compression ignition engine. Fuel 2018, 212, 462–469. [Google Scholar] [CrossRef]
- Dabros, T.M.; Stummann, M.Z.; Hoj, M.; Jensen, P.A.; Grunwaldt, J.D.; Gabrielsen, J.; Jensen, A.D. Transportation fuels from biomass fast pyrolysis, catalytic hydrodeoxygenation, and catalytic fast hydropyrolysis. Prog. Energy Combust. Sci. 2018, 68, 268–309. [Google Scholar] [CrossRef]
- Fazal, M.A.; Suhaila, N.R.; Haseeb, A.S.M.A.; Rubaiee, S. Sustainability of additive-doped biodiesel: Analysis of its aggressiveness toward metal corrosion. Clean. Prod. 2018, 181, 508–516. [Google Scholar] [CrossRef]
- Reddy, S.M.; Sharma, N.; Gupta, N.; Agarwal, A.K. Effect of non-edible oil and its biodiesel on wear of fuel injection equipment components of a genset engine. Fuel 2018, 222, 841–851. [Google Scholar] [CrossRef]
- Zainal, N.A.; Zulkifli, N.W.M.; Gulzar, M.; Masjuki, H.H. A review on the chemistry, production, and technological potential of bio-based lubricants. Renew. Sustain. Energy Rev. 2018, 82, 80–102. [Google Scholar] [CrossRef]
- Leung, D.Y.; Wu, X.; Leung, M.K.H. A review on biodiesel production using catalyzed transesterification. Appl. Energy 2010, 87, 1083–1095. [Google Scholar] [CrossRef]
- Sander, A.; Košćak, M.A.; Kosir, D.; Milosavljević, N.; Vuković, J.P.; Magić, L. The influence of animal fat type and purification conditions on biodiesel quality. Renew. Energy 2018, 118, 752–760. [Google Scholar] [CrossRef]
- Lee, H.; Liao, J.D.; Yang, J.W.; Hsu, W.D.; Liu, B.H.; Chen, T.C.; Gedanken, A. Continuous Waste Cooking Oil Transesterification with Microwave Heating and Strontium Oxide Catalyst. Chem. Eng. Technol. 2018, 41, 192–198. [Google Scholar] [CrossRef]
- Lee, H.; Liao, J.D.; Sivashanmugan, K.; Liu, B.H.C.; Su, Y.H.; Yao, C.K.; Juang, Y.D. Hydrothermal Fabrication of Highly Porous Titanium Bio-Scaffold with a Load-Bearable Property. Materials 2017, 10, 726. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Liao, J.D.; Sivashanmugan, K.; Liu, B.H.; Weng, S.L.; Juang, Y.D.; Yao, C.K. Dual properties of zirconia coated porous titanium for a stiffness enhanced bio-scaffold. Mater. Des. 2017, 132, 13–21. [Google Scholar] [CrossRef]
- Roschat, W.; Phewphong, S.; Khunchalee, J.; Moonsin, P. Biodiesel production by ethanolysis of palm oil using SrO as a basic heterogeneous catalyst. Mater. Today Proc. 2018, 5, 13916–13921. [Google Scholar] [CrossRef]
- Marwaha, A.; Dhir, A.; Mahla, S.K.; Mohapatra, S.K. An overview of solid base heterogeneous catalysts for biodiesel production. Catal. Rev. 2018, 60, 594–628. [Google Scholar] [CrossRef]
- Kumar, P.; Kaur, R.; Verma, S.; Srivastava, V.C.; Mishra, I.M. The preparation and efficacy of SrO/CeO2 catalysts for the production of dimethyl carbonate by transesterification of ethylene carbonate. Fuel 2018, 220, 706–716. [Google Scholar] [CrossRef]
- Teo, S.H.; Rashid, U.; Taufiq-Yap, Y.H. Heterogeneous catalysis of transesterification of jatropha curcas oil over calcium–cerium bimetallic oxide catalyst. RSC Adv. 2014, 4, 48836–48847. [Google Scholar] [CrossRef]
- Hammond, M.A.; Wallace, J.M. Exercise prevents [beta]-aminopropionitrile-induced morphological changes to type I collagen in murine bone. Bonekey Rep. 2015, 4, 645. [Google Scholar] [CrossRef] [PubMed]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Lander, J.J. Experimental heat contents of SrO, BaO, CaO, BaCO3 and SrCO3 at high temperatures. Dissociation pressures of BaCO3 and SrCOJ. Am. Chem. Soc. 1951, 73, 5794–5797. [Google Scholar] [CrossRef]
- Hattori, A.; Hattori, H.; Tanabe, K. Double-bond migration of allylamine to enamine over basic oxide catalysts. J. Catal. 1980, 65, 245–252. [Google Scholar] [CrossRef]
- Li, J.M. Realizing single-crystalline vertically-oriented and high-density electrospun nanofibril bundles by controlled postcalcination. CrystEngComm 2017, 19, 3392–3397. [Google Scholar] [CrossRef]
- He, G.; Lu, S.; Xu, W.; Yu, T.; Li, J.; Dai, T. Durable superhydrophobic Zn/ZnO/TiO2 surfaces on Ti6Al4V substrate with self-cleaning property and switchable wettability. Ceram. Int. 2018, 44, 638–647. [Google Scholar] [CrossRef]
- Burnett, P.J.; Rickerby, D.S. The relationship between hardness and scratch adhession. Thin Solid Films 1987, 154, 403–416. [Google Scholar] [CrossRef]
- Liu, X.; He, H.; Wang, Y.; Zhu, S. Transesterification of soybean oil to biodiesel using SrO as a solid base catalyst. Catal. Commun. 2007, 8, 1107–1111. [Google Scholar] [CrossRef]
- Abboud, J.; Schobing, J.; Legros, G.; Matynia, A.; Bonnety, J.; Tschamber, V.; Da Costa, P. Impacts of ester’s carbon chain length and concentration on sooting propensities and soot oxidative reactivity: Application to Diesel and Biodiesel surrogates. Fuel 2018, 222, 586–598. [Google Scholar] [CrossRef]
- Bouchet, M.I.D.B.; Martin, J.M.; Forest, C.; le Mogne, T.; Mazarin, M.; Avila, J.; Fisher, G.L. Tribochemistry of unsaturated fatty acids as friction modifiers in (bio) diesel fuel. RSC Adv. 2017, 7, 33120–33131. [Google Scholar] [CrossRef]
- Zagonel, G.F.; Peralta-Zamora, P.; Ramos, L.P. Multivariate monitoring of soybean oil ethanolysis by FTIR. Talanta 2004, 63, 1021–1025. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.R.; Kugelmeier, C.L.; Pinheiro, R.S.; Batalha, M.O.; da Silva César, A. Glycerol from biodiesel production: Technological paths for sustainability. Renew. Sustain. Energy Rev. 2018, 88, 109–122. [Google Scholar] [CrossRef]
- Ghesti, G.F.; de Macedo, J.L.; Braga, V.S.; de Souza, A.T.; Parente, V.C.; Figuerêdo, E.S.; Dias, S.C. Application of raman spectroscopy to monitor and quantify ethyl esters in soybean oil transesterification. J. Am. Oil Chem. Soc. 2006, 83, 597–601. [Google Scholar] [CrossRef]
- Maru, M.M.; Lucchese, M.M.; Legnani, C.; Quirino, W.G.; Balbo, A.; Aranha, I.B.; dos Santos Cruz, T. Biodiesel compatibility with carbon steel and HDPE parts. Fuel Process. Technol. 2009, 90, 1175–1182. [Google Scholar] [CrossRef]
- Russo, V.; Ghidelli, M.; Gondoni, P.; Casari, C.S.; Bassi, A.L. Multi-wavelength Raman scattering of nanostructured Al-doped zinc oxide. J. Appl. Phys. 2014, 115, 073508. [Google Scholar] [CrossRef] [Green Version]
- Gondoni, P.; Ghidelli, M.; Di Fonzo, F.; Carminati, M.; Russo, V.; Bassi, A.L.; Casari, C.S. Structure-dependent optical and electrical transport properties of nanostructured Al-doped ZnO. Nanotechnology 2012, 23, 365706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Lv, Y.; Zhang, M.; Liu, Y.; Zhu, Y.; Zong, R.; Zhu, Y. Defect-related photoluminescence and photocatalytic properties of porous ZnO nanosheets. J. Mater. Chem. 2014, 2, 15377–15388. [Google Scholar] [CrossRef]
- Fuentes, S.; Chávez, E.; Padilla-Campos, L.; Diaz-Droguett, D.E. Influence of reactant type on the Sr incorporation grade and structural characteristics of Ba1− xSrxTiO3 (x = 0–1) grown by sol–gel-hydrothermal synthesis. Ceram. Int. 2013, 39, 8823–8831. [Google Scholar] [CrossRef]
- Voelkel, J. Improvement in quality of sol-gel derived zirconia multilayer coatings by polymeric additive. Mol. Cryst. Liquid Cryst. Sci. Technol. Sect. A Mol. Cryst. Liquid Cryst. 2000, 354, 503–509. [Google Scholar] [CrossRef]
- Lin, C.; Zhang, C.; Lin, J. Phase transformation and photoluminescence properties of nanocrystalline ZrO2 powders prepared via the pechini-type sol− gel process. J. Phys. Chem. C 2007, 111, 3300–3307. [Google Scholar] [CrossRef]
- Fereidooni, L.; Tahvildari, K.; Mehrpooya, M. Trans-esterification of waste cooking oil with methanol by electrolysis process using KOH. Renew. Energy 2018, 116, 183–193. [Google Scholar] [CrossRef]
- Lamba, N.; Adhikari, S.; Modak, J.M.; Madras, G. Catalytic synthesis of fatty acid methyl esters from Madhuca indica oil in supercritical methanol. Energy Convers. Manag. 2018, 173, 412–425. [Google Scholar] [CrossRef]
- Bai, Z.; Liu, Q.; Lei, J.; Jin, H. Investigation on the mid-temperature solar thermochemical power generation system with methanol decomposition. Appl. Energy 2018, 217, 56–65. [Google Scholar] [CrossRef]
- Wang, Y.T.; Fang, Z.; Zhang, F. Esterification of oleic acid to biodiesel catalyzed by a highly acidic carbonaceous catalyst. Catal. Today 2019, 319, 172–181, in press. [Google Scholar] [CrossRef]
- Koberg, M.; Abu-Much, R.; Gedanken, A. Optimization of bio-diesel production from soybean and wastes of cooked oil: Combining dielectric microwave irradiation and a SrO catalyst. Bioresour. Technol. 2011, 102, 1073–1078. [Google Scholar] [CrossRef] [PubMed]


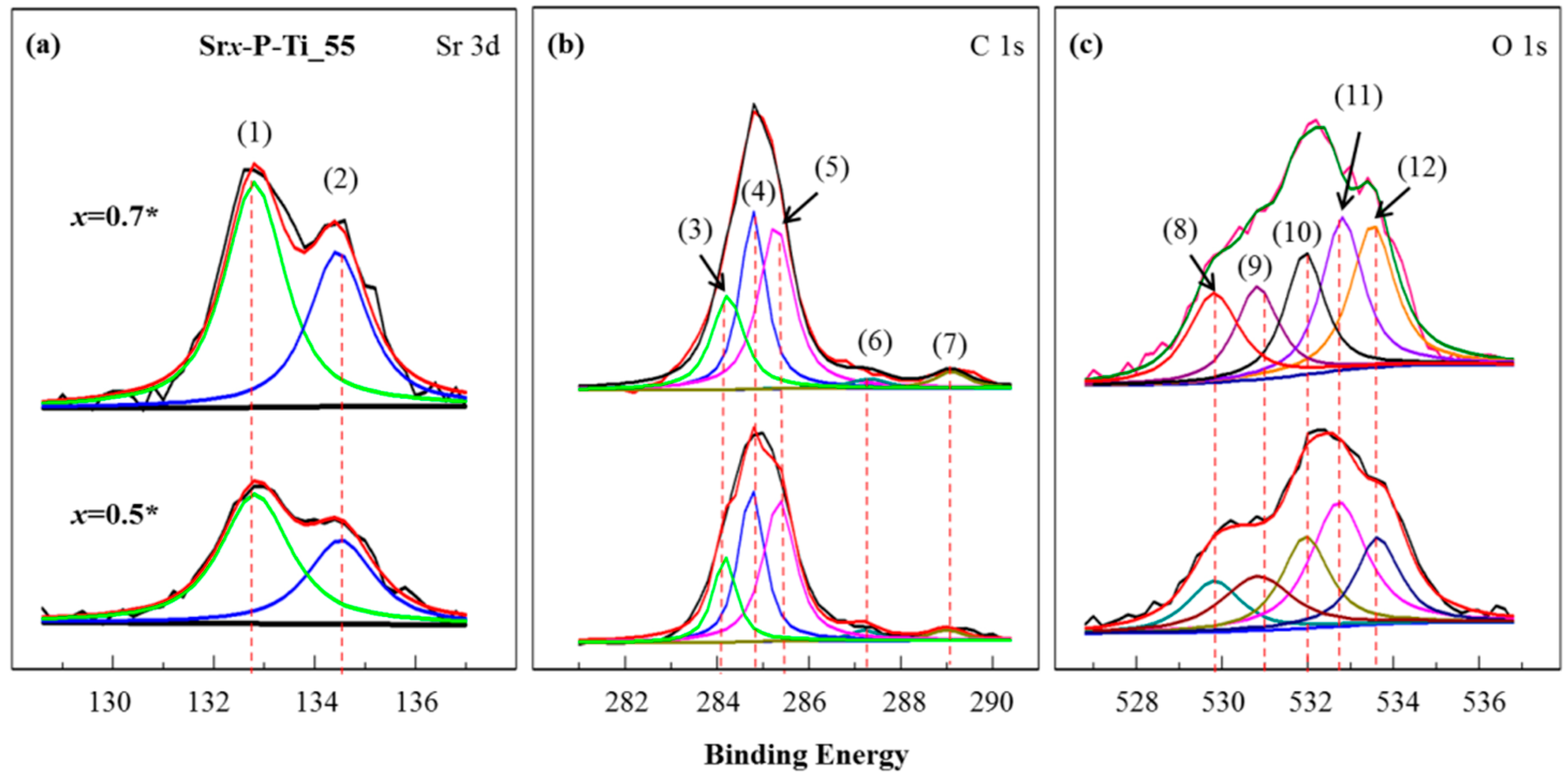

| Samples | Peak | Bond | Bonding Energy (eV) |
|---|---|---|---|
| Srx-P_Ti_55 (x = 0.5*, and 0.7*) | Sr 3d | (1) Sr with O a | 132.9 |
| (2) Sr with O a | 134.6 | ||
| C 1s | (3) C=O b | 284.2 | |
| (4) C–C | 284.8 | ||
| (5) C–H | 285.5 | ||
| (6) C–O b | 287.1 | ||
| (7) COO b | 289.2 | ||
| O 1s | (8) O-containing ions in SrO a | 529.8 | |
| (9) C–O | 531.0 | ||
| (10) M–OH | 532.0 | ||
| (11) C=O b | 532.8 | ||
| (12) C–O b | 533.7 |
| Srx-P_Ti_55 | x = 0.5* with Oil | x = 0.7* with Oil | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sr–O | C–O | M–OH | C=O | C–O | Sr–O | C–O | M–OH | C=O | C–O | |
| BE (eV) | 529.8 | 531.0 | 532.0 | 532.8 | 533.7 | 529.8 | 531 | 532 | 532.8 | 533.7 |
| Peak area ratio (Opeak(y)/Ototal%) | 12.9 y = 8 | 31.6 y = 9 | 20.2 y = 10 | 17.5 y = 11 | 17.8 y = 12 | 24 y = 8 | 15.4 y = 9 | 24.4 y = 10 | 18.6 y = 11 | 17.6 y = 12 |
| Compare (0.5* and 0.7*) | Inc. | Dec. | Inc. | Inc. | Dec. | |||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Liao, J.-D.; Lee, M.H.; Liu, B.H.; Fu, W.-E.; Sivashanmugan, K.; Juang, Y.-D. Strontium Oxide Deposited onto a Load-Bearable and Porous Titanium Matrix as Dynamic and High-Surface-Contact-Area Catalysis for Transesterification. Nanomaterials 2018, 8, 973. https://doi.org/10.3390/nano8120973
Lee H, Liao J-D, Lee MH, Liu BH, Fu W-E, Sivashanmugan K, Juang Y-D. Strontium Oxide Deposited onto a Load-Bearable and Porous Titanium Matrix as Dynamic and High-Surface-Contact-Area Catalysis for Transesterification. Nanomaterials. 2018; 8(12):973. https://doi.org/10.3390/nano8120973
Chicago/Turabian StyleLee, Han, Jiunn-Der Liao, Mu Hsuan Lee, Bernard Haochih Liu, Wei-En Fu, Kundan Sivashanmugan, and Yung-Der Juang. 2018. "Strontium Oxide Deposited onto a Load-Bearable and Porous Titanium Matrix as Dynamic and High-Surface-Contact-Area Catalysis for Transesterification" Nanomaterials 8, no. 12: 973. https://doi.org/10.3390/nano8120973






