Fabrication of InxGa1−xN Nanowires on Tantalum Substrates by Vapor-Liquid-Solid Chemical Vapor Deposition
Abstract
:1. Introduction
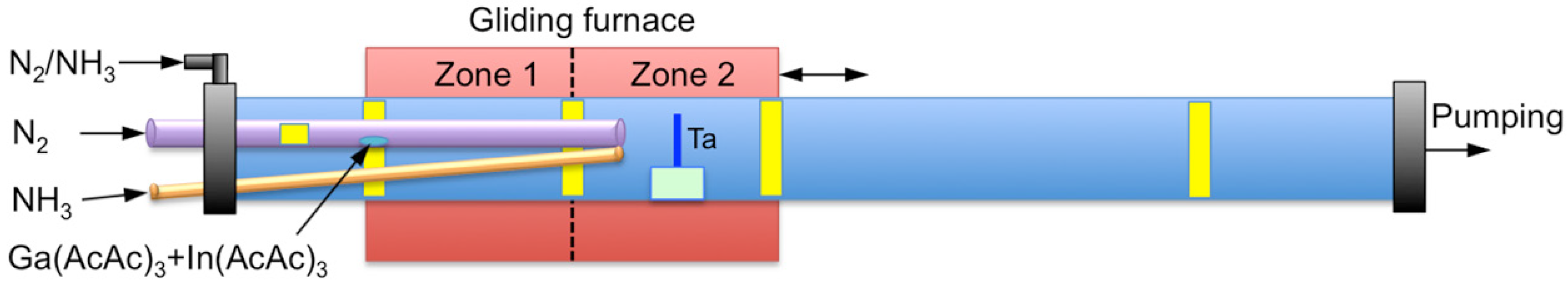
2. Materials and Methods
3. Results and Discussion
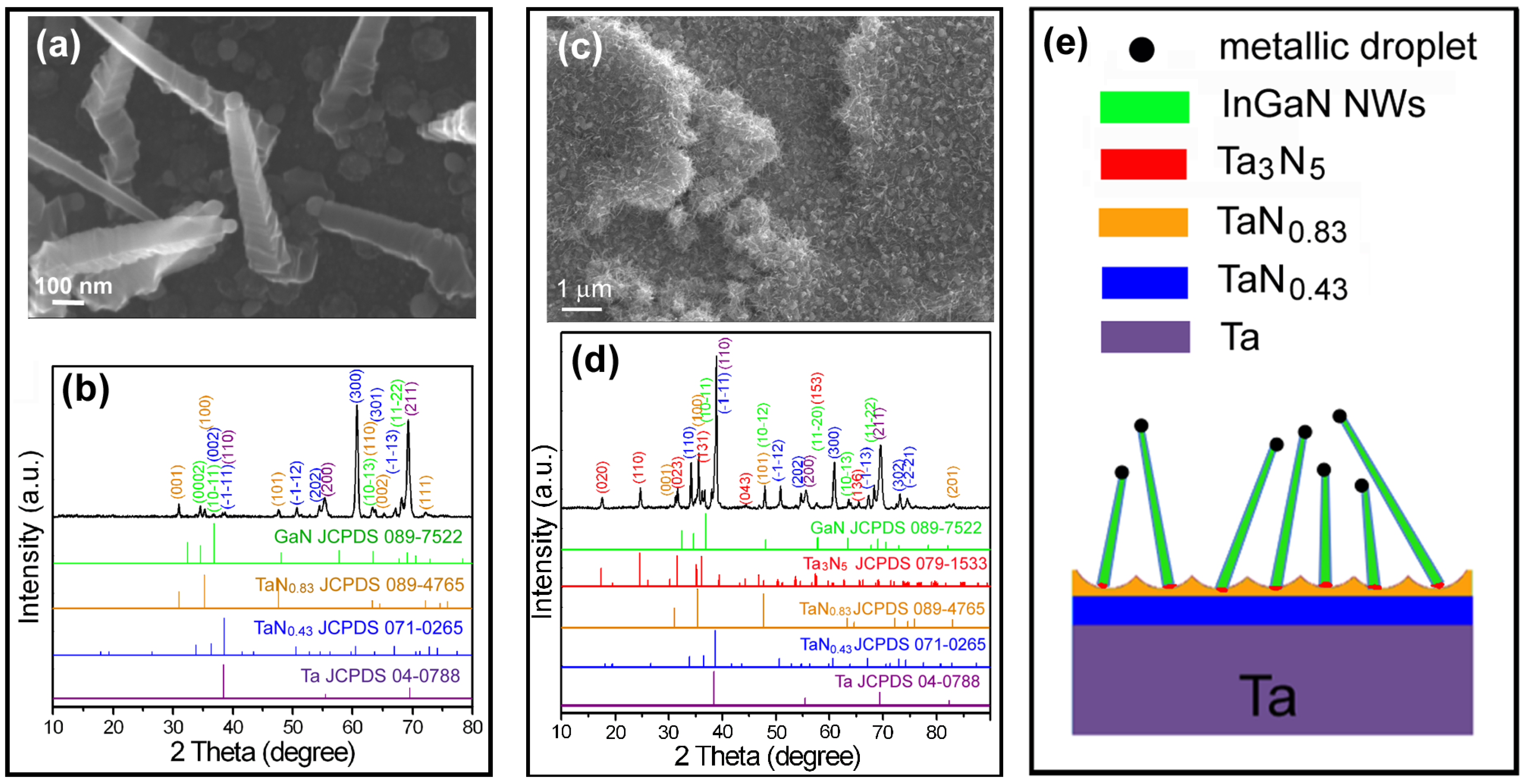
3.1. CVD Growth on the Native and Oxidative Ta Substrates
3.2. CVD Growth on the Nitrided Ta Substrates
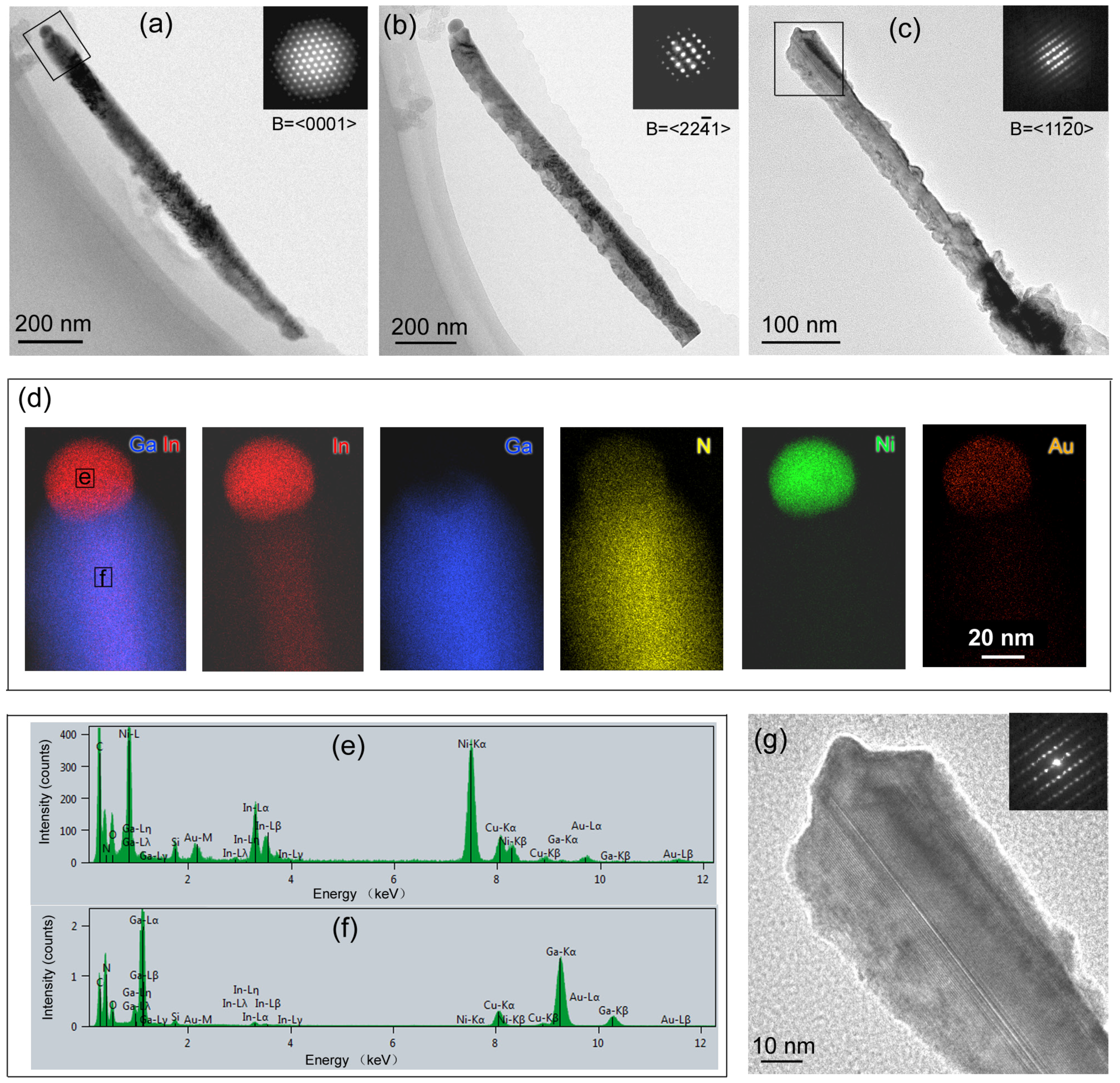
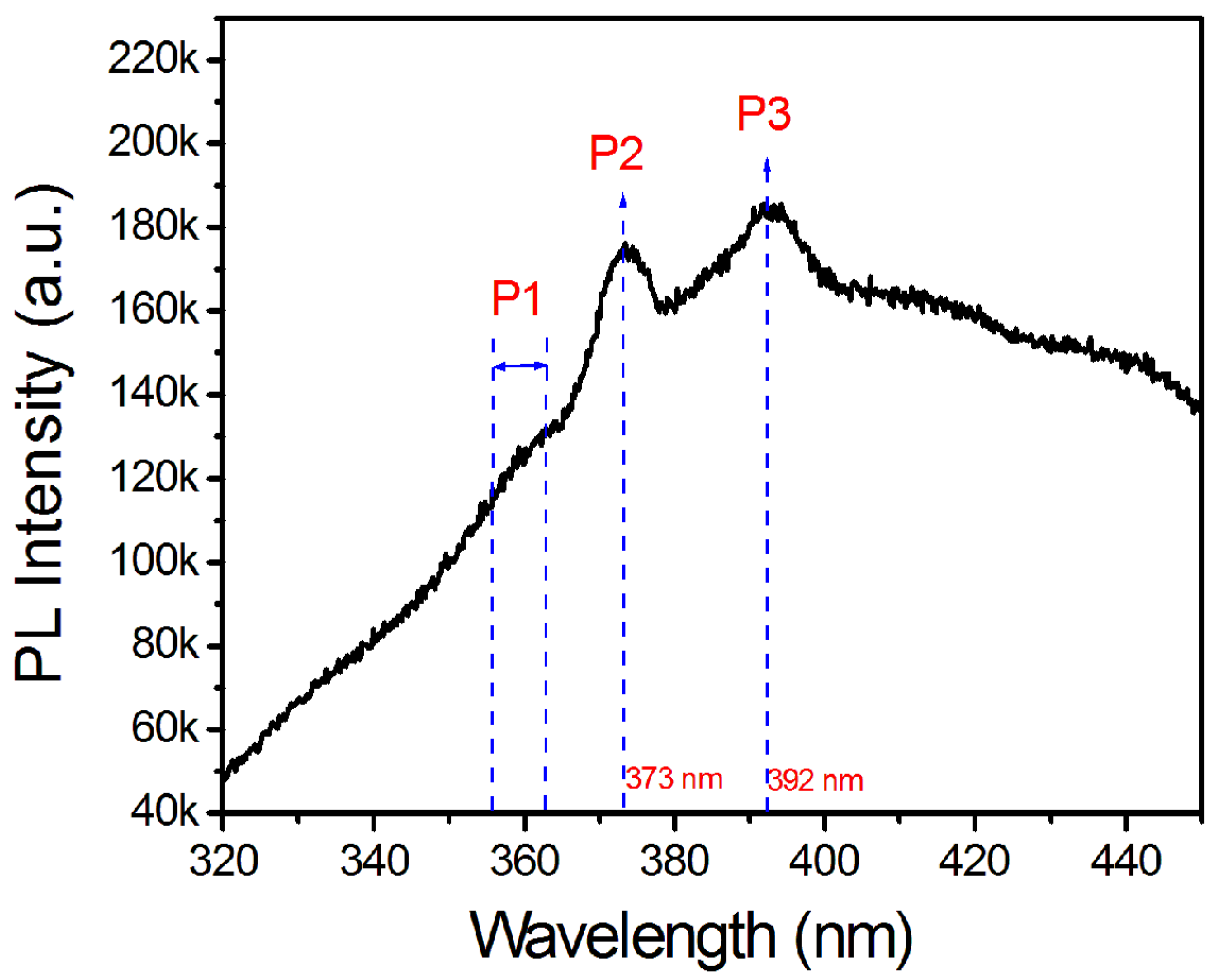
3.3. TEM Characterization of InxGa1−xN NWs on Nitrided Ta Substrates
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nakamura, S.; Fasol, G. The Blue Laser Diode: GaN Based Light Emitters and Lasers; Springer: Berlin/Heidelberg, Germany, 1997; ISBN-10: 3540615903. [Google Scholar]
- Fujii, K.; Kusakabe, K.; Ohkawa, K. Photoelectrochemical Properties of InGaN for H2 Generation from Aqueous Water. Jpn. J. Appl. Phys. 2005, 44, 7433–7435. [Google Scholar] [CrossRef]
- AlOtaibi, B.; Fan, S.; Wang, D.; Ye, J.; Mi, Z. Wafer-Level Artificial Photosynthesis for CO2 Reduction into CH4 and CO Using GaN Nanowires. ACS Catal. 2015, 5, 5342–5348. [Google Scholar] [CrossRef]
- Wang, S.; Sun, C.; Shao, Y.; Wu, Y.; Zhang, L.; Hao, X. Self-Supporting GaN Nanowires/Graphite Paper: Novel High-Performance Flexible Supercapacitor Electrodes. Small 2017, 13, 1603330. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, L.; Sun, C.; Shao, Y.; Wu, Y.; Lv, J.; Hao, X. Gallium Nitride Crystals: Novel Supercapacitor Electrode Materials. Adv. Mater. 2016, 28, 3768–3776. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Pierre, A.; Kibria, M.G.; Cui, K.; Han, X.; Bevan, K.H.; Guo, H.; Paradis, S.; Hakima, A.-R.; Mi, Z. Wafer-Level Photocatalytic Water Splitting on GaN Nanowire Arrays Grown by Molecular Beam Epitaxy. Nano Lett. 2011, 11, 2353–2357. [Google Scholar] [CrossRef] [PubMed]
- Kuykendall, T.; Ulrich, P.; Aloni, S.; Yang, P. Complete Composition Tunability of InGaN Nanowires Using a Combinatorial Approach. Nat. Mater. 2007, 6, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Kuykendall, T.R.; Schwartzberg, A.M.; Aloni, S. Gallium Nitride Nanowires and Heterostructures: Toward Color-Tunable and White-Light Sources. Adv. Mater. 2015, 27, 5805–5812. [Google Scholar] [CrossRef] [PubMed]
- Ra, Y.-H.; Wang, R.; Woo, S.Y.; Djavid, M.; Sadaf, S.Md.; Lee, J.; Botton, G.A.; Mi, Z. Full-Color Single Nanowire Pixels for Projection Displays. Nano Lett. 2016, 16, 4608–4615. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Woo, S.Y.; Vanka, S.; Botton, G.A.; Mi, Z. An In0.5Ga0.5N Nanowire Photoanode for Harvesting Deep Visible Light Photons. APL Mater. 2016, 4, 076106. [Google Scholar] [CrossRef]
- Zhao, C.; Ng, T.K.; Wei, N.; Prabaswara, A.; Alias, M.S.; Janjua, B.; Shen, C.; Ooi, B.S. Facile Formation of High-Quality InGaN/GaN Quantum-Disks-in-Nanowires on Bulk-Metal Substrates for High-Power Light-Emitters. Nano Lett. 2016, 16, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Wolz, M.; Hauswald, C.; Flissikowski, T.; Gotschke, T.; Fernandez-Garrido, S.; Brandt, O.; Grahn, H.T.; Geelhaar, L.; Riechert, H. Epitaxial Growth of GaN Nanowires with High Structural Perfection on a Metallic TiN Film. Nano Lett. 2015, 15, 3743–3747. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, A.G.; Carnevale, S.D.; Yang, F.; Kent, T.F.; Jamison, J.J.; McComb, D.W.; Myers, R.C. Semiconductor Nanowire Light-Emitting Diodes Grown on Metal: A Direction Toward Large-Scale Fabrication of Nanowire Devices. Small 2015, 11, 5402–5408. [Google Scholar] [CrossRef] [PubMed]
- May, B.J.; Sarwar, A.T.M.G.; Myers, R.C. Nanowire LEDs Grown Directly on Flexible Metal Foil. Appl. Phys. Lett. 2016, 108, 141103. [Google Scholar] [CrossRef]
- Bae, S.-Y.; Lekhal, K.; Lee, H.-J.; Mitsunari, T.; Min, J.-W.; Lee, D.-S.; Kushimoto, M.; Honda, Y.; Amano, H. Selective-area Growth of Vertically Oriented GaN Nanostructures with a Hafnium Pre-orienting Layer. J. Cryst. Growth 2017, 468, 110–113. [Google Scholar] [CrossRef]
- Zhong, M.M.; Qin, F.W.; Liu, Y.M.; Wang, C.; Bian, J.M.; Wang, E.P.; Wang, H.; Zhang, D. Low-temperature Growth of High c-orientated Crystalline GaN Films on Amorphous Ni/glass Substrates with ECR-PEMOCVD. J. Alloy. Compd. 2014, 583, 39–42. [Google Scholar] [CrossRef]
- Li, H.; Zhao, G.; Wang, L.; Chen, Z.; Yang, S. Morphology Controlled Fabrication of InN Nanowires on Brass Substrates. Nanomaterials 2016, 6, 195. [Google Scholar] [CrossRef] [PubMed]
- Pendyala, C.; Jasinski, J.B.; Kim, J.H.; Vendra, V.K.; Lisenkov, S.; Menond, M.; Sunkara, M.K. Nanowires as Semi-rigid Substrates for Growth of Thick, InxGa1−xN (x > 0.4) Epi-layers without Phase Segregation for Photoelectrochemical Water Splitting. Nanoscale 2012, 4, 6269–6275. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Alfaraj, N.; Subedi, R.C.; Liang, J.W.; Alatawi, A.A.; Alhamoud, A.A.; Ebaid, M.; Alias, M.S.; Ng, T.K.; Ooi, B.S. III-nitride Nanowires on Unconventional Substrates: From Materials to Optoelectronic Device Applications. Progress Quantum Electron. 2018, 61, 1–31. [Google Scholar] [CrossRef]
- Chaneliere, C.; Autran, J.L.; Devine, R.A.B.; Balland, B. Tantalum Pentoxide (Ta2O5) Thin Films for Advanced Dielectric Application. Mater. Sci. Eng. R 1998, 22, 269–322. [Google Scholar] [CrossRef]
- Allam, N.K.; Feng, X.J.; Grimes, C.A. Self-Assembled Fabrication of Vertically Oriented Ta2O5 Nanotube Arrays, and Membranes Thereof, by One-Step Tantalum Anodization. Chem. Mater. 2008, 20, 6477–6481. [Google Scholar] [CrossRef]
- El-Sayed, H.A.; Molero, H.M.; Birss, V.I. The Impact of Fabrication Conditions on the Quality of Au Nanoparticle Arrays on Dimpled Ta Templates. Nanotechnology 2012, 23, 435602. [Google Scholar] [CrossRef] [PubMed]
- Dabirian, A.; Krol, R. High-Temperature Ammonolysis of Thin Film Ta2O5 Photoanodes: Evolution of Structural, Optical, and Photoelectrochemical Properties. Chem. Mater. 2015, 27, 708–715. [Google Scholar] [CrossRef]
- Hara, M.; Chiba, E.; Ishikawa, A.; Takata, T.; Kondo, J.N.; Domen, K. Ta3N5 and TaON Thin Films on Ta Foil: Surface Composition and Stability. J. Phys. Chem. B 2003, 107, 13441–13445. [Google Scholar] [CrossRef]
- Chun, W.-J.; Ishikawa, A.; Fujisawa, H.; Takata, T.; Kondo, J.N.; Hara, M.; Kawai, M.; Matsumoto, Y.; Domen, K. Conduction and Valence Band Positions of Ta2O5, TaON, and Ta3N5 by UPS and Electrochemical Methods. J. Phys. Chem. B 2003, 107, 1798–1803. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, X.; Nguyen, N.T.; Hwang, I.; Schmuki, P. Strongly Enhanced Water Splitting Performance of Ta3N5 Nanotube Photoanodes with Subnitrides. Adv. Mater. 2016, 28, 2432–2438. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-Y.; Liao, C.-I.; Peng, H.-Y. Influence of Gas Flow Rates on the Formation of III-nitride Nanowires. Phys. Status Solidi C 2010, 7, 40–43. [Google Scholar] [CrossRef]
- Li, M.; Luo, W.; Cao, D.; Zhao, X.; Li, Z.; Yu, T.; Zou, Z. A Co-catalyst-Loaded Ta3N5 Photoanode with a High Solar Photocurrent for Water Splitting upon Facile Removal of the Surface Layer. Angew. Chem. Int. Ed. 2013, 52, 11016–11020. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.-C.; Chen, L.-Y.; Tang, W.-C.; Hong, F.C.N. Control of Seed Detachment in Au-Assisted GaN Nanowire Growths. Cryst. Growth Des. 2011, 11, 990–994. [Google Scholar] [CrossRef]
- Calabrese, G.; Corfdir, P.; Gao, G.; Pfuller, C.; Trampert, A.; Brandt, O.; Geelhaar, L.; Fernández-Garrido, S. Molecular beam epitaxy of single crystalline GaN nanowires on a flexible Ti foil. Appl. Phys. Lett. 2016, 108, 202101. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Bell, A.; Ponce, F.A.; Chen, C.Q.; Yang, J.W.; Khan, M.A. Luminescence from stacking faults in gallium nitride. Appl. Phys. Lett. 2005, 86, 021908. [Google Scholar] [CrossRef]
- Moses, P.G.; Van de Walle, C.G. Band Bowing and Band Alignment in InGaN Alloys. Appl. Phys. Lett. 2010, 96, 021908. [Google Scholar] [CrossRef]
- Aschenbrenner, T.; Figge, S.; Schowalter, M.; Rosenauer, A.; Hommel, D. Photoluminescence and Structural Analysis of a-plane InGaN Layers. J. Cryst. Growth 2008, 310, 4992–4995. [Google Scholar] [CrossRef]
- Fischer, A.M.; Wu, Z.; Sun, K.; Wei, Q.; Huang, Y.; Senda1, R.; Iida, D.; Iwaya, M.; Amano, H.; Ponce, F.A. Misfit Strain Relaxation by Stacking Fault Generation in InGaN Quantum Wells Grown on m-Plane GaN. Appl. Phys. Express 2009, 2, 041002. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.-L.; Zhu, Y.; Ji, H.; Luo, Q.; Fu, A.; Wang, X.; Xu, G.; Yang, H.; Lian, J.; Sun, J.; et al. Fabrication of InxGa1−xN Nanowires on Tantalum Substrates by Vapor-Liquid-Solid Chemical Vapor Deposition. Nanomaterials 2018, 8, 990. https://doi.org/10.3390/nano8120990
Hu Y-L, Zhu Y, Ji H, Luo Q, Fu A, Wang X, Xu G, Yang H, Lian J, Sun J, et al. Fabrication of InxGa1−xN Nanowires on Tantalum Substrates by Vapor-Liquid-Solid Chemical Vapor Deposition. Nanomaterials. 2018; 8(12):990. https://doi.org/10.3390/nano8120990
Chicago/Turabian StyleHu, Yan-Ling, Yuqin Zhu, Huayu Ji, Qingyuan Luo, Ao Fu, Xin Wang, Guiyan Xu, Haobin Yang, Jiqiong Lian, Jingjing Sun, and et al. 2018. "Fabrication of InxGa1−xN Nanowires on Tantalum Substrates by Vapor-Liquid-Solid Chemical Vapor Deposition" Nanomaterials 8, no. 12: 990. https://doi.org/10.3390/nano8120990




