Determination of Non-Transferrin Bound Iron, Transferrin Bound Iron, Drug Bound Iron and Total Iron in Serum in a Rats after IV Administration of Sodium Ferric Gluconate Complex by Simple Ultrafiltration Inductively Coupled Plasma Mass Spectrometric Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Instrumentation
2.3. Proposed Bioanalytical Method
2.3.1. Ultrafiltration Method
2.3.2. Sample Digestion
2.3.3. ICP-MS Method
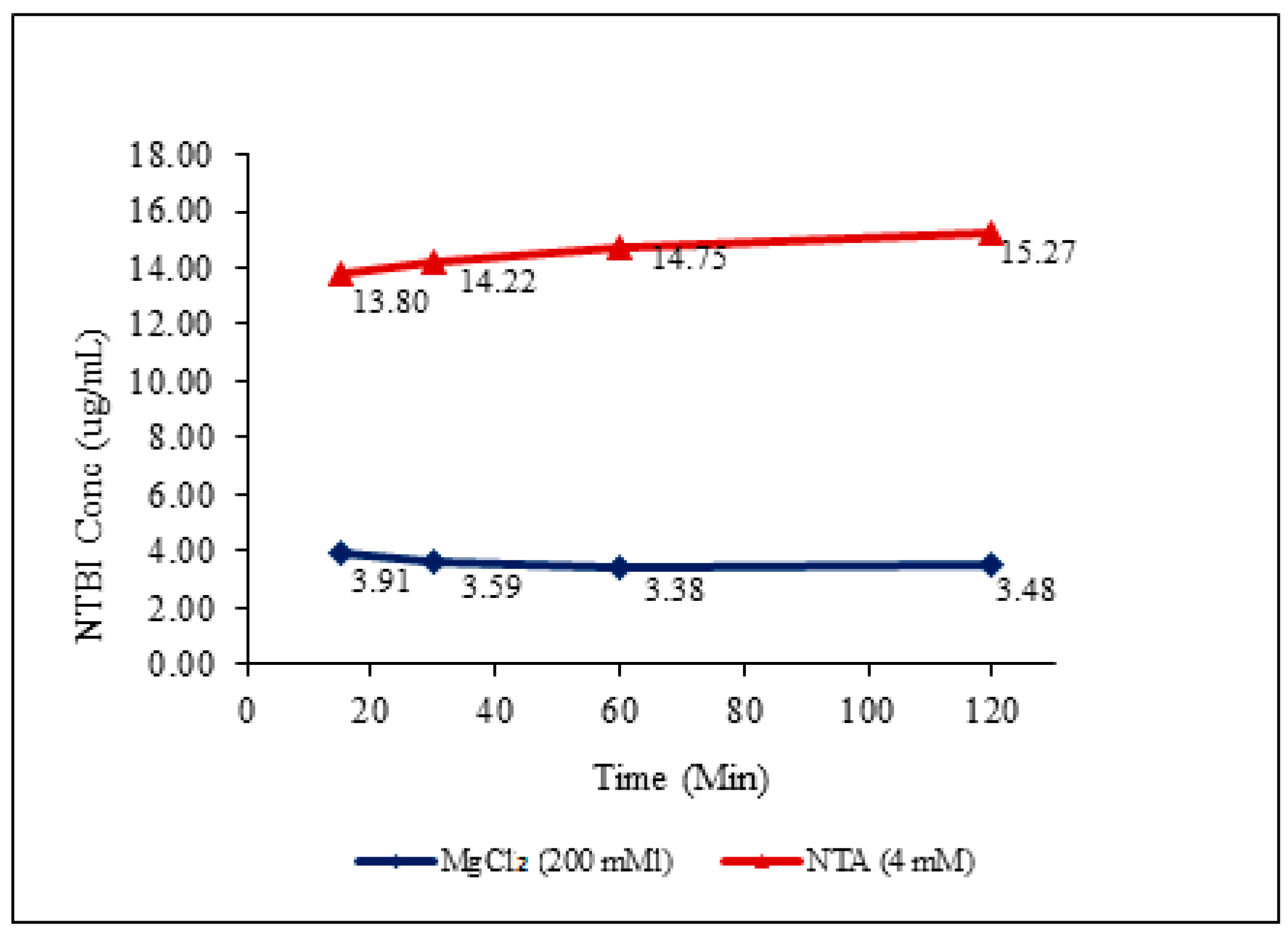
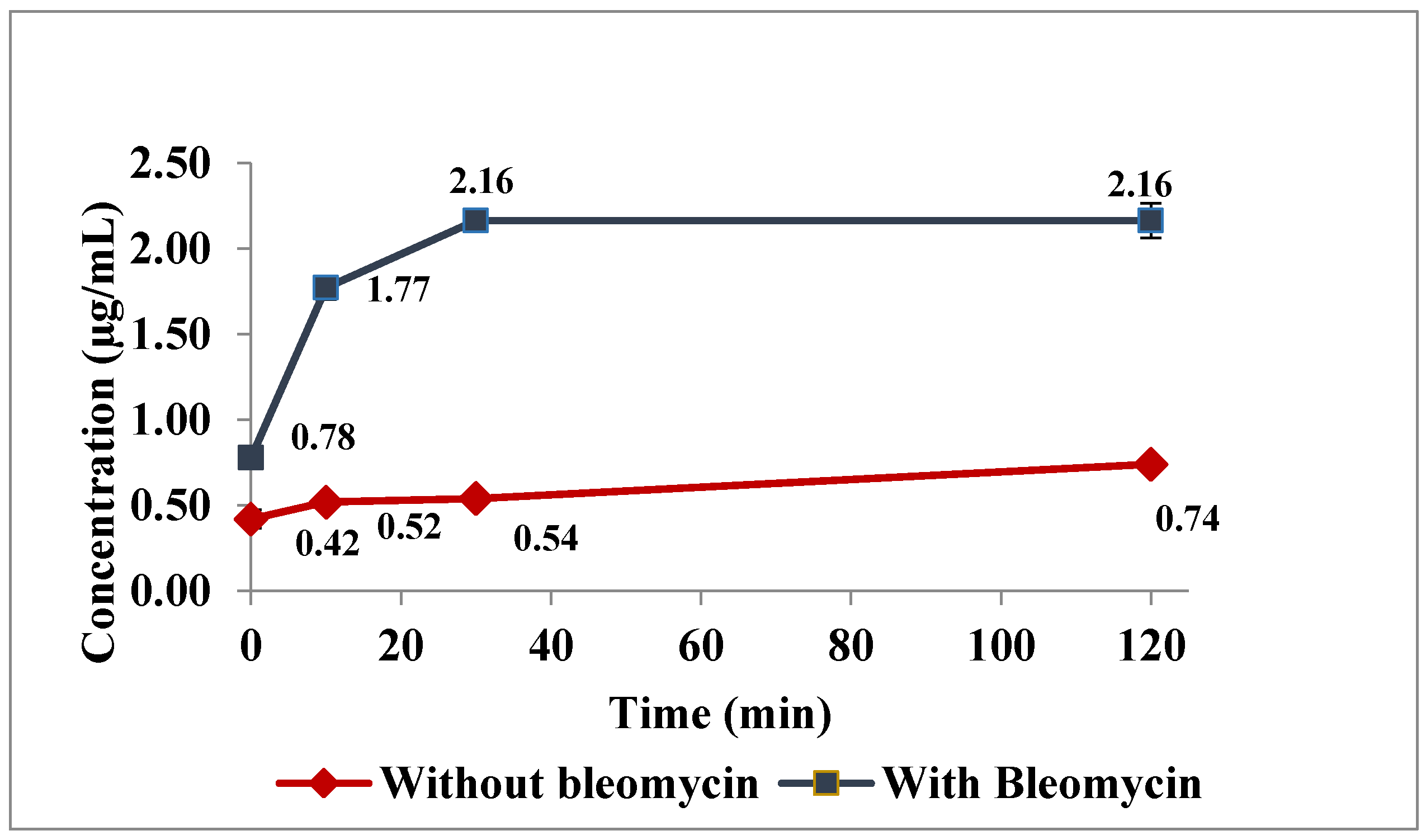
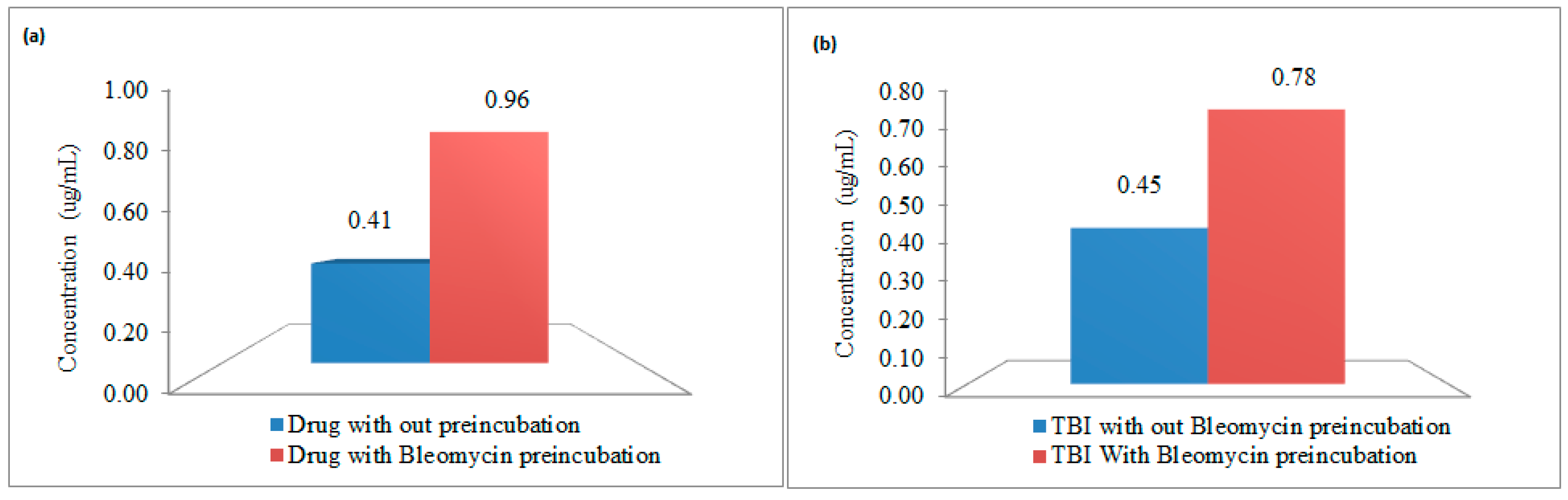
2.4. In Vitro Incuation Studies to Evaluate Advantages of Proposed Method
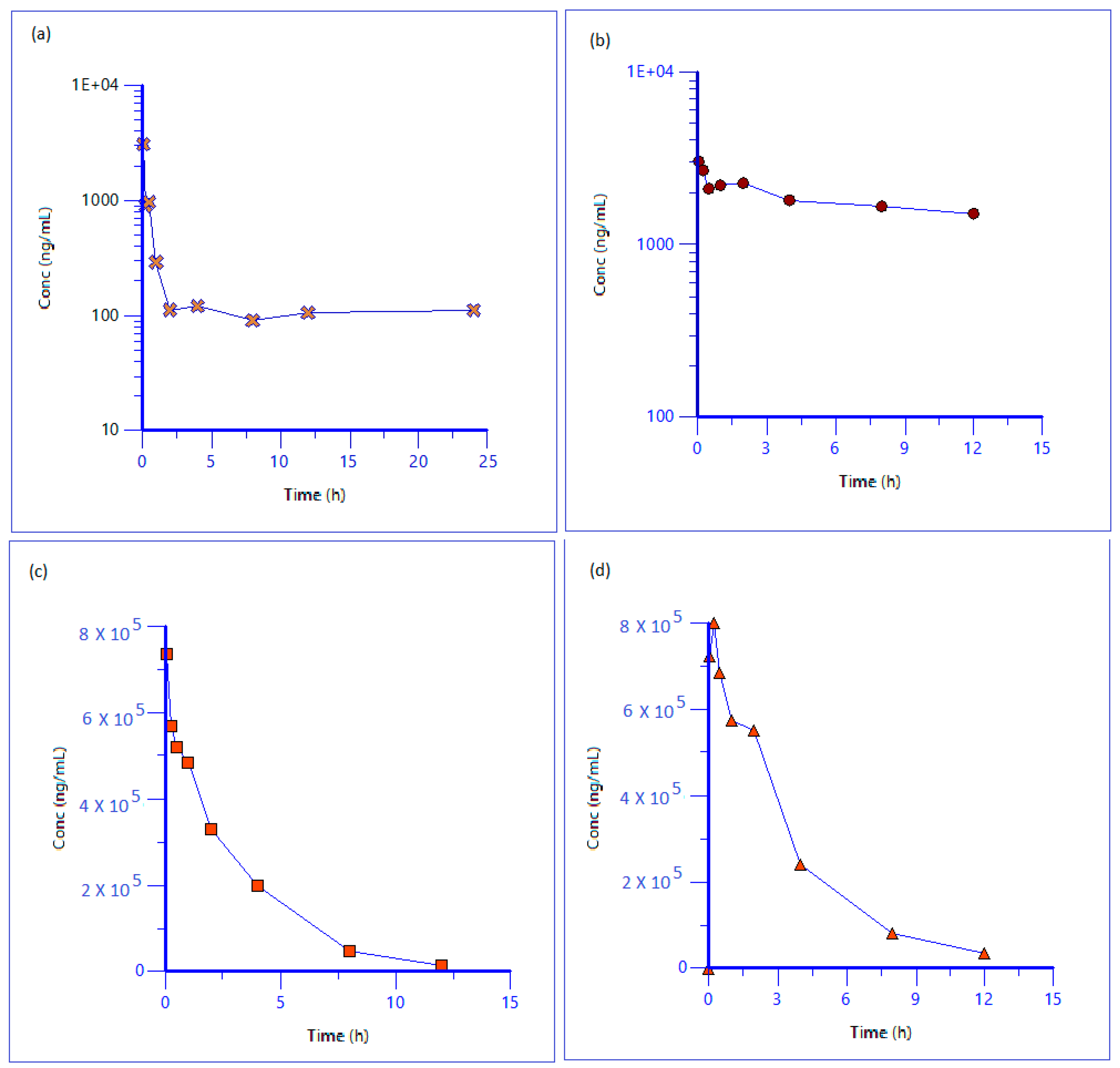
2.5. Pharmacokinetic Study in Sprague Drawley Rats
3. Results
3.1. Proposed Bioanalytical Method
3.1.1. Ultrafiltration Method and Sample Digestion
3.1.2. ICP-MS Method
3.2. In Vitro Incubation Studies
3.3. Pharmacokinetic Study in Sprague Drawley Rats
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Geisser, P.; Burckhardt, S. Pharmacokinetics and pharmacodynamics of iron preparations. Pharmaceutics 2011, 3, 12–13. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Streja, E.; Miller, J.E.; Nissenson, A.R. Intravenous iron versus erythropoiesis-stimulating agents: Friends or foes in treating chronic kidney disease anemia. Adv. Chronic Kidney Dis. 2009, 16, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Gotloib, L.; Silverberg, D.; Fudin, R.; Shostak, A. Iron deficiency is a common cause of anemia in chronic kidney disease and can often be corrected with intravenous iron. J. Nephrol. 2006, 19, 161–167. [Google Scholar] [PubMed]
- Nanas, P.N.; Matsouka, C.; Karageorgopoulos, D.; Leonti, A.; Tsolakis, E.; Drakos, S.G.; Tsagalou, E.P.; Maroulidis, G.D.; Alexopoulos, G.P.; Kanakakis, J.E. Etiology of anemia in patients with advanced heart failure. J. Am. Coll. Cardiol. 2006, 48, 2485–2489. [Google Scholar] [CrossRef] [PubMed]
- Danielson, J. Structure, chemistry, and pharmacokinetics of intravenous iron agents. Am. Soc. Nephrol. 2004, 15, 93–98. [Google Scholar]
- Brissot, P.; Ropert, M.; Lan, C.L.; Loreal, O. Non-transferrin bound iron: A key role in iron overload and iron toxicity. Biochim. Biophys. Acta 2012, 1820, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Dobhal, R.; Nayak, A.; Agarwal, S.; Uniyal, D.P.; Singh, P.; Sharma, B.; Tyagi, S.; Singh, R. Advanced and Hyphenated Techniques for Nano-Level Analysis of Iron in Water. Crit. Rev. Anal. Chem. 2012, 42, 245–256. [Google Scholar] [CrossRef]
- Leuenberger, N.; Baume, N.; Robinson, N.; Saugy, M. Pre-analytical and analytical aspects of EDTA-plasma iron measurement. Drug Test. Anal. 2016, 10, 1077–1079. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.A.; Fernandez, D.T.; Alvarez, E.A.; Gonzalez, E.B.; Montes-Bayon, M.; Sanz-Medel, A. Iron speciation, ferritin concentrations and Fe:ferritin ratios in different malignant breast cancer cell lines: On the search for cancer biomarkers. Metallomics 2016, 10, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Leao, D.J.; Junior, M.M.; Brandao, G.C.; Ferreira, S.L. Simultaneous determination of cadmium, iron and tin in canned foods using high-resolution continuum source graphite furnace atomic absorption spectrometry. Talanta 2016, 153, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Szabo, S.T.; Harry, G.J.; Hayden, K.M.; Szabo, D.T.; Birnbaum, L. Comparison of Metal Levels between Postmortem Brain and Ventricular Fluid in Alzheimer’s Disease and Nondemented Elderly Controls. Toxicol. Sci. 2016, 150, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Agustina, E.; Goak, J.; Lee, S.; Seo, Y.; Park, J.Y.; Lee, N. Simple and Precise Quantification of Iron Catalyst Content in Carbon Nanotubes Using UV/Visible Spectroscopy. Chemistryopen 2015, 4, 613–619. [Google Scholar] [CrossRef] [PubMed]
- De Swart, L.; Hendriks, J.C.; van der Vorm, L.N.; Cabantchik, Z.I.; Evans, P.J.; Hod, E.A.; Brittenham, G.M.; Furman, Y.; Wojczyk, B.; Janssen, M.C.; et al. Second international round robin for the quantification of serum non-transferrin-bound iron and labile plasma iron in patients with iron-overload disorders. Haematologica 2016, 101, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.F.; Yang, Y.; Yan, J.; Xie, X.Q.; Zhang, H.; Chai, W.M.; Wang, L.; Schmidt, B.; Yan, F.H. Virtual iron concentration imaging based on dual-energy CT for noninvasive quantification and grading of liver iron content: An iron overload rabbit model study. Eur. Radiol. 2015, 25, 2657–2664. [Google Scholar] [CrossRef] [PubMed]
- Sussulini, A.; Becker, J.S. Application of laser microdissection ICP-MS for high resolution elemental mapping in mouse brain tissue: A comparative study with laser ablation ICP-MS. Talanta 2015, 132, 579–582. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Liu, T.; Wu, Y.; Thacker, J.; Shenkar, R.; Mikati, A.G.; Shi, C.; Dykstra, C.; Wang, Y.; Prasad, P.V.; et al. Evaluation of iron content in human cerebral cavernous malformation using quantitative susceptibility mapping. Investig. Radiol. 2014, 49, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Kolb, A.M.; Smit, N.P.M.; Lentz-Ljuboje, R.; Osanto, S.; van Pelt, J. Non-transferrin bound iron measurement is influenced by chelators concentration. Anal. Chem. 2009, 385, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Goggin, M.M.; Gozum, S.D.; Burrows, D.M.; Nguyen, A.; Corcoran, H.F.; Dotzauer, H.; Tann, C.M.; Lundberg, R.A.; Janis, G.C. Analysis of total and transferrin-bound iron in human serum for pharmacokinetic studies of iron-sucrose formulations. Bioanalysis 2011, 16, 1837–1846. [Google Scholar] [CrossRef] [PubMed]
- Busto, M.E.C.; Bayon, M.M.; Bettmer, J.; Medel, A.S. Stable isotope labelling and FPLC-ICP-SFMS for the accurate determination of clinical iron status parameters in human serum. Anlayst 2008, 133, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Bonsdorff, L.V.; Lindeberg, E.; Sahlstedt, L.; Lehto, J.; Parkkinen, J. Bleomycin-detectable Iron Assay for Non Transferrin Bound Iron in Hematologic Malignancies. Clin. Chem. 2002, 48, 307–314. [Google Scholar]
- Bernat, X.; Fortuny, A.; Stuber, F.; Bengoa, C.; Fabregat, A.; Font, J. Recovery of iron (III) from aqueous streams by ultrafiltration. Desalination 2008, 221, 413–418. [Google Scholar] [CrossRef]
- Beekman, C.; Matta, M.K.; Thomas, C.; Mohammad, A.; Stewart, S.; Xu, L.; Chockalingam, A.; Shea, K.; Sun, D.; Jiang, W.; et al. Comparative Evaluation of U.S. Brand and Generic Intravenous Sodium Ferric Gluconate Complex in Sucrose Injection: Biodistribution after Intravenous Dosing in Rats. Nanomaterials 2018, 8, 10. [Google Scholar] [CrossRef] [PubMed]

| ICP Parameters | |
| RF Power | 1600 W |
| Plasma gas flow rate | 18.0 L/min |
| Auxiliary gas flow rate | 1.2 L/min |
| Nebulizer gas flow rate | 1.0 L/min |
| Spray chamber | Peltier-cooled (2 °C) baffled quartz cyclonic spray chamber |
| Torch | Quartz |
| Sampler and skimmer cones | Platinum |
| Hyper skimmer cones | Nickel |
| Mass Spectrometer Parameters | |
| Resolution | 0.7 amu at 10% peak maximum |
| Dwell time | 50 ms |
| Sweeps | 20 |
| Readings | 1 |
| Replicates | 3 |
| Autolens | ON |
| Internal Standard | In (115 amu) |
| Mode of detection | Kinetic Energy Differentiation (KED) |
| QC | Nominal Concentration (ug/mL) | Calculated Concentration Mean ± SD (ug/mL) | Accuracy (%) | % RSD |
|---|---|---|---|---|
| NTBI | ||||
| Low QC | 2.2 | 1.8 ± 0.1 | 85 | 8.0 |
| Mid QC | 3.3 | 3.6 ± 0.3 | 109 | 9.3 |
| High QC | 3.3 | 7.3 ± 0.2 | 106 | 2.6 |
| TBI | ||||
| Low QC | 6.0 | 7.0 ± 0.1 | 117 | 1.4 |
| Mid QC | 10.0 | 10.4 ± 0.1 | 104 | 1.1 |
| High QC | 18.0 | 18.3 ± 0.7 | 101 | 3.7 |
| DI | ||||
| Low QC | 50.0 | 56.3 ± 2.3 | 113 | 4.0 |
| Mid QC | 100.0 | 107.3 ± 1.8 | 107 | 1.7 |
| High QC | 250.0 | 249.8 ± 5.3 | 100 | 2.1 |
| QC | Nominal Concentration (ng/mL) | Calculated Concentration (ng/mL) | Calculated Concentration Mean ± SD (ng/mL) | Accuracy | % CV |
|---|---|---|---|---|---|
| QC-1 | 5 | 4.6 | 4.8 ± 0.4 | 96 | 9.1 |
| 4.5 | |||||
| 5.3 | |||||
| QC-2 | 50 | 48.2 | 48.6 ± 0.6 | 97 | 1.3 |
| 48.3 | |||||
| 49.3 | |||||
| QC-3 | 200 | 196.2 | 196.7 ± 0.4 | 98 | 0.2 |
| 197.0 | |||||
| 196.8 | |||||
| QC-4 | 500 | 588.7 | 489.5 ± 4.7 | 98 | 1.0 |
| 494.6 | |||||
| 485.3 |
| Type of Iron | Half Life (h) | Vss (L/kg) | MRT (h) | AUClast (h·ng/mL) | Cl (mL/min/kg) |
|---|---|---|---|---|---|
| Free Iron (NTBI) | - | - | - | 3552.2 | - |
| Transferrin Bound Iron | - | - | - | 21,880.3 | - |
| Drug Iron | 2.2 | 0.05 | 3.0 | 2,120,035.7 | 0.3 |
| Total Iron | - | - | - | 2,886,456.9 | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matta, M.K.; Beekman, C.R.; Gandhi, A.; Narayanasamy, S.; Thomas, C.D.; Mohammad, A.; Stewart, S.; Xu, L.; Chockalingam, A.; Shea, K.; et al. Determination of Non-Transferrin Bound Iron, Transferrin Bound Iron, Drug Bound Iron and Total Iron in Serum in a Rats after IV Administration of Sodium Ferric Gluconate Complex by Simple Ultrafiltration Inductively Coupled Plasma Mass Spectrometric Detection. Nanomaterials 2018, 8, 101. https://doi.org/10.3390/nano8020101
Matta MK, Beekman CR, Gandhi A, Narayanasamy S, Thomas CD, Mohammad A, Stewart S, Xu L, Chockalingam A, Shea K, et al. Determination of Non-Transferrin Bound Iron, Transferrin Bound Iron, Drug Bound Iron and Total Iron in Serum in a Rats after IV Administration of Sodium Ferric Gluconate Complex by Simple Ultrafiltration Inductively Coupled Plasma Mass Spectrometric Detection. Nanomaterials. 2018; 8(2):101. https://doi.org/10.3390/nano8020101
Chicago/Turabian StyleMatta, Murali K., Christopher R. Beekman, Adarsh Gandhi, Suresh Narayanasamy, Christopher D. Thomas, Adil Mohammad, Sharron Stewart, Lin Xu, Ashok Chockalingam, Katherine Shea, and et al. 2018. "Determination of Non-Transferrin Bound Iron, Transferrin Bound Iron, Drug Bound Iron and Total Iron in Serum in a Rats after IV Administration of Sodium Ferric Gluconate Complex by Simple Ultrafiltration Inductively Coupled Plasma Mass Spectrometric Detection" Nanomaterials 8, no. 2: 101. https://doi.org/10.3390/nano8020101
APA StyleMatta, M. K., Beekman, C. R., Gandhi, A., Narayanasamy, S., Thomas, C. D., Mohammad, A., Stewart, S., Xu, L., Chockalingam, A., Shea, K., Patel, V., & Rouse, R. (2018). Determination of Non-Transferrin Bound Iron, Transferrin Bound Iron, Drug Bound Iron and Total Iron in Serum in a Rats after IV Administration of Sodium Ferric Gluconate Complex by Simple Ultrafiltration Inductively Coupled Plasma Mass Spectrometric Detection. Nanomaterials, 8(2), 101. https://doi.org/10.3390/nano8020101





