Using Polarized Spectroscopy to Investigate Order in Thin-Films of Ionic Self-Assembled Materials Based on Azo-Dyes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Bulk Structure
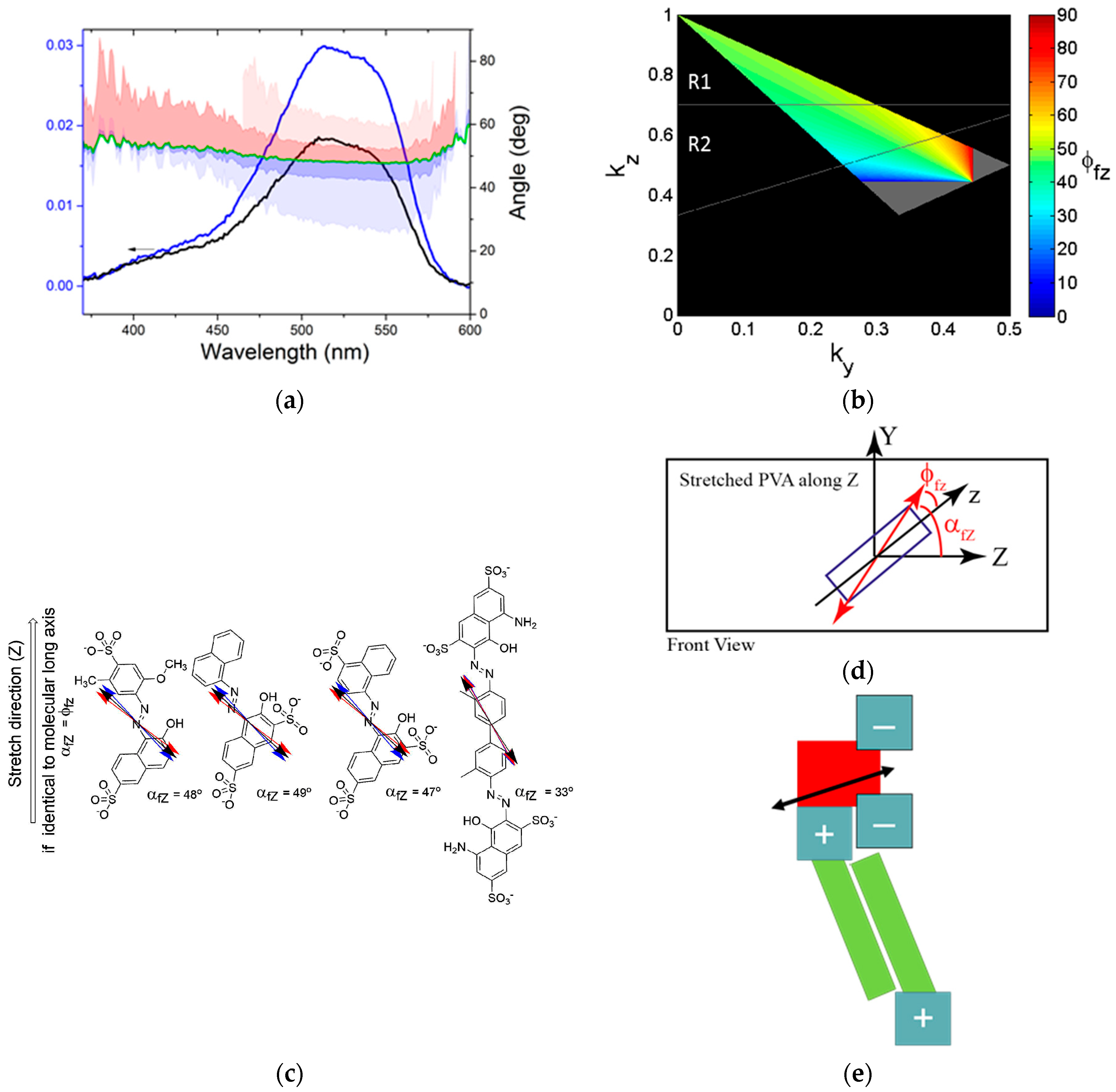
2.3. Polarized Spectroscopy
2.4. Stretched Polymers
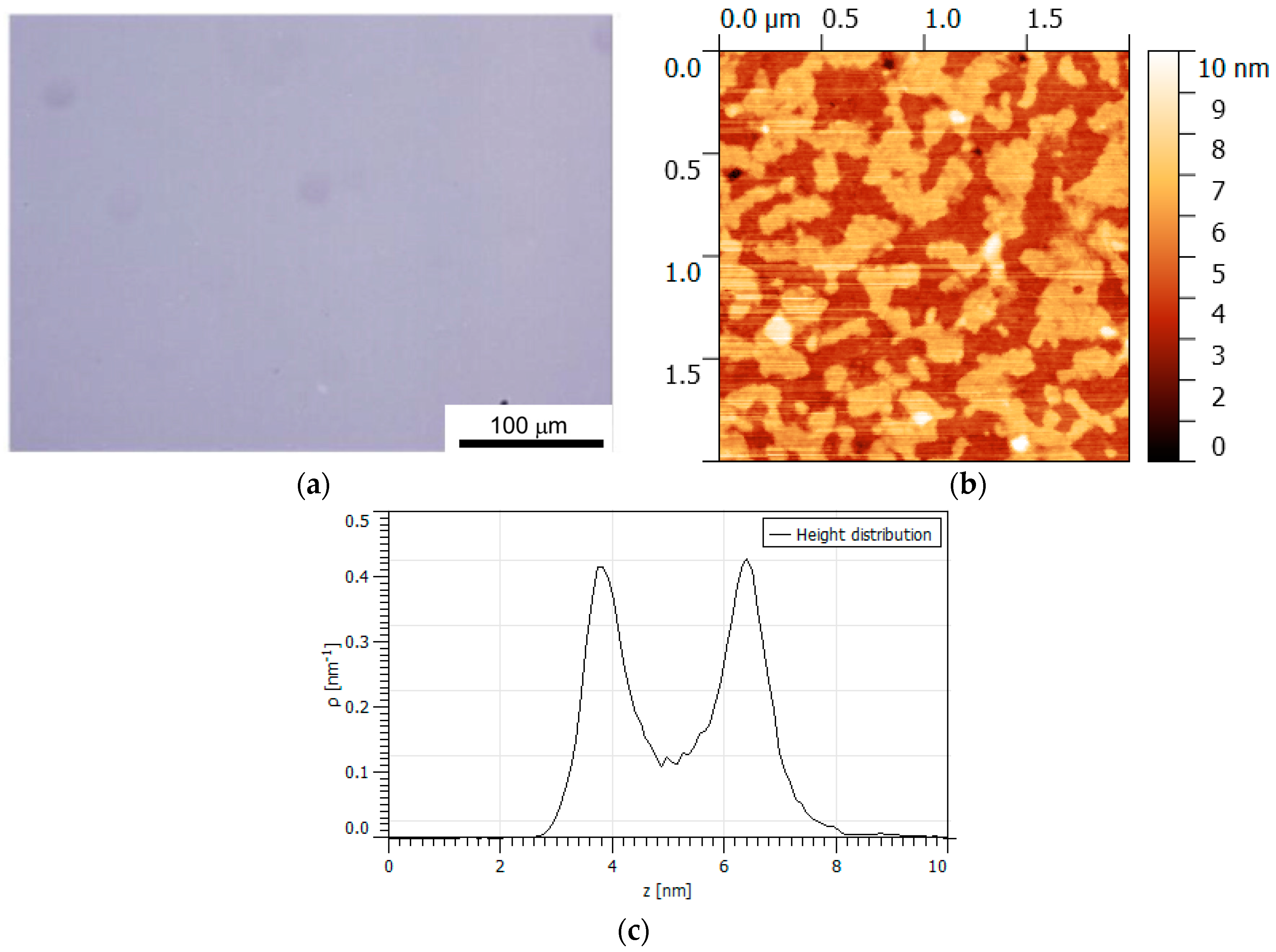
2.5. Thin Film Structure
2.6. Molecular Structure in Thin Films
3. Materials and Methods
3.1. Synthesis and Characterization
3.2. Polarized Spectroscopy
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A. Theory Used in Polarized Spectroscopy
Appendix A.1 Stretched Polymers
Appendix A.2. Thin Film Data

References
- Minemawari, H.; Yamada, T.; Matsui, H.; Tsutsumi, J.; Haas, S.; Chiba, R.; Kumai, R.; Hasegawa, T. Inkjet printing of single-crystal films. Nature 2011, 475, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, T.J.; Hildebrandt, C.B.; Elm, J.; Andreasen, J.W.; Madsen, A.O.; Westerlund, F.; Laursen, B.W. Large area, soft crystalline thin films of N,N′,N″-trialkyltriazatriangulenium salts with homeotropic alignment of the discotic cores in a lamellar lattice. J. Mater. Chem. 2012, 22, 4797–4805. [Google Scholar] [CrossRef]
- Nitschke, J.R. Construction, substitution, and sorting of metallo-organic structures via subcomponent self-assembly. Acc. Chem. Res. 2006, 40, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Northrop, B.H.; Zheng, Y.R.; Chi, K.W.; Stang, P.J. Self-organization in coordination-driven self-assembly. Acc. Chem. Res. 2009, 42, 1554–1563. [Google Scholar] [CrossRef] [PubMed]
- Dubois, L.H.; Nuzzo, R.G. Synthesis, structure, and properties of model organic surfaces. Annu. Rev. Phys. Chem. 1992, 43, 437–463. [Google Scholar] [CrossRef]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem. Rev. 2005, 105, 1103–1170. [Google Scholar] [CrossRef] [PubMed]
- Collier, C.P.; Mattersteig, G.; Wong, E.W.; Luo, Y.; Beverly, K.; Sampaio, J.; Raymo, F.M.; Stoddart, J.F.; Heath, J.R. A [2]catenane-based solid state electronically reconfigurable switch. Science 2000, 289, 1172–1175. [Google Scholar] [CrossRef] [PubMed]
- Pisula, W.; Menon, A.; Stepputat, M.; Lieberwirth, I.; Kolb, U.; Tracz, A.; Sirringhaus, H.; Pakula, T.; Mullen, K. A zone-casting technique for device fabrication of field-effect transistors based on discotic hexa-peri-hexabenzoeoronene. Adv. Mater. 2005, 17, 684–689. [Google Scholar] [CrossRef]
- van de Craats, A.M.; Stutzmann, N.; Bunk, O.; Nielsen, M.M.; Watson, M.; Mullen, K.; Chanzy, H.D.; Sirringhaus, H.; Friend, R.H. Meso-epitaxial solution-growth of self-organizing discotic liquid-crystalline semiconductors. Adv. Mater. 2003, 15, 495–499. [Google Scholar] [CrossRef]
- Hansen, C.R.; Sørensen, T.J.; Glyvradal, M.; Larsen, J.; Eisenhardt, S.H.; Bjørnholm, T.; Nielsen, M.M.; Feidenhans’l, R.; Laursen, B.W. Structure of the buried metal-molecule interface in organic thin film devices. Nano Lett. 2009, 9, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- Laursen, B.W.; Norgaard, K.; Reitzel, N.; Simonsen, J.B.; Nielsen, C.B.; Als-Nielsen, J.; Bjornholm, T.; Solling, T.I.; Nielsen, M.M.; Bunk, O.; et al. Macroscopic alignment of graphene stacks by langmuir-blodgett deposition of amphiphilic hexabenzocoronenes. Langmuir 2004, 20, 4139–4146. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.B.; Giri, G.; Ayzner, A.L.; Zoombelt, A.P.; Mannsfeld, S.C.B.; Chen, J.H.; Nordlund, D.; Toney, M.F.; Huang, J.S.; Bao, Z.N. Ultra-high mobility transparent organic thin film transistors grown by an off-centre spin-coating method. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Mizoshita, N.; Kishimoto, K. Functional liquid-crystalline assemblies: Self-organized soft materials. Angew. Chem. 2005, 45, 38–68. [Google Scholar] [CrossRef] [PubMed]
- Binnemans, K. Ionic liquid crystals. Chem. Rev. 2005, 105, 4148–4204. [Google Scholar] [CrossRef] [PubMed]
- Alvarez Fernandez, A.; Kouwer, P.H. Key developments in ionic liquid crystals. Int. J. Mol. Sci. 2016, 17, 731. [Google Scholar] [CrossRef] [PubMed]
- Demus, D.; Goodby, J.; Gray, G.W.; Spiess, H.-W.; Vill, V. Handbook of Liquid Crystals; Wiley-VCH: New York, NY, USA, 1998; Volume 1. [Google Scholar]
- Kato, T. Self-assembly of phase-segregated liquid crystal structures. Science 2002, 295, 2414–2418. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.-X.; Pagliaro, M.; Xu, Y.-J.; Liu, B. Layer-by-layer assembly of versatile nanoarchitectures with diverse dimensionality: A new perspective for rational construction of multilayer assemblies. Chem. Soc. Rev. 2016, 45, 3088–3121. [Google Scholar] [CrossRef] [PubMed]
- Decher, G.; Hong, J.D. Buildup of ultrathin multilayer films by a self-assembly process, 1 Consecutive adsorption of anionic and cationic bipolar amphiphiles on charged surfaces. Makromol. Chem. Makromol. Symp. 1991, 46, 321–327. [Google Scholar] [CrossRef]
- Decher, G.; Hong, J.D. Buildup of ultrathin multilayer films by a self-assembly process: 11. Consecutive adsorption of anionic and cationic bipolar amphiphiles and polyelectrolytes on charged surfaces. Ber. Bunsetlges. Phys. 1991, 95, 1430–1434. [Google Scholar] [CrossRef]
- Decher, G.; Hong, J.D.; Schmitt, J. Buildup of ultrathin multilayer films by a self-assembly process: Iii. Consecutively alternating adsorption of anionic and cationic polyelectrolytes on charged surfaces. Thin Solid Films 1992, 210–211, 831–835. [Google Scholar] [CrossRef]
- Faul, C.F.J.; Antonietti, M. Facile synthesis of optically functional, highly organized nanostructures: Dye-surfactant complexes. Chemistry 2002, 8, 2764–2768. [Google Scholar] [CrossRef]
- Guan, Y.; Antonietti, M.; Faul, C.F.J. Ionic self-assembly of dye-surfactant complexes: Influence of tail lengths and dye architecture on the phase morphology. Langmuir 2002, 18, 5939–5945. [Google Scholar] [CrossRef]
- Faul, C.F.J.; Antonietti, M. Ionic self-assembly: Facile synthesis of supramolecular materials. Adv. Mater. 2003, 15, 673–683. [Google Scholar] [CrossRef]
- Guan, Y.; Zakrevskyy, Y.; Stumpe, J.; Antonietti, M.; Faul, C.F.J. Perylenediimide-surfactant complexes: Thermotropic liquid-crystalline materials via ionic self-assembly. Chem. Commun. 2003, 7, 894–895. [Google Scholar] [CrossRef]
- Johal, M.S.; Casson, J.L.; Chiarelli, P.A.; Liu, D.-G.; Shaw, J.A.; Robinson, J.M.; Wang, H.-L. Polyelectrolyte trilayer combinations using spin-assembly and ionic self-assembly. Langmuir 2003, 19, 8876–8881. [Google Scholar] [CrossRef]
- Wang, Z.; Ho, K.J.; Medforth, C.J.; Shelnutt, J.A. Porphyrin nanofiber bundles from phase-transfer ionic self-assembly and their photocatalytic self-metallization. Adv. Mater. 2006, 18, 2557–2560. [Google Scholar] [CrossRef]
- Ferreira, M.; Cheung, J.H.; Rubner, M.F. Molecular self-assembly of conjugated polyions—A new process for fabricating multilayer thin-film heterostructures. Thin Solid Films 1994, 244, 806–809. [Google Scholar] [CrossRef]
- MacDonald, J.C.; Dorrestein, P.C.; Pilley, M.M. Design of supramolecular layers via self-assembly of imidazole and carboxylic acids. Cryst. Growth Des. 2001, 1, 29–38. [Google Scholar] [CrossRef]
- Wang, Z.; Medforth, C.J.; Shelnutt, J.A. Porphyrin nanotubes by ionic self-assembly. J. Am. Chem. Soc. 2004, 126, 15954–15955. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Yu, S.H.; Antonietti, M.; Bottcher, C.; Faul, C.F.J. Synthesis of supramolecular polymers by ionic self-assembly of oppositely charged dyes. Chemistry 2005, 11, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Zakrevskyy, Y.; Stumpe, J.; Faul, C.F.J. A supramolecular approach to optically anisotropic materials: Photosensitive ionic self-assembly complexes. Adv. Mater. 2006, 18, 2133–2136. [Google Scholar] [CrossRef]
- Ma, T.; Li, C.; Shi, G. Optically active supramolecular complex formed by ionic self-assembly of cationic perylenediimide derivative and adenosine triphosphate. Langmuir 2008, 24, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Brown, J.; Oakley, R.J.; Faul, C.F.J. Towards functional nanostructures: Ionic self-assembly of polyoxometalates and surfactants. Curr. Opin. Colloid Interface Sci. 2009, 14, 62–70. [Google Scholar] [CrossRef]
- Rybtchinski, B. Adaptive supramolecular nanomaterials based on strong noncovalent interactions. ACS Nano 2011, 5, 6791–6818. [Google Scholar] [CrossRef] [PubMed]
- Faul, C.F.J. Ionic self-assembly for functional hierarchical nanostructured materials. Acc. Chem. Res. 2014, 47, 3428–3438. [Google Scholar] [CrossRef] [PubMed]
- Santella, M.; Amini, F.; Andreasen, K.B.; Aswad, D.S.; Ausar, H.; Austin, L.M.; Bora, I.; Boye, I.M.I.; Brinkenfeldt, N.K.; Bøe, M.F.; et al. Template-guided ionic self-assembled molecular materials and thin films with nanoscopic order. ChemNanoMat 2015, 1, 253–258. [Google Scholar] [CrossRef]
- Sørensen, T.J.; Hildebrandt, C.B.; Glyvradal, M.; Laursen, B.W. Synthesis, optical properties and lamellar self-organization of new N,N′,N″-trialkyl-triazatriangulenium tetrafluoroborate salts. Dyes Pigment. 2013, 98, 297–303. [Google Scholar] [CrossRef]
- IUPAC. Compendium of Chemical Terminology, 2nd ed.; McNaught, A.D., Wilkinson, A., Eds.; Blackwell Scientific Publications: Oxford, UK, 1997. [Google Scholar]
- Michl, J.; Thulstrup, E.W. Spectroscopy with Polarized Light; Wiley-VCH: New York, NY, USA, 1986; p. 573. [Google Scholar]
- Thulstrup, E.W.; Michl, J. Elementary Polarization Spectroscopy; Wiley-VCH: New York, NY, USA, 1989; p. 167. [Google Scholar]
- Samori, B.; Thulstrup, E. Polarized Spectroscopy of Ordered Systems; Springer Netherlands: Dordrecht, The Netherlands, 1988. [Google Scholar]
- Graf, H.M.; Zobel, O.; East, A.J.; Haarer, D. The polarized absorption spectroscopy as a novel method for determining the orientational order of poled nonlinear optical polymer films. J. Appl. Phys. 1994, 75, 3335–3339. [Google Scholar] [CrossRef]
- Era, M.; Tsutsui, T.; Saito, S. Polarized electroluminescence from oriented p-sexiphenyl vacuum-deposited film. Appl. Phys. Lett. 1995, 67, 2436–2438. [Google Scholar] [CrossRef]
- Grell, M.; Bradley, D.D.C. Polarized luminescence from oriented molecular materials. Adv. Mater. 1999, 11, 895–905. [Google Scholar] [CrossRef]
- Miller, E.K.; Maskel, G.S.; Yang, C.Y.; Heeger, A.J. Polarized ultraviolet absorption by an oriented derivative of poly(para-phenylene). Phys. Rev. B 1999, 60, 8028–8033. [Google Scholar] [CrossRef]
- Miller, E.K.; Yoshida, D.; Yang, C.Y.; Heeger, A.J. Polarized ultraviolet absorption of highly oriented poly(2-methoxy, 5-(2′-ethyl)-hexyloxy)paraphenylene vinylene. Phys. Rev. B 1999, 59, 4661–4664. [Google Scholar] [CrossRef]
- Westerlund, F.; Lemke, H.T.; Hassenkam, T.; Simonsen, J.B.; Laursen, B.W. Self-assembly and near perfect macroscopic alignment of fluorescent triangulenium salt in spin cast thin films on ptfe. Langmuir 2013, 29, 6728–6736. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Gommans, H.H.; Ugawa, A.; Tashiro, H.; Haggenmueller, R.; Winey, K.I.; Fischer, J.E.; Tanner, D.B.; Rinzler, A.G. Polarized spectroscopy of aligned single-wall carbon nanotubes. Phys. Rev. B 2000, 62, R13310–R13313. [Google Scholar] [CrossRef]
- Van Beer, H.C.A.; Heertjes, P.M. Photochemical Reactions of Azo Dyes in Solution with Different Substrates. J. Soc. Dyers Colourists. 1963, 79, 661–670. [Google Scholar] [CrossRef]
- Dane, T.G.; Cresswell, P.T.; Bikondoa, O.; Newby, G.E.; Arnold, T.; Faul, C.F.J.; Briscoe, W.H. Structured oligo(aniline) nanofilms via ionic self-assembly. Soft Matter 2012, 8, 2824–2832. [Google Scholar] [CrossRef]
- Available online: www.gwyddion.net (accessed on 15 February 2018).
- CRC. CRC Handbook of Chemistry and Physics, 73rd ed.; CRC Press: Ann Arbor, MI, USA, 1992. [Google Scholar]





| Entry | Material | H2O | C | H | N | |||
|---|---|---|---|---|---|---|---|---|
| Allura Red AllR | Calcd | Found | Calcd | Found | Calcd | Found | ||
| 1 | BZK10 precipitated | ½ | 66.44 | 66.63 | 8.26 | 8.42 | 5.53 | 5.52 |
| 2 | BZK12 precipitated | ½ | 67.44 | 67.44 | 8.58 | 8.83 | 5.24 | 5.18 |
| 3 | BZK14 extracted | 0.00 | 68.90 | 69.06 | 8.85 | 9.33 | 5.02 | 4.77 |
| 4 | BZK16 extracted | 1.00 | 67.11 | 67.02 | 9.13 | 9.20 | 4.74 | 4.60 |
| 5 | BZK18 extracted | 0.00 | 70.43 | 70.12 | 9.36 | 9.37 | 4.56 | 4.54 |
| Bordeaux Red BorR | ||||||||
| 6 | BZK10 extracted | ½ | 68.40 | 68.67 | 8.02 | 7.28 | 5.50 | 5.55 |
| 7 | BZK12 precipitated | 0.00 | 69.90 | 69.61 | 8.33 | 8.30 | 5.26 | 5.25 |
| 8 | BZK14 extracted | 0.00 | 69.70 | 69.75 | 8.91 | 8.66 | 5.16 | 4.96 |
| 9 | BZK16 extracted | wet2 | - | 61.24 | - | 9.24 | - | 3.64 |
| 10 | BZK18 extracted | 4.00 | 68.06 | 68.31 | 9.26 | 9.32 | 4.29 | 4.11 |
| Amaranth Amar | ||||||||
| 11 | BZK10 extracted | 2.00 | 66.01 | 65.87 | 8.42 | 8.28 | 5.00 | 4.91 |
| 12 | BZK12 extracted | ½ | 68.37 | 68.29 | 8.71 | 9.28 | 4.80 | 4.60 |
| 13 | BZK14 extracted | ½ | 69.31 | 69.24 | 9.02 | 9.01 | 4.54 | 4.45 |
| 14 | BZK16 extracted | 2.00 | 69.01 | 69.29 | 9.33 | 9.82 1 | 4.24 | 4.12 |
| 15 | BZK18 precipitated | 0.00 | 71.23 | 71.06 | 9.54 | 10.30 1 | 4.12 | 3.95 |
| Trypan Blue TryB | ||||||||
| 16 | BZK10 precipitated | 1.00 | 66.96 | 66.86 | 8.13 | 8.32 | 7.10 | 6.83 |
| Entry | Material | αfZ,max a (deg) | λmax (nm) | dXRD (nm) | ∆d | d’AFM (nm) |
|---|---|---|---|---|---|---|
| Allura red AllR | ||||||
| 1 | BZK10 | 54.4 | 500 | 4.42 | - | - |
| 2 | BZK12 | 53.2 | 500 | 4.71 | 0.3 | 2.65 |
| 3 | BZK14 | 55.9 | 500 | - | - | 5.85 |
| 4 | BZK16 | 60.1 | 500 | - | - | 2.5&1.5 |
| 5 | BZK18 | 63.2 | 500 | 5.75 | - | 2.8–3.2 |
| Bordeaux red BorR | ||||||
| 6 | BZK10 | 62.1 | 510 | 3.63 | - | 2.8 & 2.2 |
| 7 | BZK12 | 63.9 | 510 | 3.87 | 0.2 | - |
| 8 | BZK14 | 61.4 | 510 | 4.06 | 0.2 | - |
| 9 | BZK16 | 59.9 | 510 | - | - | - |
| 10 | BZK18 | 55.2 | 510 | 4.66 | 0.2 | 2.5–3.3 |
| Amaranth Amar | ||||||
| 11 | BZK10 | 64.9 | 520 | 3.72 | - | 7.5–8.0 |
| 12 | BZK12 | 62.2 | 520 | 4.18 | 0.4 | - |
| 13 | BZK14 | 63.3 | 520 | 4.38 | 0.2 | 2.1 |
| 14 | BZK16 | 64.8 | 520 | 4.96 | 0.6 | 3.9 |
| 15 | BZK18 | 63.2 | 520 | 5.18 | 0.2 | 2.6 & 3.7 |
| Trypan Blue TrypB | ||||||
| 16 | BZK10 | 80 | 617 | 2.51 | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kühnel, M.R.C.-T.M.; Ahmad, M.; Andersen, F.; Bech, Á.B.; Bendixen, H.K.L.; Nawrocki, P.R.; Bloch, A.J.; Bora, I.; Bukhari, T.A.; Bærentsen, N.V.; et al. Using Polarized Spectroscopy to Investigate Order in Thin-Films of Ionic Self-Assembled Materials Based on Azo-Dyes. Nanomaterials 2018, 8, 109. https://doi.org/10.3390/nano8020109
Kühnel MRC-TM, Ahmad M, Andersen F, Bech ÁB, Bendixen HKL, Nawrocki PR, Bloch AJ, Bora I, Bukhari TA, Bærentsen NV, et al. Using Polarized Spectroscopy to Investigate Order in Thin-Films of Ionic Self-Assembled Materials Based on Azo-Dyes. Nanomaterials. 2018; 8(2):109. https://doi.org/10.3390/nano8020109
Chicago/Turabian StyleKühnel, Miguel R. Carro-Temboury Martin, Mariam Ahmad, Frederik Andersen, Ári Brend Bech, H. Krestian L. Bendixen, Patrick R. Nawrocki, Anders J. Bloch, Ilkay Bora, Tahreem A. Bukhari, Nicolai V. Bærentsen, and et al. 2018. "Using Polarized Spectroscopy to Investigate Order in Thin-Films of Ionic Self-Assembled Materials Based on Azo-Dyes" Nanomaterials 8, no. 2: 109. https://doi.org/10.3390/nano8020109





