Toxicity of Pristine and Chemically Functionalized Fullerenes to White Rot Fungus Phanerochaete chrysosporium
Abstract
:1. Introduction
2. Materials and Methods
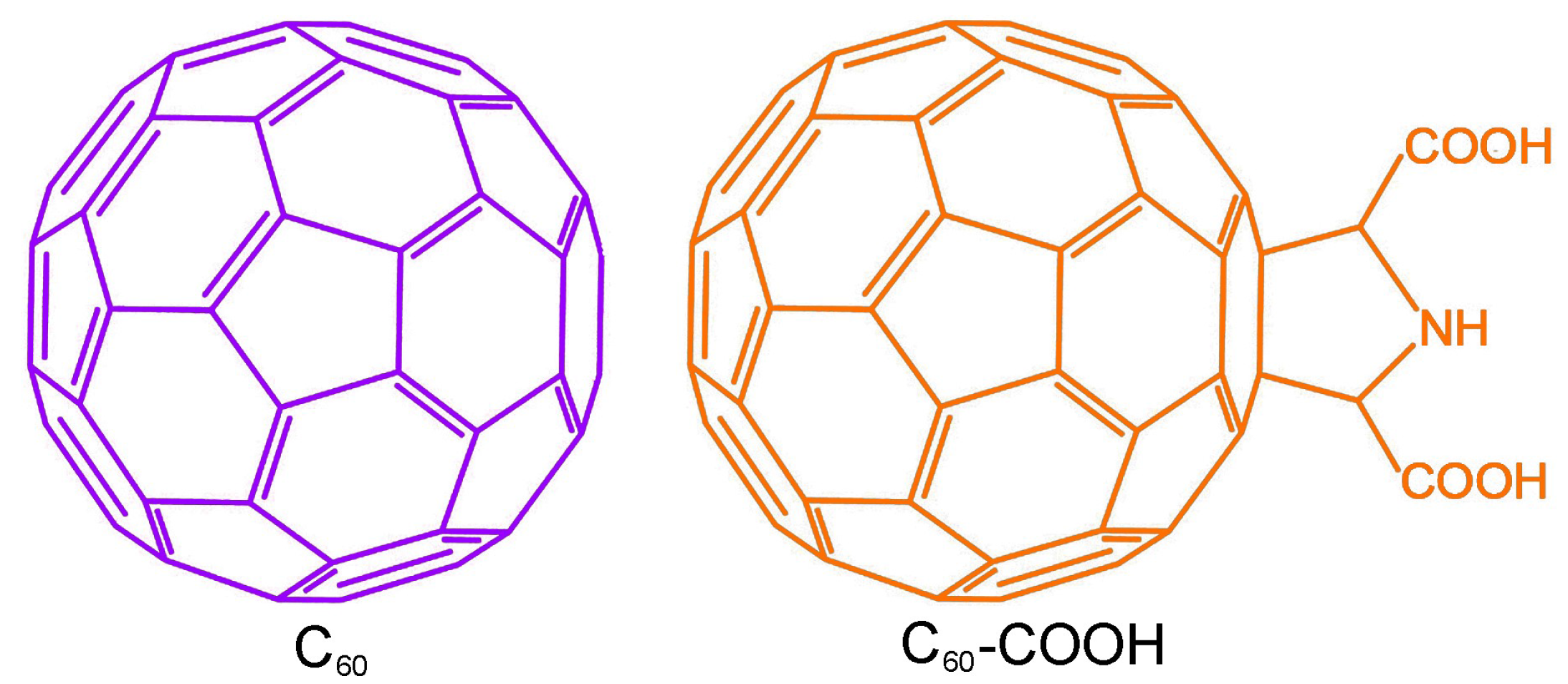
2.1. Materials
2.2. Toxicity Evaluations
2.3. Enzyme Activities
2.4. Degradation Capabilities
2.5. Statistical Analysis
3. Results and Discussion
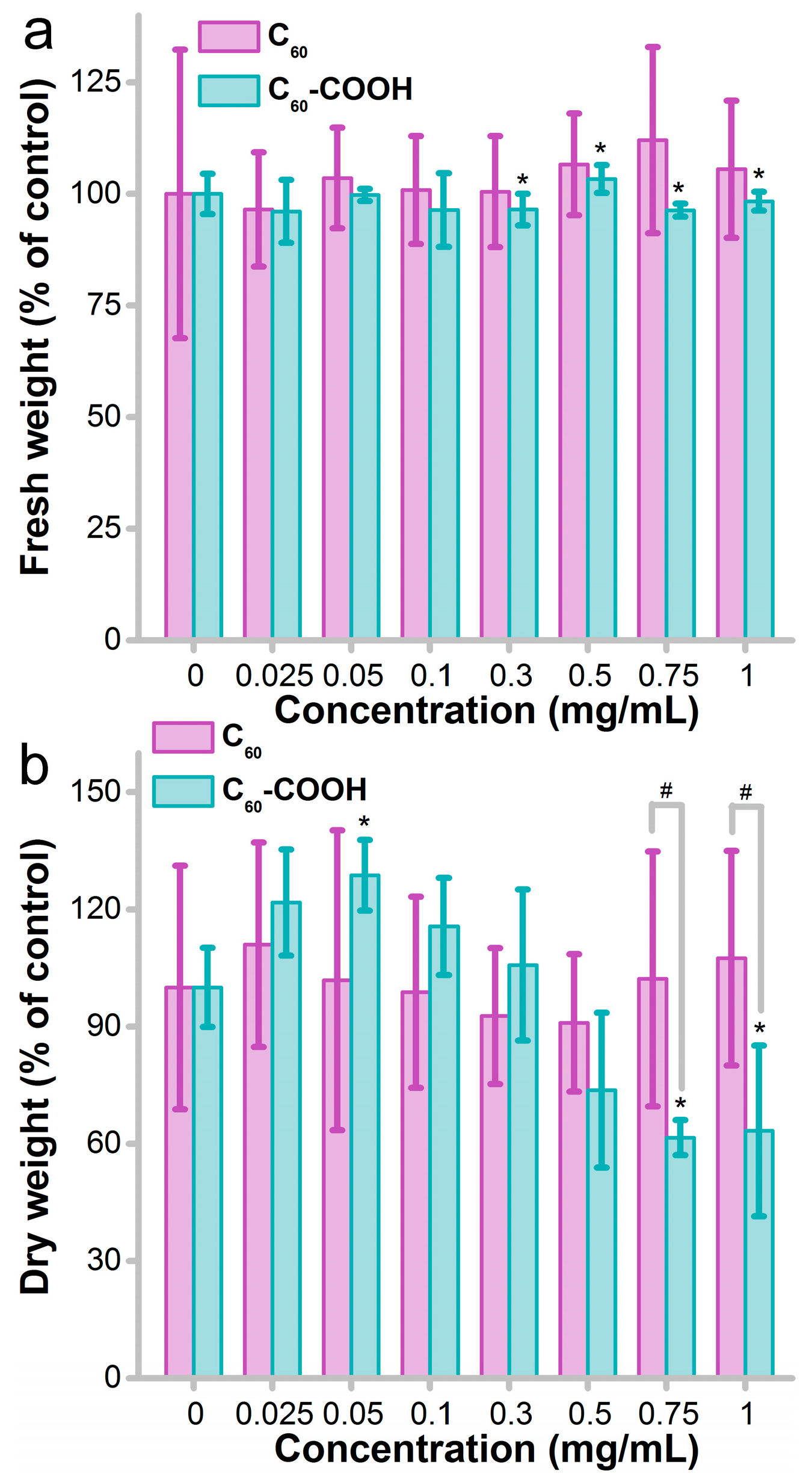
3.1. Influence of Fullerene on Fungus Growth
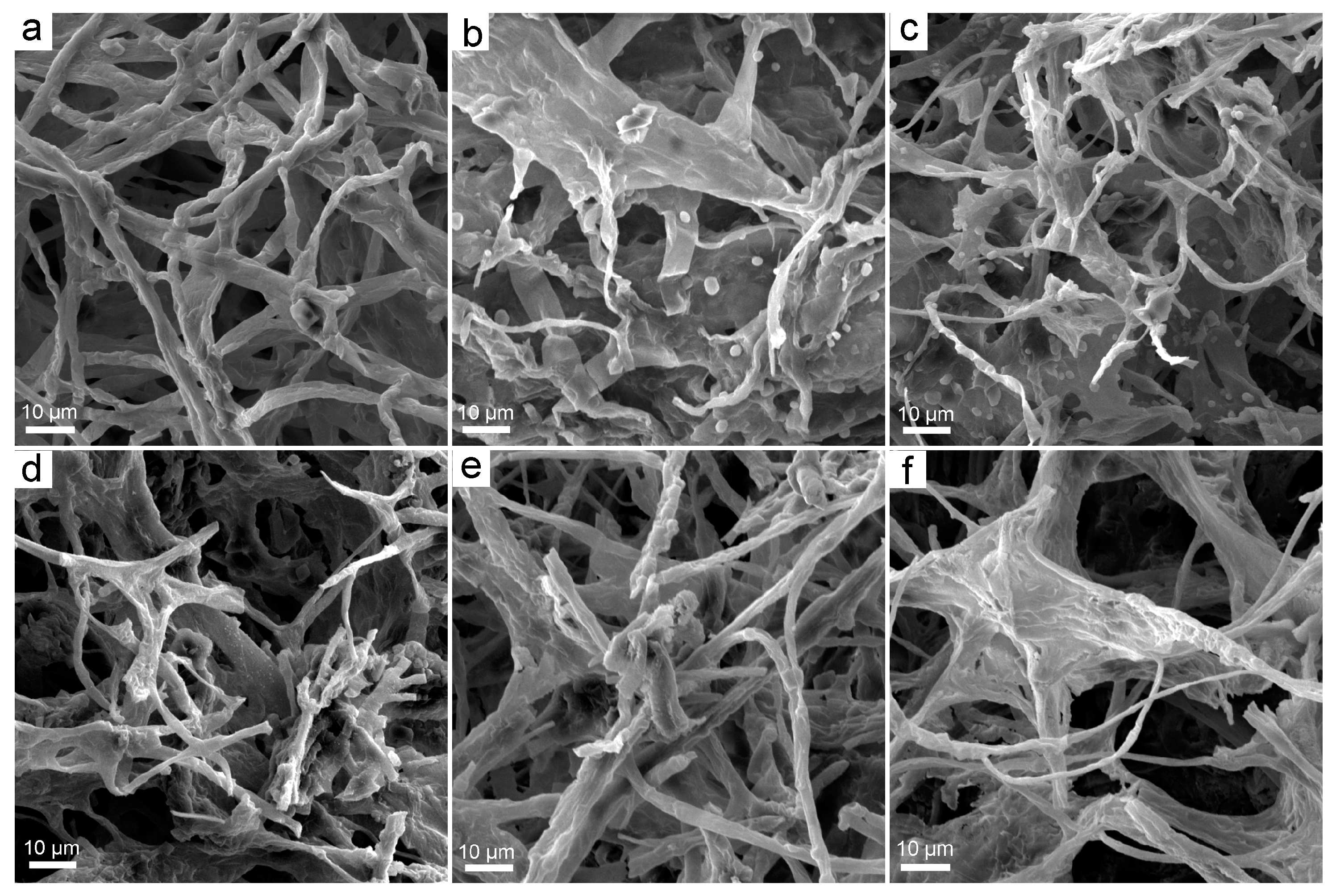
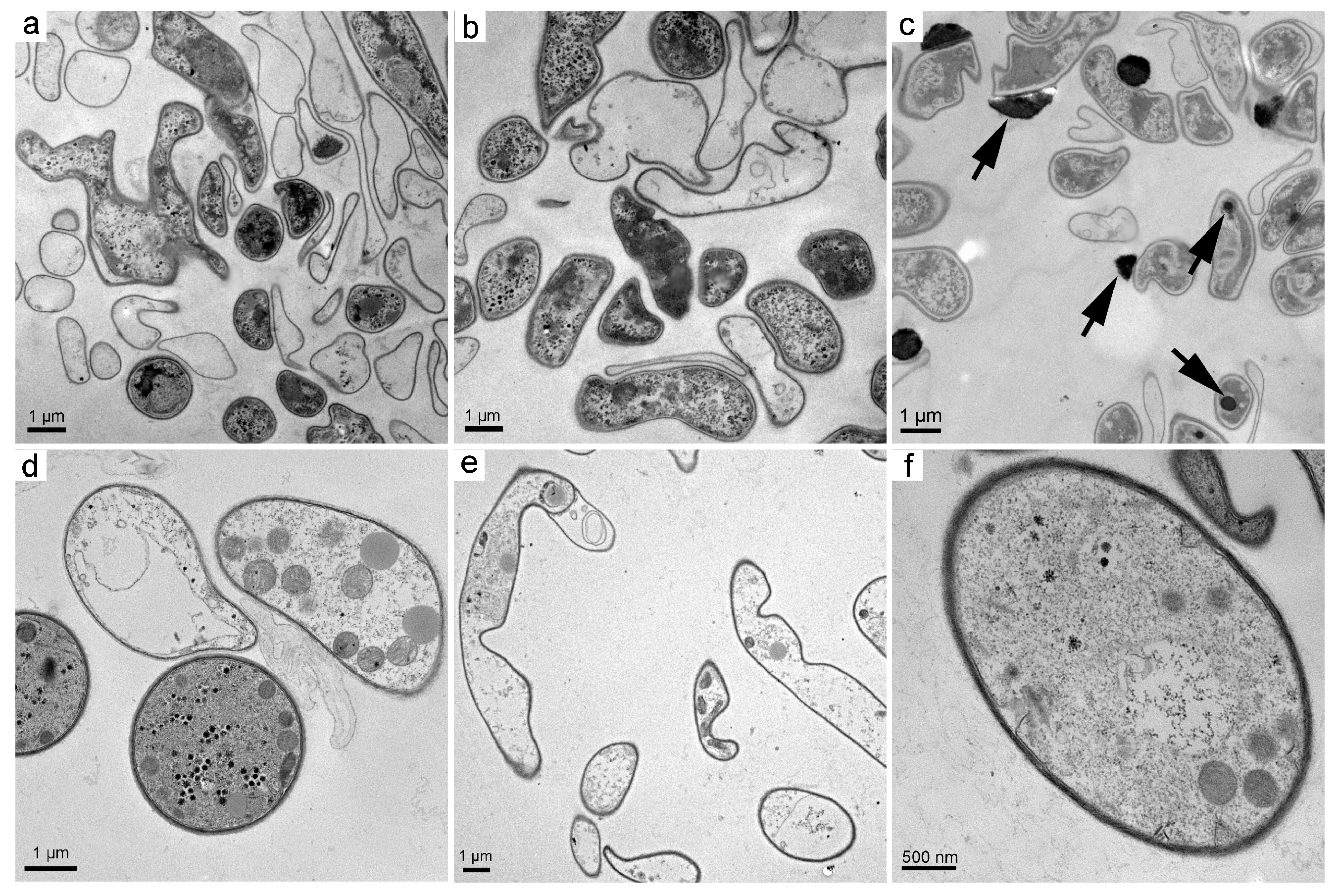
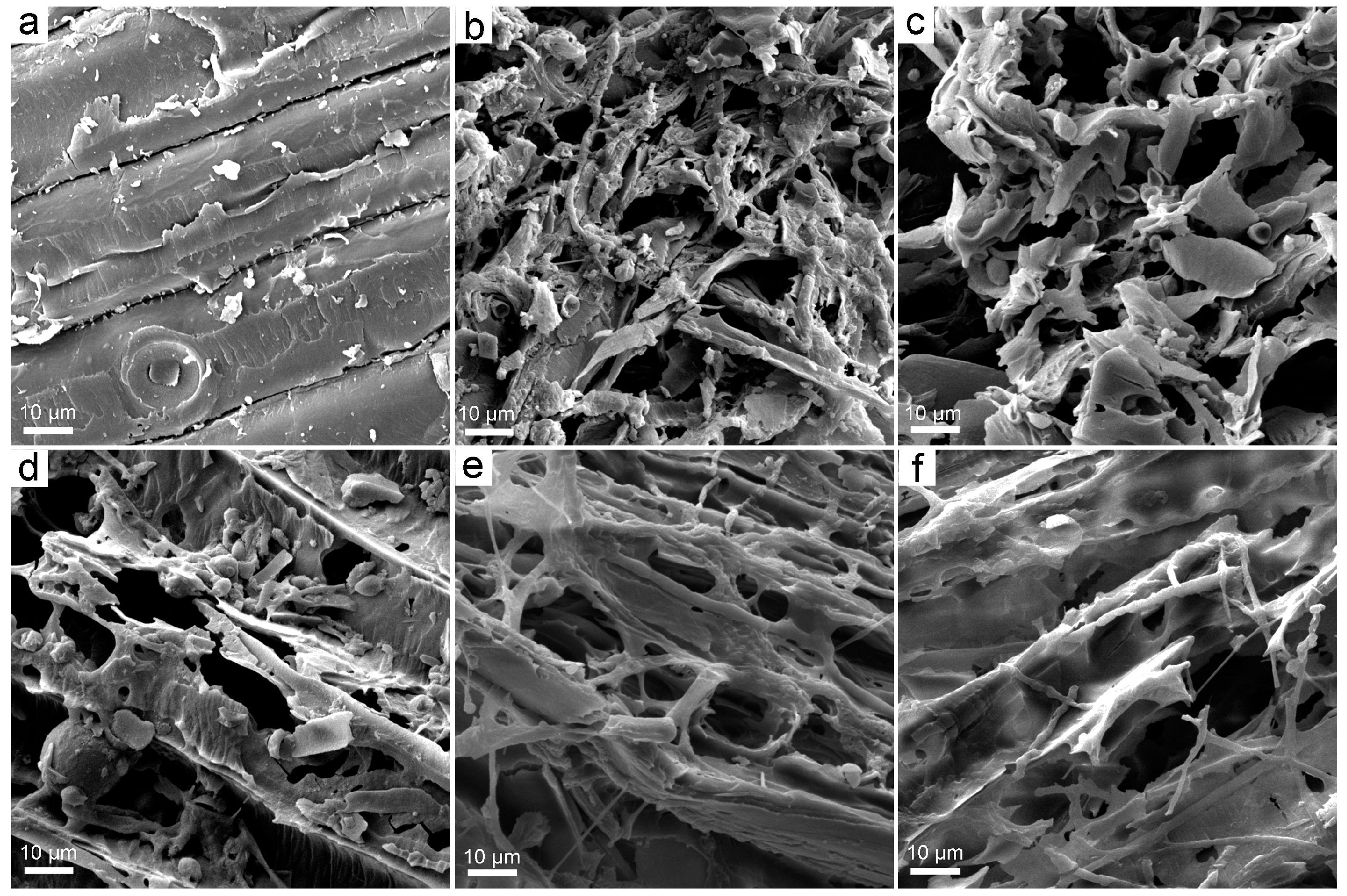
3.2. Structural Changes upon the Exposure to Fullerene
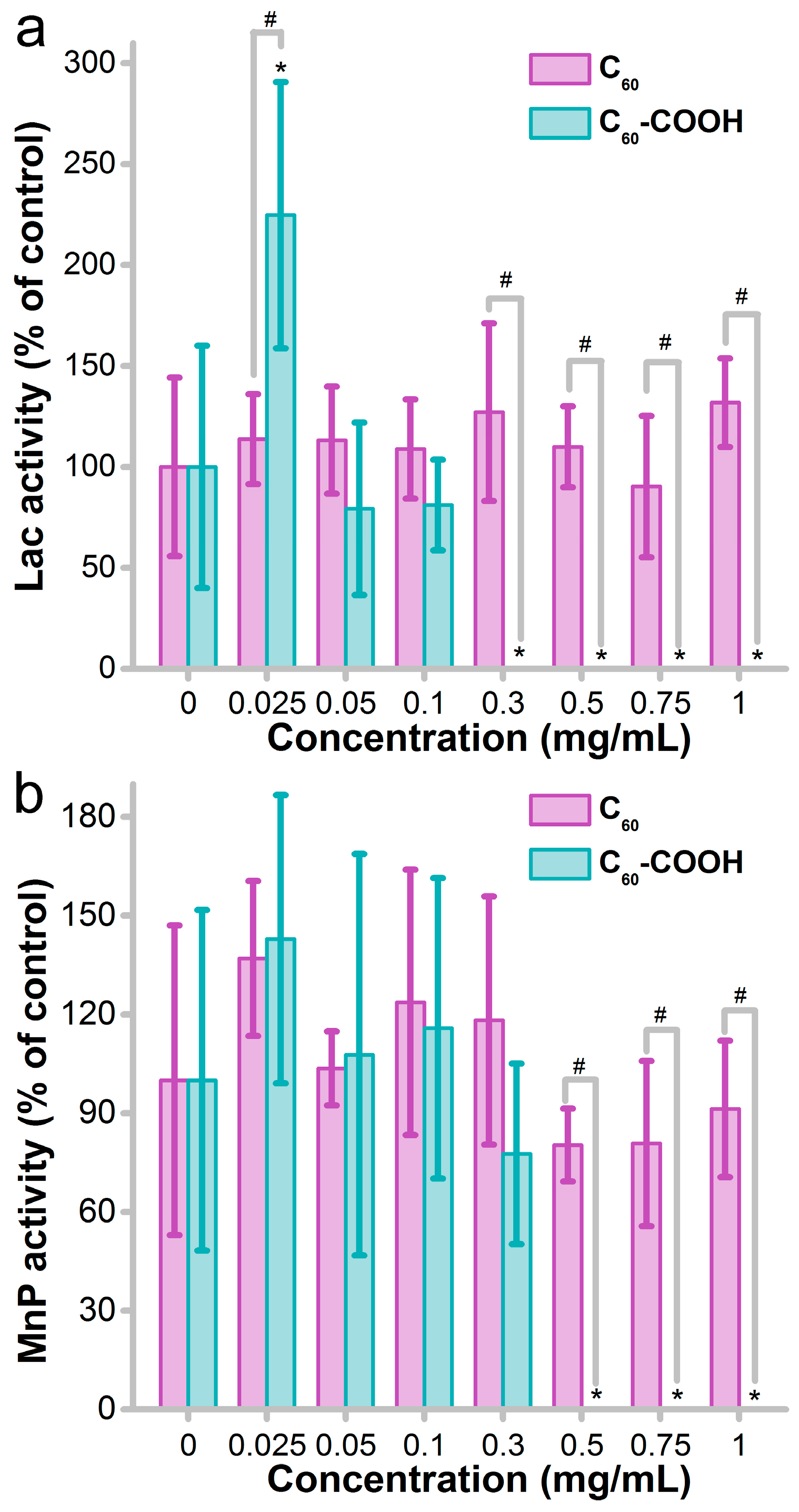
3.3. Impact of Fullerene on Enzyme Activity
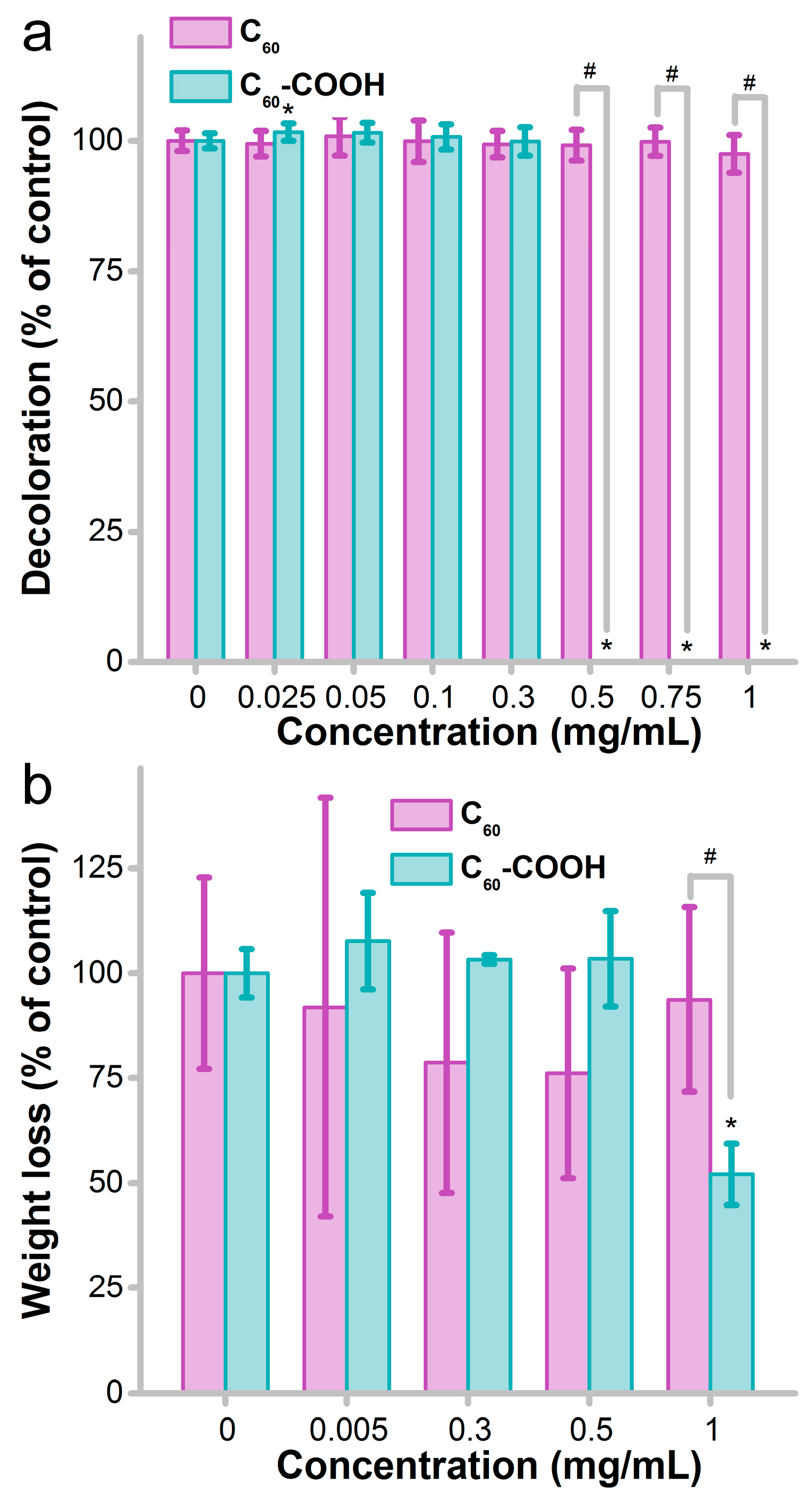
3.4. Influence of Fullerene on the Decomposition Activity
3.5. Implications
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Song, L.; Cao, X.; Li, L.; Wang, Q.; Ye, H.; Gu, L.; Mao, C.; Song, J.; Zhang, S.; Niu, H. General method for large-area films of carbon nanomaterials and application of a self-assembled carbon nanotube film as a high-performance electrode material for an all-solid-state supercapacitor. Adv. Funct. Mater. 2017, 27, 1700474. [Google Scholar] [CrossRef]
- Bernstein, R.; Ferran Prat, A.; Foote, C.S. On the mechanism of DNA cleavage by fullerenes investigated in model systems: Electron transfer from guanosine and 8-oxo-guanosine derivatives to C60. J. Am. Chem. Soc. 1999, 121, 464–465. [Google Scholar] [CrossRef]
- Tuček, J.; Kemp, K.C.; Kim, K.S.; Zbořil, R. Iron-oxide-supported nanocarbon in lithium-ion batteries, medical, catalytic, and environmental applications. ACS Nano 2014, 8, 7571–7612. [Google Scholar] [CrossRef] [PubMed]
- Levchenko, I.; Ostrikov, K.K.; Zheng, J.; Li, X.; Keidar, M.; Teo, K.B.K. Scalable graphene production: Perspectives and challenges of plasma applications. Nanoscale 2016, 8, 10511–10527. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Niu, A.; Li, J.; Fu, J.W.; Xu, Q.; Pei, D.S. In vivo characterization of hair and skin derived carbon quantum dots with high quantum yield as longterm bioprobes in zebrafish. Sci. Rep. 2016, 6, 37860. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.S.; Tzeng, Y.; Liu, Y.K.; Chen, Y.C.; Walker, K.R.; Guntupalli, R.; Liu, C. Immobilization of antibodies and bacterial binding on nanodiamond and carbon nanotubes for biosensor applications. Diam. Relat. Mater. 2004, 13, 1098–1102. [Google Scholar] [CrossRef]
- Chang, X.; Yang, S.-T.; Xing, G. Molecular toxicity of nanomaterials. J. Biomed. Nanotechnol. 2014, 10, 2828–2851. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Yang, S.-T.; Chen, L.; Wang, X.; Wang, Z. Quantification of sp2 carbon nanomaterials in biological systems: Pharmacokinetics, biodistribution and ecological uptake. Rev. Inorg. Chem. 2015, 35, 225–247. [Google Scholar] [CrossRef]
- Chen, L.; Wang, C.; Li, H.; Qu, X.; Yang, S.-T.; Chang, X.-L. Bioaccumulation and toxicity of 13C-skeleton labeled graphene oxide in wheat. Environ. Sci. Technol. 2017, 51, 10146–10153. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Yang, S.-T.; He, T.; Yang, S.; Tang, X. Bioaccumulation and toxicity of carbon nanoparticles suspension injection in intravenously exposed mice. Int. J. Mol. Sci. 2017, 18, 2562. [Google Scholar] [CrossRef] [PubMed]
- Prato, M. Fullerene chemistry for materials science applications. J. Mater. Chem. 1997, 7, 1097–1109. [Google Scholar] [CrossRef]
- Chakravarty, S.; Gelfand, M.P.; Kivelson, S. Electronic correlation effects and superconductivity in doped fullerenes. Science 1991, 254, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.C.; Lin, Y.S.; Tseng, Z.L.; Wu, C.; Kao, F.S.; Chen, S.H. Overcoming the intrinsic difference between hydrophilic CH3NH3PbI3 and hydrophobic C60 thin films to improve the photovoltaic performance. Nanomaterials 2017, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- Markovic, Z.; Trajkovic, V. Biomedical potential of the reactive oxygen species generation and quenching by fullerenes (C60). Biomaterials 2008, 29, 3561–3573. [Google Scholar] [CrossRef] [PubMed]
- Flores-Cervantes, D.X.; Bucheli, T.D. Testing the resistance of fullerenes to chemothermal oxidation used to isolate soots from environmental samples. Environ. Pollut. 2011, 159, 3793–3796. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.C.; Jiang, X.; Wang, J.; Chen, C.; Liu, R.S. Nano-bio effects: Interaction of nanomaterials with cells. Nanoscale 2013, 5, 3547–3569. [Google Scholar] [CrossRef] [PubMed]
- Trpkovic, A.; Todorovic-Markovic, B.; Trajkovic, V. Toxicity of pristine versus functionalized fullerenes: Mechanisms of cell damage and the role of oxidative stress. Arch. Toxicol. 2012, 86, 1809–1827. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Bai, Y.; Li, H.; Liao, R.; Li, J.; Zhang, H.; Zhang, X.; Zhang, S.; Yang, S.-T.; Chang, X.-L. Surface modification-mediated biodistribution of 13C-fullerene C60 in vivo. Part. Fibre Toxicol. 2016, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, H.; Ruan, L.; Chen, L.; Li, H.; Chang, X.-L.; Zhang, X.; Yang, S.-T. Bioaccumulation of 13C-fullerenol nanomaterials in wheat. Environ. Sci. Nano 2016, 3, 799–805. [Google Scholar] [CrossRef]
- Schuhmann, M.K.; Fluri, F. Effects of fullerenols on mouse brain microvascular endothelial cells. Int. J. Mol. Sci. 2017, 18, 1783. [Google Scholar] [CrossRef] [PubMed]
- Navarro, E.; Baun, A.; Behra, R.; Hartmann, N.B.; Filser, J.; Miao, A.; Quigg, A.; Santschi, P.H.; Sigg, L. Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 2008, 17, 372–386. [Google Scholar] [CrossRef] [PubMed]
- Kovač, T.; Šarkanj, B.; Klapec, T.; Borišev, I.; Kovač, M.; Nevistić, A.; Strelec, I. Fullerol C60(OH)24 nanoparticles and mycotoxigenic fungi: A preliminary investigation into modulation of mycotoxin production. Environ. Sci. Pollut. Res. Int. 2017, 24, 16673–16681. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, Y.; Folta, K.M.; Krishna, V.; Bai, W.; Indeglia, P.; Georgieva, A.; Nakamura, H.; Koopman, B.; Moudgil, B. Polyhydroxy fullerenes (fullerols or fullerenols): Beneficial effects on growth and lifespan in diverse biological models. PLoS ONE 2011, 6, e19976. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, X.; Chen, J.; Han, H.; Yuan, Z. Evaluation and mechanism of antifungal effects of carbon nanomaterials in controlling plant fungal pathogen. Carbon 2014, 68, 798–806. [Google Scholar] [CrossRef]
- Schreiner, K.M.; Filley, T.R.; Blanchette, R.A.; Bowen, B.B.; Bolskar, R.D.; Hockaday, W.C.; Masiello, C.A.; Raebiger, J.W. White-rot basidiomycete-mediated decomposition of C60 fullerol. Environ. Sci. Technol. 2009, 43, 3162–3168. [Google Scholar] [CrossRef] [PubMed]
- Goswami, L.; Kim, K.H.; Deep, A.; Das, P.; Bhattacharya, S.S.; Kumar, S.; Adelodun, A.A. Engineered nano particles: Nature, behavior, and effect on the environment. J. Environ. Manag. 2017, 196, 297–315. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Ming, Z.; Li, H.; Yang, H.; Yu, B.; Wu, R.; Liu, X.; Bai, Y.; Yang, S.-T. Toxicity of graphene oxide to white rot fungus Phanerochaete chrysosporium. Chemosphere 2016, 151, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Schimel, D.S. Terrestrial ecosystem and the carbon cycle. Glob. Chang. Biol. 1995, 1, 77–91. [Google Scholar] [CrossRef]
- Cox, P.; Betts, R.; Jones, C.; Spall, S.; Totterdell, I. Will carbon-cycle feedbacks accelerate global warming in the 21st century. Nature 2000, 408, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.W.S. Structure and action mechanism of ligninolytic enzymes. Appl. Biochem. Biotechnol. 2009, 157, 174–209. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Feng, S.; Ma, Q.; Ming, Z.; Bai, Y.; Chen, L.; Yang, S.-T. Influence of reduced graphene oxide on the growth, structure and decomposition activity of white rot fungus Phanerochaete chrysosporium. RSC Adv. 2018, 8, 5026–5033. [Google Scholar] [CrossRef]
- Rodriguezcouto, S.; Arzac, A.; Leal, G.P.; Tomovska, R. Reduced graphene oxide hydrogels and xerogels provide efficient platforms for immobilization and laccase production by Trametes pubescens. Biotechnol. J. 2014, 9, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Berry, T.D.; Filley, T.R.; Blanchette, R.A. Oxidative enzymatic response of white-rot fungi to single-walled carbon nanotubes. Environ. Pollut. 2014, 193, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Eggert, C.; Temp, U.; Eriksson, K.E. The ligninolytic system of the white rot fungus Pycnoporus cinnabarinus: Purification and characterization of the laccase. Appl. Environ. Micro. 1996, 62, 1151–1158. [Google Scholar]
- Périé, F.H.; Gold, M.H. Manganese regulation of manganese peroxidase expression and lignin degradation by the white rot fungus Dichomitus squalens. Appl. Environ. Micro. 1991, 57, 2240–2245. [Google Scholar]
- Huang, D.L.; Wang, C.; Xu, P.; Zeng, G.M.; Lu, B.A.; Li, N.J.; Huang, C.; Lai, C.; Zhao, M.H.; Xu, J.J.; et al. A coupled photocatalytic-biological process for phenol degradation in the Phanerochaete chrysosporium-oxalate-Fe3O4 system. Int. Biodeter. Biodegr. 2015, 97, 115–123. [Google Scholar] [CrossRef]
- Bai, Y.; Ming, Z.; Cao, Y.; Feng, S.; Yang, H.; Chen, L.; Yang, S.-T. Influence of graphene oxide and reduced graphene oxide on the activity and conformation of lysozyme. Colloid Surf. B 2017, 154, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zeng, G.; Chen, G.; Huang, Z.; Wan, J.; Chen, A.; Yu, Z.; Yang, J.; He, K.; Qin, L. Bioaccumulation and toxicity of CdSe@ZnS quantum dots in Phanerochaete chrysosporium. Colloid Surf. B 2017, 159, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Chen, G.; Zeng, G.; Guo, Z.; He, K.; Hu, L.; Wu, J.; Zhang, L.; Zhu, Y.; Song, Z. Toxicity mechanisms and synergies of silver nanoparticles in 2,4-dichlorophenol degradation by Phanerochaete chrysosporium. J. Hazard. Mater. 2017, 321, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-T.; Liu, Y.; Wang, Y.; Cao, A. Biosafety and bioapplication of nanomaterials by designing protein-nanoparticle interactions. Small 2013, 9, 1635–1653. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yang, S.-T.; Wang, H.; Wang, L.; Hu, W.; Cao, A.; Liu, Y. Influences of the size and hydroxyl number of fullerenes/fullerenols on their interactions with proteins. J. Nanosci. Nanotechnol. 2010, 10, 6298–6304. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-T.; Wang, H.; Guo, L.; Gao, Y.; Liu, Y.; Cao, A. Interaction of fullerenol with lysozyme investigated by experimental and computational approaches. Nanotechnology 2008, 19, 395101. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Dobiásová, P.; Baldrian, P.; Nerud, F.; Kumar, A.; Seal, S. Influence of iron and copper nanoparticle powder on the production of lignocellulose degrading enzymes in the fungus Trametes versicolor. J. Hazard. Mater. 2010, 178, 1141–1145. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, C. γ-Fe2O3 nanoparticle-facilitated bisphenol A degradation by white rot fungus. Sci. Bull. 2016, 61, 468–472. [Google Scholar] [CrossRef]
- Filpo, G.D.; Palermo, A.M.; Rachiele, F.; Nicoletta, F.P. Preventing fungal growth in wood by titanium dioxide nanoparticles. Int. Biodeter. Biodegr. 2013, 85, 217–222. [Google Scholar] [CrossRef]
- Taghiyari, H.R.; Moradi-Malek, B.; Kookandeh, M.G.; Bibalan, O.F. Effects of silver and copper nanoparticles in particleboard to control Trametes versicolor fungus. Int. Biodeter. Biodegr. 2014, 94, 69–72. [Google Scholar] [CrossRef]
- Akhtari, M.; Taghiyari, H.R.; Kokandeh, M.G. Effect of some metal nanoparticles on the spectroscopy analysis of Paulownia wood exposed to white-rot fungus. Eur. J. Wood Prod. 2013, 71, 283–285. [Google Scholar] [CrossRef]
- Tiwari, A.J.; Ashrafkhorassani, M.; Marr, L.C. C60 fullerenes from combustion of common fuels. Sci. Total Environ. 2016, 547, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Onasch, T.B.; Ge, X.; Collier, S.; Zhang, Q.; Sun, Y.; Yu, H.; Chen, M.; Prévôt, A.S.H.; Worsnop, D.R. Observation of fullerene soot in eastern china. Environ. Sci. Technol. Lett. 2016, 3, 121–126. [Google Scholar] [CrossRef]
- Wu, J.; Goodwin, D.G.; Peter, K.; Benoit, D.; Li, W.; Fairbrother, D.H.; Fortner, J.D. Photo-oxidation of hydrogenated fullerene (fullerane) in water. Environ. Sci. Technol. Lett. 2014, 1, 490–494. [Google Scholar] [CrossRef]
- Iwata, N.; Mukai, T.; Yamakoshi, Y.N.; Haraa, S.; Yanase, T.; Shoji, M.; Endo, T.; Miyata, N. Effects of C60, a fullerene, on the activities of glutathione S-transferase and glutathione-related enzymes in rodent and human livers. Fuller. Sci. Technol. 1998, 6, 213–226. [Google Scholar] [CrossRef]
- Kolosnjaj, J.; Szwarc, H.; Moussa, F. Toxicity studies of fullerenes and derivatives. Adv. Exp. Med. Biol. 2007, 620, 168–180. [Google Scholar] [PubMed]
- Xia, X.R.; Monteiro-Riviere, N.A.; Riviere, J.E. Intrinsic biological property of colloidal fullerene nanoparticles (nC60): lack of lethality after high dose exposure to human epidermal and bacterial cells. Toxicol. Lett. 2010, 197, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Hadduck, A.N.; Hindagolla, V.; Contreras, A.E.; Li, Q.L.; Bakalinsky, A.T. Does aqueous fullerene inhibit the growth of Saccharomyces cerevisiae or Escherichia coli? Appl. Environ. Microbiol. 2010, 76, 8239–8942. [Google Scholar] [CrossRef] [PubMed]
- Maas, M. Carbon nanomaterials as antibacterial colloids. Materials 2016, 9, 617. [Google Scholar] [CrossRef] [PubMed]
- Khanna, P.; Ong, C.; Bay, B.; Baeg, G. Nanotoxicity: An interplay of oxidative stress, inflammation and cell death. Nanomaterials 2015, 5, 1163–1180. [Google Scholar] [CrossRef] [PubMed]
- Waissi, G.C.; Bold, S.; Pakarinen, K.; Akkanen, J.; Leppänen, M.T.; Petersen, E.J.; Kukkonen, J.V.K. Chironomus riparius exposure to fullerene-contaminated sediment results in oxidative stress and may impact life cycle parameters. J. Hazard. Mater. 2017, 322, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Horie, M.; Nishio, K.; Kato, H.; Shinohara, N.; Nakamura, A.; Fujita, K.; Kinugasa, S.; Endoh, S.; Yoshida, Y.; Hagihara, Y.; et al. In vitro evaluation of cellular influences induced by stable fullerene C-70 medium dispersion: Induction of cellular oxidative stress. Chemosphere 2013, 93, 1182–1188. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, A.; Srdjenovic, B.; Seke, M.; Petrovic, D.; Injac, R.; Mrdjanovic, J. Review of synthesis and antioxidant potential of fullerenol nanoparticles. J. Nanomater. 2015, 16, 280. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ming, Z.; Feng, S.; Yilihamu, A.; Ma, Q.; Yang, S.; Yang, S.-T. Toxicity of Pristine and Chemically Functionalized Fullerenes to White Rot Fungus Phanerochaete chrysosporium. Nanomaterials 2018, 8, 120. https://doi.org/10.3390/nano8020120
Ming Z, Feng S, Yilihamu A, Ma Q, Yang S, Yang S-T. Toxicity of Pristine and Chemically Functionalized Fullerenes to White Rot Fungus Phanerochaete chrysosporium. Nanomaterials. 2018; 8(2):120. https://doi.org/10.3390/nano8020120
Chicago/Turabian StyleMing, Zhu, Shicheng Feng, Ailimire Yilihamu, Qiang Ma, Shengnan Yang, and Sheng-Tao Yang. 2018. "Toxicity of Pristine and Chemically Functionalized Fullerenes to White Rot Fungus Phanerochaete chrysosporium" Nanomaterials 8, no. 2: 120. https://doi.org/10.3390/nano8020120




