Fabrication of Two Polyester Nanofiber Types Containing the Biobased Monomer Isosorbide: Poly (Ethylene Glycol 1,4-Cyclohexane Dimethylene Isosorbide Terephthalate) and Poly (1,4-Cyclohexane Dimethylene Isosorbide Terephthalate)
Abstract
:1. Introduction
2. Materials and Methods
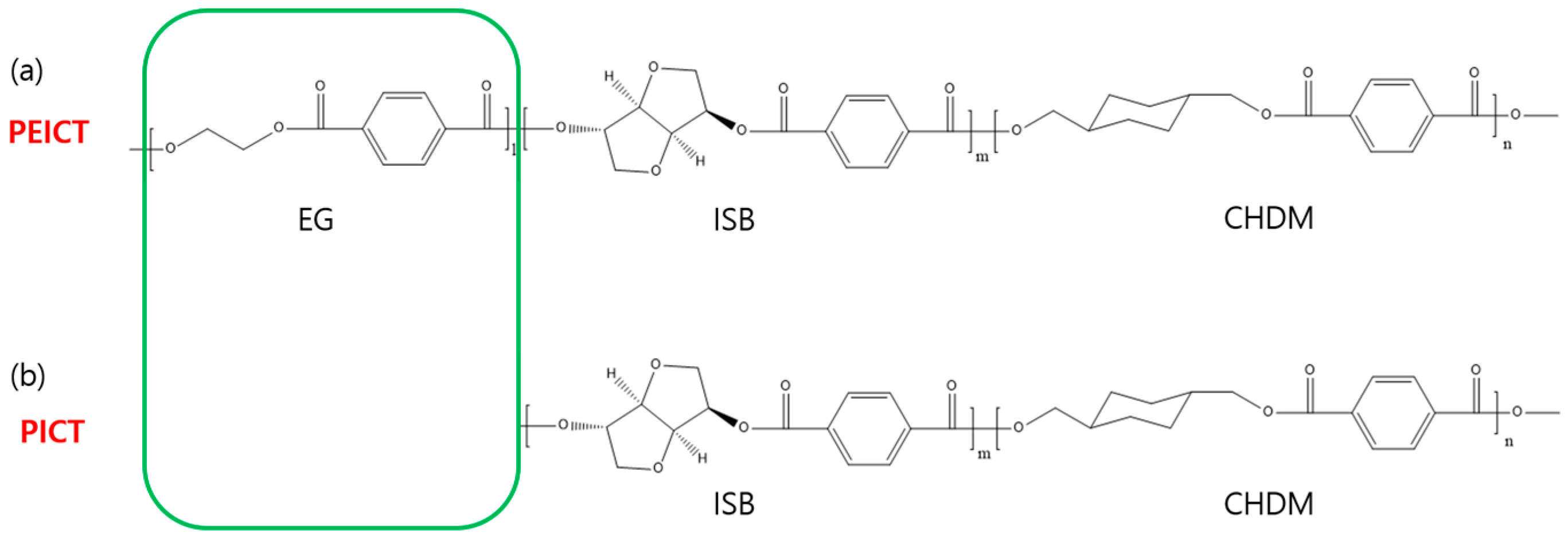
2.1. Materials
2.2. Characterization
2.3. Electrospinning
3. Results and Discussion
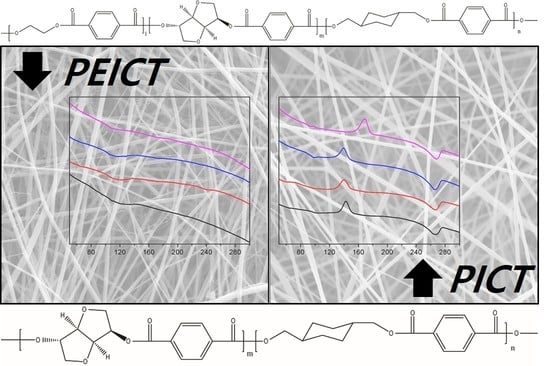
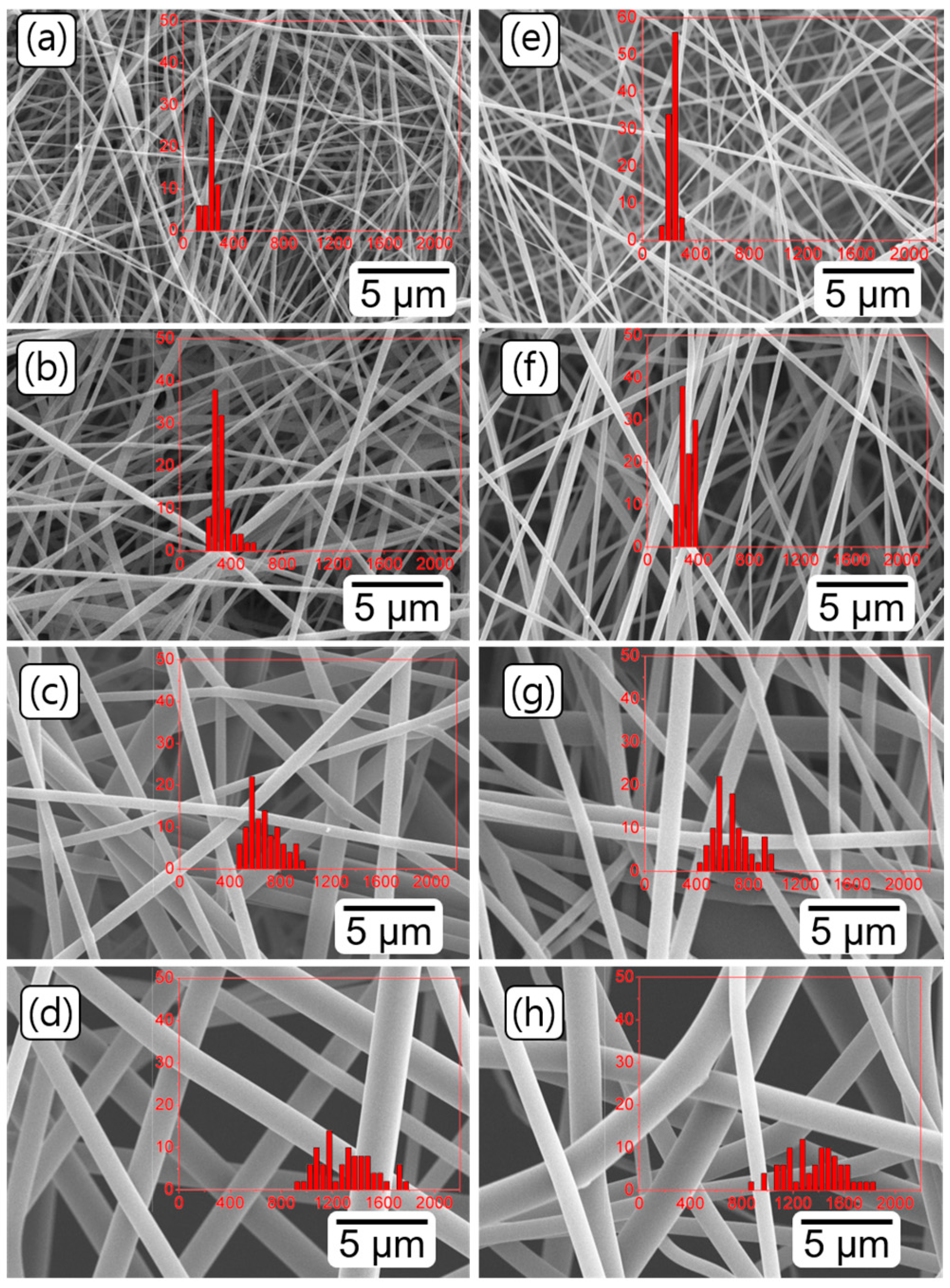
3.1. Morphology of PEICT and PICT Nanofibers
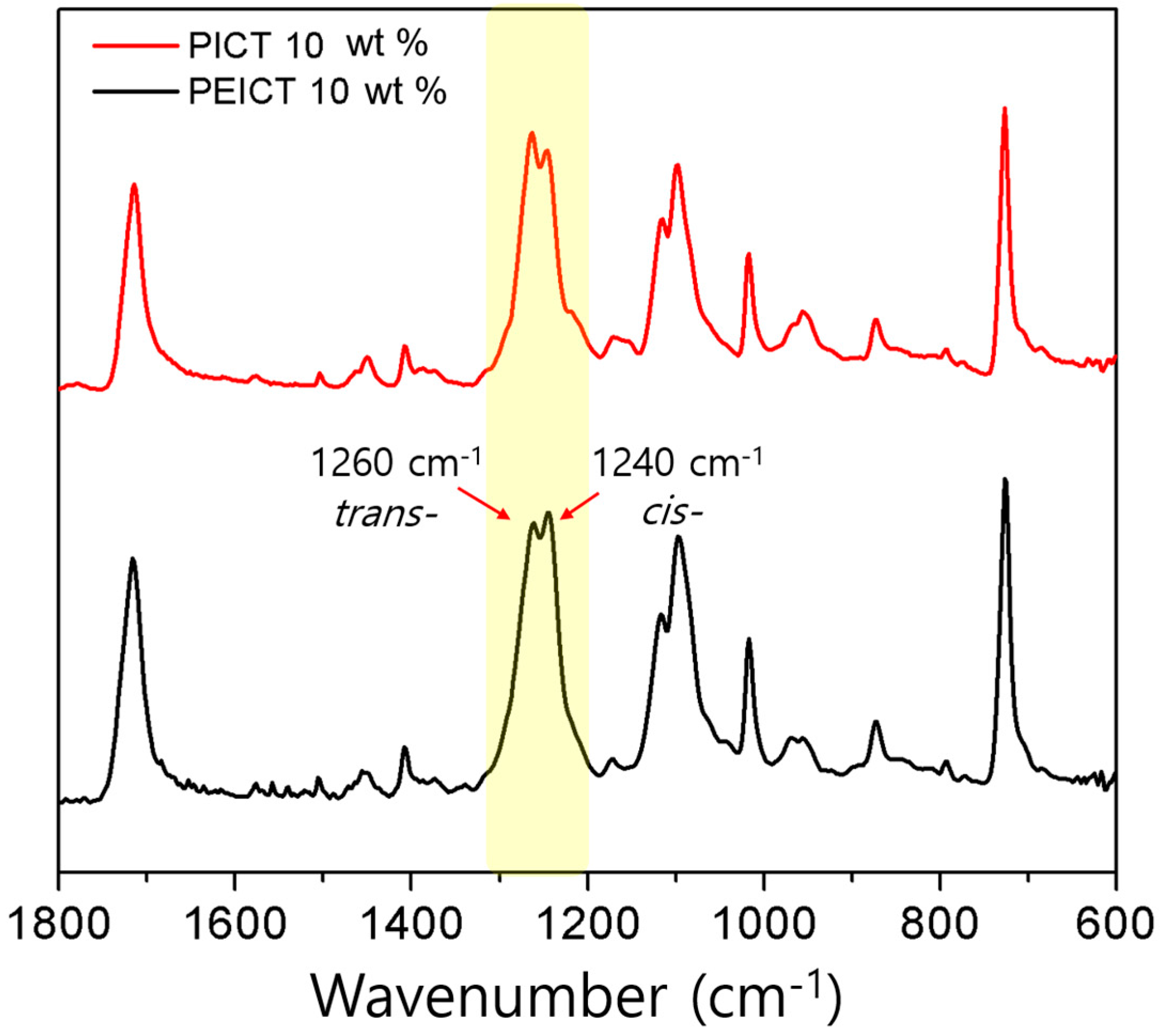
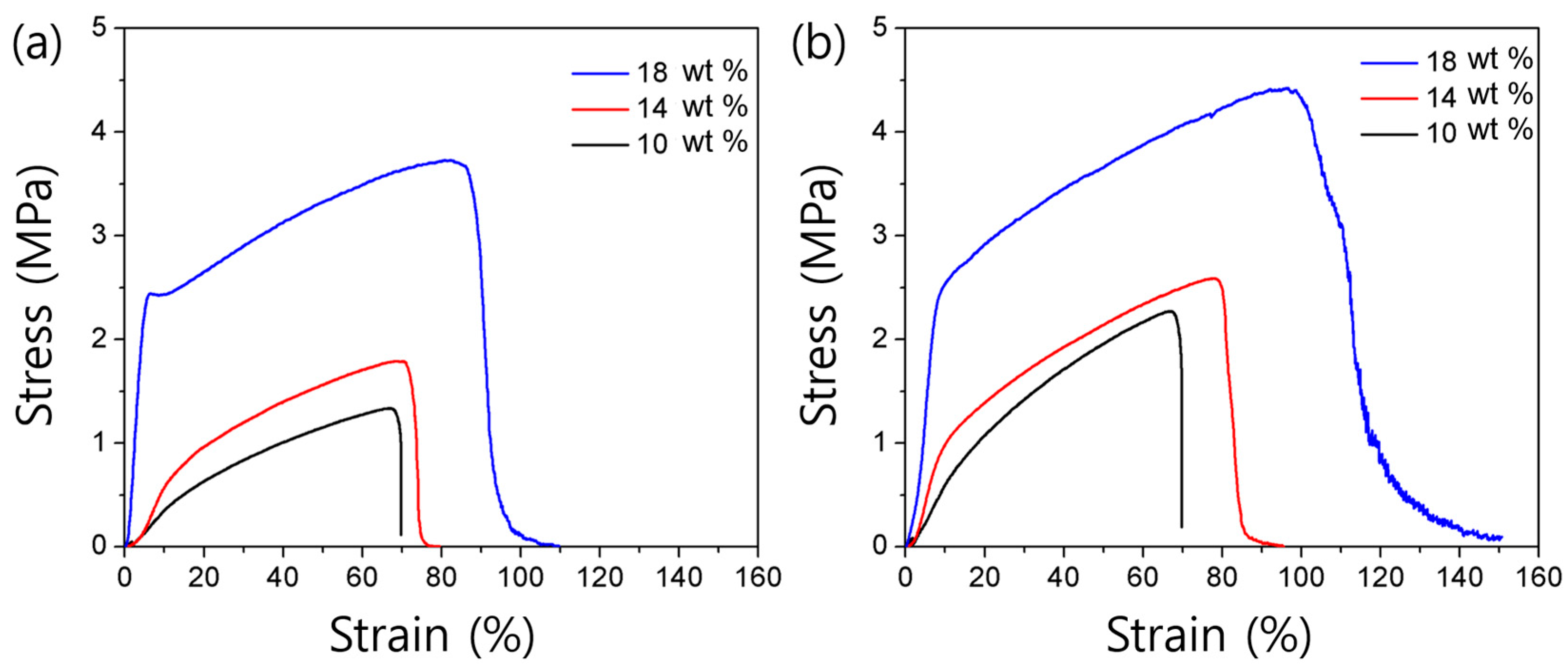
3.2. Chemical Structures and Mechanical Properties of PICT and PEICT Nanofibers
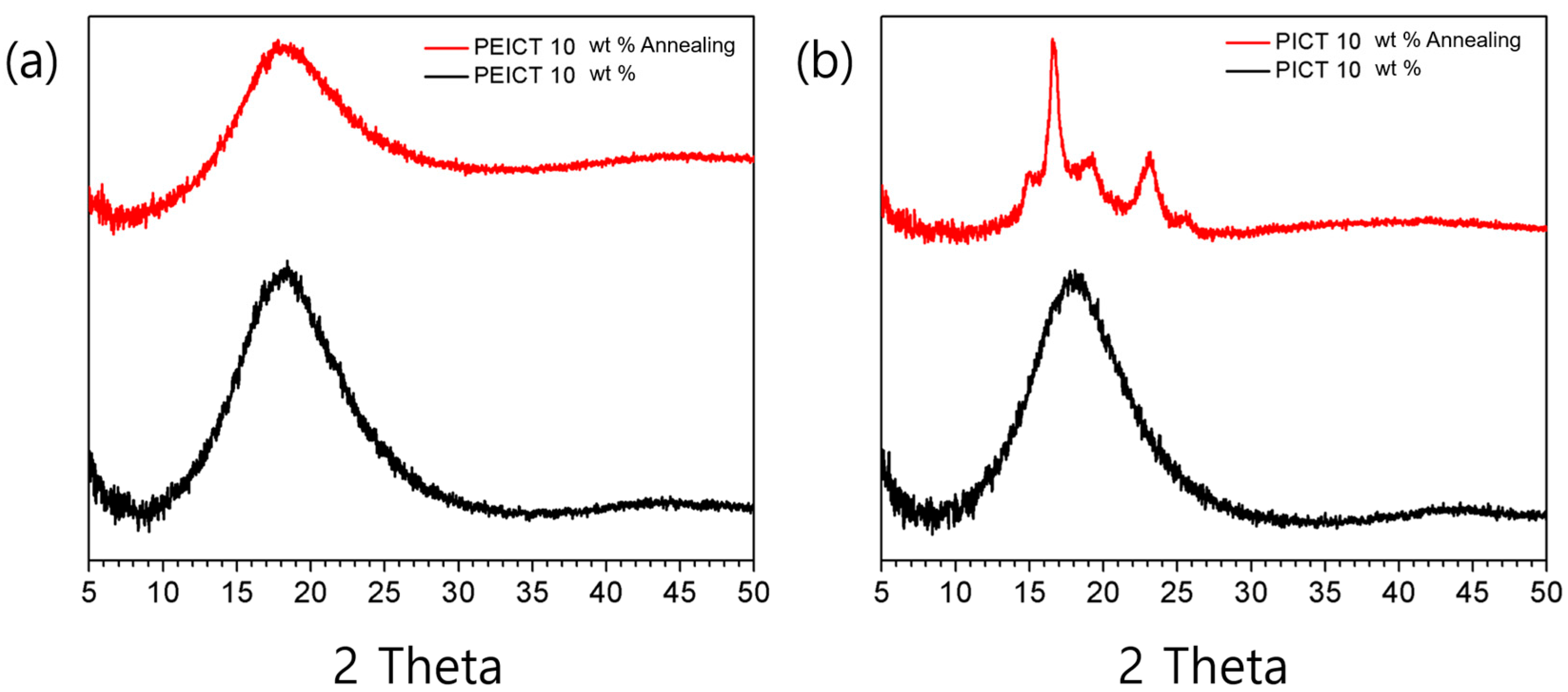
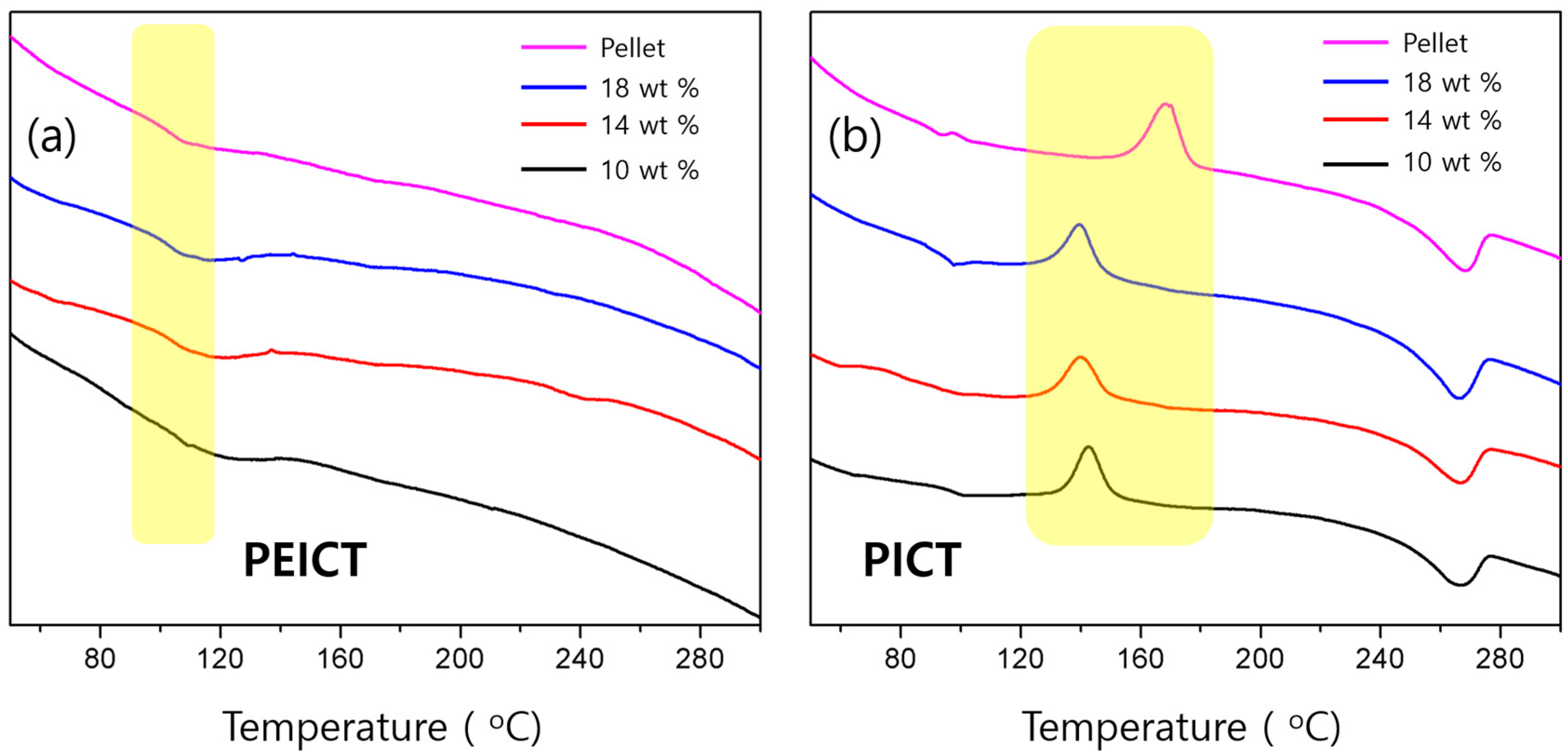
3.3. Thermal Crystallization Behavior
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Giacomini, D.; Torricelli, P.; Gentilomi, G.A.; Boanini, E.; Gazzano, M.; Bonvicini, F.; Benetti, E.; Soldati, R.; Martelli, G.; Rubini, K.; et al. Monocyclic β-lactams loaded on hydroxyapatite: New biomaterials with enhanced antibacterial activity against resistant strains. Sci. Rep. 2017, 7, 2712. [Google Scholar] [CrossRef] [PubMed]
- Alakpa, E.V.; Jayawarna, V.; Burgess, K.E.V.; West, C.C.; Péault, B.; Ulijn, R.V.; Dalby, M.J. Improving cartilage phenotype from differentiated pericytes in tunable peptide hydrogels. Sci. Rep. 2017, 7, 6895. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Koo, J.M.; Sohn, D.; Kim, I.-S.; Im, S.S. High thermal stability and high tensile strength terpolyester nanofibers containing biobased monomer: Fabrication and characterization. RSC Adv. 2016, 6, 40383–40388. [Google Scholar] [CrossRef]
- Koo, J.M.; Hwang, S.Y.; Yoon, W.J.; Lee, Y.G.; Kim, S.H.; Im, S.S. Structural and thermal properties of poly(1,4-cyclohexane dimethylene terephthalate) containing isosorbide. Polym. Chem. 2015, 6, 6973–6986. [Google Scholar] [CrossRef]
- Chatti, S.; Kricheldorf, H.R.; Schwarz, G. Copolycarbonates of isosorbide and various diols. J. Polym. Sci. A 2006, 44, 3616–3628. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, J.; Luo, M.; Xing, J.; Wu, J.; Pan, H.; Ruan, C.; Luo, Y. Incorporating isosorbide as the chain extender improves mechanical properties of linear biodegradable polyurethanes as potential bone regeneration materials. RSC Adv. 2017, 7, 13886–13895. [Google Scholar] [CrossRef]
- Yoon, W.J.; Hwang, S.Y.; Koo, J.M.; Lee, Y.J.; Lee, S.U.; Im, S.S. Synthesis and Characteristics of a Biobased High-Tg Terpolyester of Isosorbide, Ethylene Glycol, and 1,4-Cyclohexane Dimethanol: Effect of Ethylene Glycol as a Chain Linker on Polymerization. Macromolecules 2013, 46, 7219–7231. [Google Scholar] [CrossRef]
- Lee, H.; Watanabe, K.; Kim, M.; Gopiraman, M.; Song, K.-H.; Lee, J.S.; Kim, I.S. Handspinning enabled highly concentrated carbon nanotubes with controlled orientation in nanofibers. Sci. Rep. 2016, 6, 37590. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Xu, G.; Kharaghani, D.; Nishino, M.; Song, K.H.; Lee, J.S.; Kim, I.S. Electrospun tri-layered zein/PVP-GO/zein nanofiber mats for providing biphasic drug release profiles. Int. J. Pharm. 2017, 531, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, M.; Sohn, D.; Kim, S.H.; Oh, S.-G.; Im, S.S.; Kim, I.S. Electrospun tungsten trioxide nanofibers decorated with palladium oxide nanoparticles exhibiting enhanced photocatalytic activity. RSC Adv. 2017, 7, 6108–6113. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Electrospinning of nanofibers: Reinventing the wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Lee, H.; Yamaguchi, K.; Nagaishi, T.; Murai, M.; Kim, M.; Wei, K.; Zhang, K.-Q.; Kim, I.S. Enhancement of mechanical properties of polymeric nanofibers by controlling crystallization behavior using a simple freezing/thawing process. RSC Adv. 2017, 7, 43994–44000. [Google Scholar] [CrossRef]
- Lee, H.; Hun Song, K.; Soon Im, S.; Jung, J.-S.; Jatoi, A.W.; Kim, I.S. Fabrication of poly(vinyl alcohol)/cellulose nanofiber derivative from kenaf bast fiber via electrospinning. Nanosci. Nanotechnol. Lett. 2016, 8, 168–172. [Google Scholar] [CrossRef]
- Lee, H.; Nagaishi, T.; Phan, D.-N.; Kim, M.; Zhang, K.-Q.; Wei, K.; Kim, I.S. Effect of graphene incorporation in carbon nanofiber decorated with TiO2 for photoanode applications. RSC Adv. 2017, 7, 6574–6582. [Google Scholar] [CrossRef]
- Feng, Y.-C.; Zhao, H.; Hao, T.-H.; Hu, G.-H.; Jiang, T.; Zhang, Q.-C. Effects of poly(cyclohexanedimethylene terephthalate) on microstructures, crystallization behavior and properties of the poly(ester ether) elastomers. Materials 2017, 10, 694. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W.J.; Oh, K.S.; Koo, J.M.; Kim, J.R.; Lee, K.J.; Im, S.S. Advanced Polymerization and Properties of Biobased High Tg polyester of Isosorbide and 1,4-Cyclohexanedicarboxylic Acid Through in situ Acetylation. Macromolecules 2013, 46, 2930–2940. [Google Scholar] [CrossRef]
- Wu, J.; Eduard, P.; Jasinska-Walc, L.; Rozanski, A.; Noordover, B.A.J.; van Es, D.S.; Koning, C.E. Fully isohexide-based polyesters: Synthesis, characterization, and structure–properties relations. Macromolecules 2013, 46, 384–394. [Google Scholar] [CrossRef]
- Fenouillot, F.; Rousseau, A.; Colomines, G.; Saint-Loup, R.; Pascault, J.P. Polymers from renewable 1,4:3,6-dianhydrohexitols (isosorbide, isomannide and isoidide): A review. Prog. Polym. Sci. 2010, 35, 578–622. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phan, D.-N.; Lee, H.; Choi, D.; Kang, C.-Y.; Im, S.S.; Kim, I.S. Fabrication of Two Polyester Nanofiber Types Containing the Biobased Monomer Isosorbide: Poly (Ethylene Glycol 1,4-Cyclohexane Dimethylene Isosorbide Terephthalate) and Poly (1,4-Cyclohexane Dimethylene Isosorbide Terephthalate). Nanomaterials 2018, 8, 56. https://doi.org/10.3390/nano8020056
Phan D-N, Lee H, Choi D, Kang C-Y, Im SS, Kim IS. Fabrication of Two Polyester Nanofiber Types Containing the Biobased Monomer Isosorbide: Poly (Ethylene Glycol 1,4-Cyclohexane Dimethylene Isosorbide Terephthalate) and Poly (1,4-Cyclohexane Dimethylene Isosorbide Terephthalate). Nanomaterials. 2018; 8(2):56. https://doi.org/10.3390/nano8020056
Chicago/Turabian StylePhan, Duy-Nam, Hoik Lee, Dongeun Choi, Chang-Yong Kang, Seung Soon Im, and Ick Soo Kim. 2018. "Fabrication of Two Polyester Nanofiber Types Containing the Biobased Monomer Isosorbide: Poly (Ethylene Glycol 1,4-Cyclohexane Dimethylene Isosorbide Terephthalate) and Poly (1,4-Cyclohexane Dimethylene Isosorbide Terephthalate)" Nanomaterials 8, no. 2: 56. https://doi.org/10.3390/nano8020056