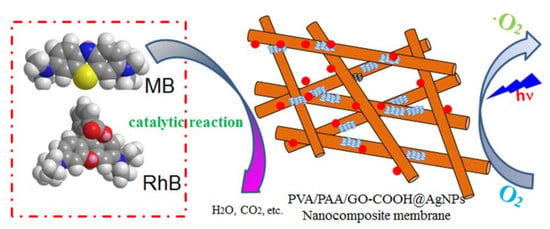
Self-Assembled AgNP-Containing Nanocomposites Constructed by Electrospinning as Efficient Dye Photocatalyst Materials for Wastewater Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
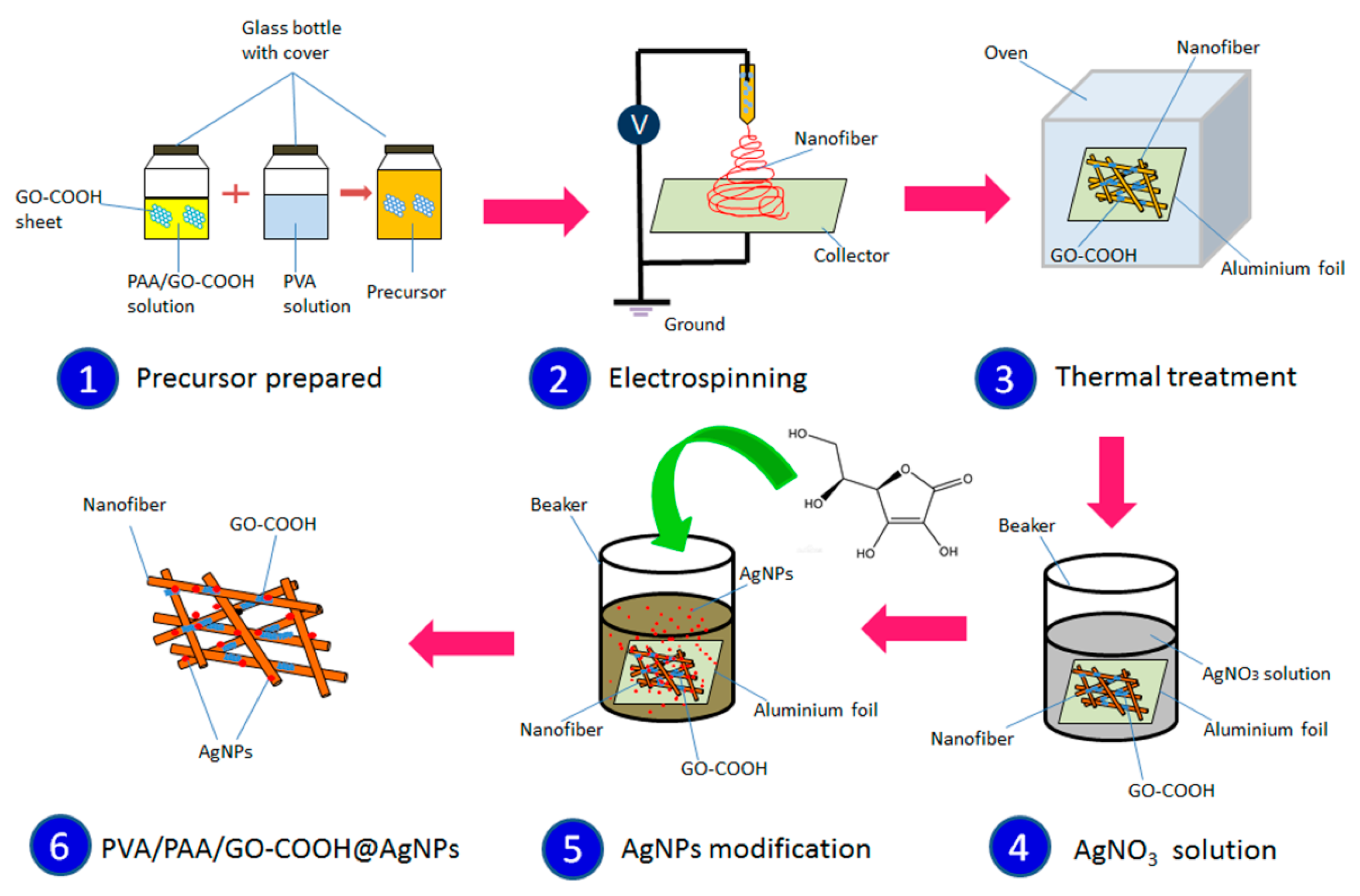
2.2. Preparation of Electrospun Composites
2.3. Catalytic Tests
2.4. Characterization
3. Results and Discussion
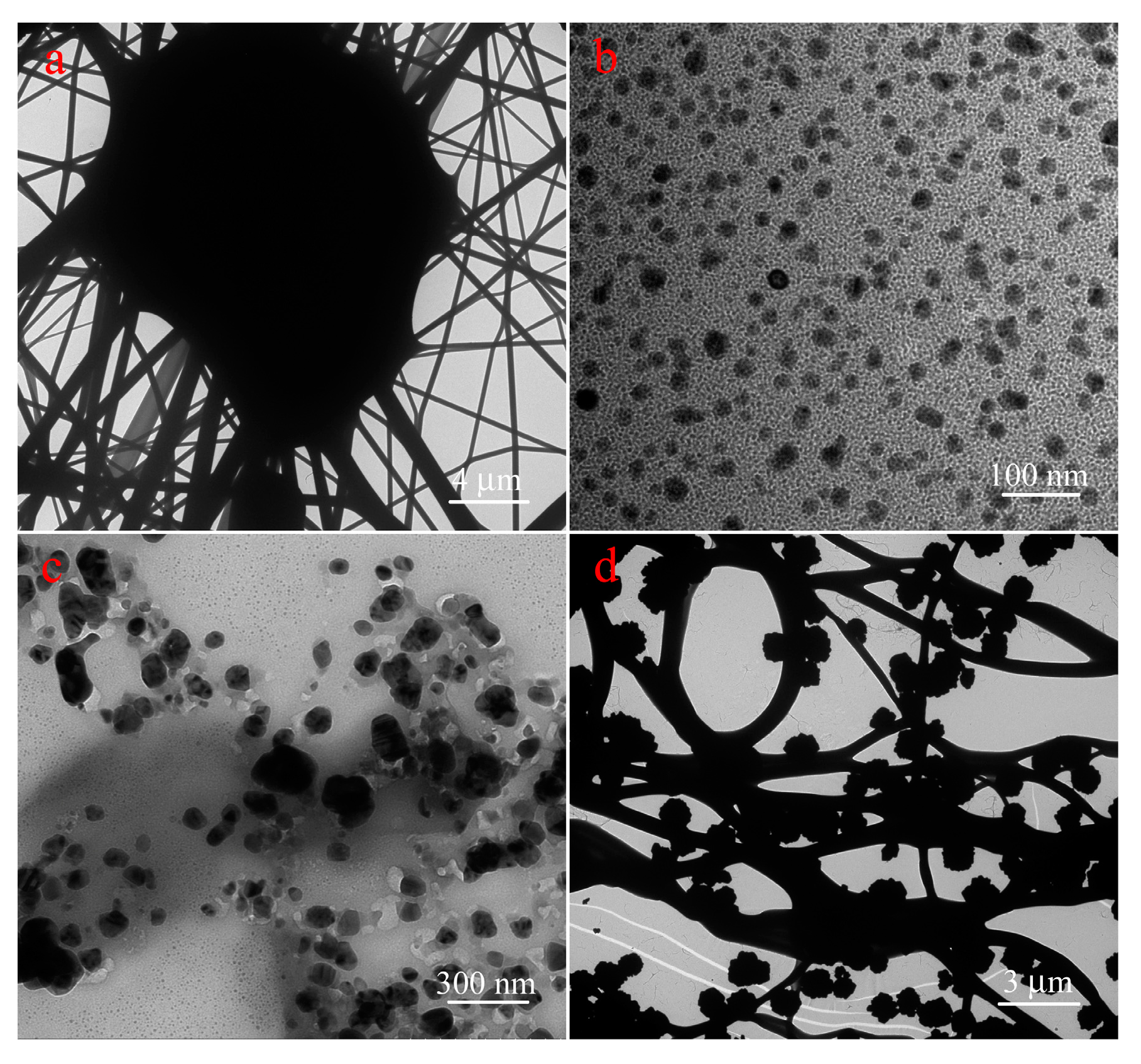
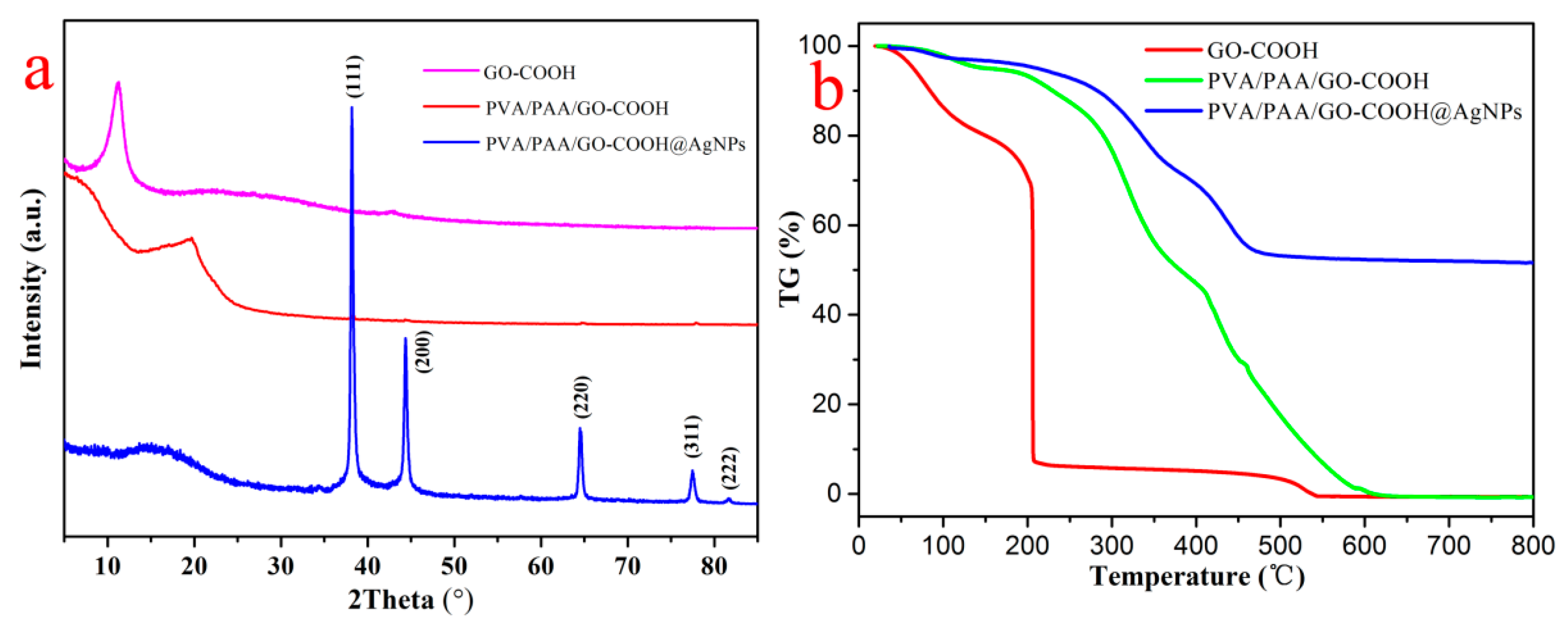
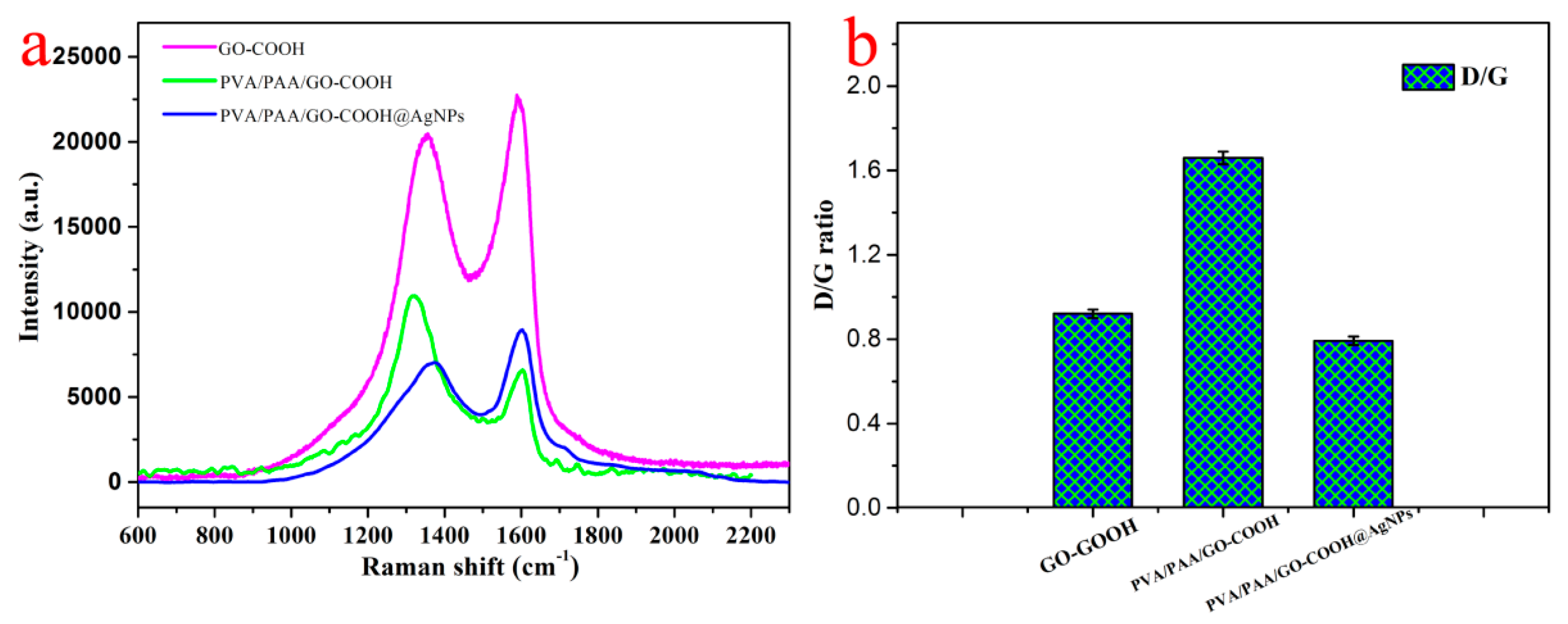
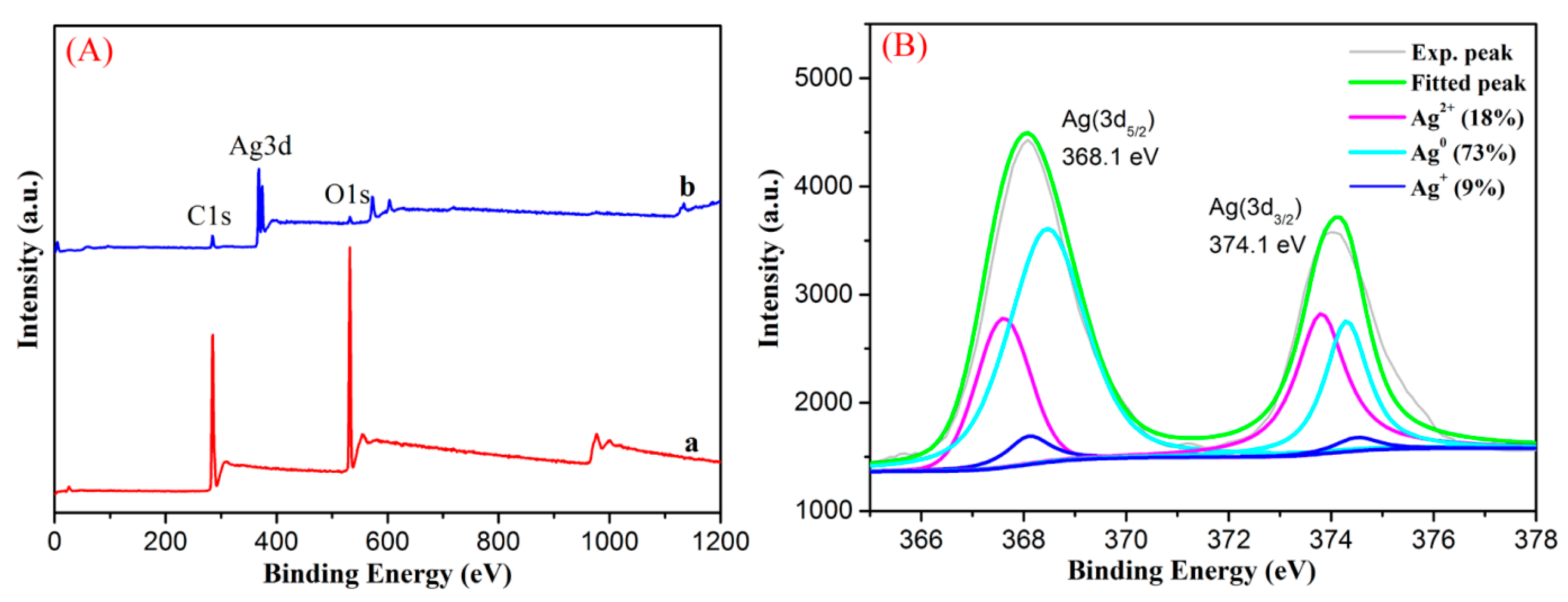
3.1. Characterization of Nanocomposites
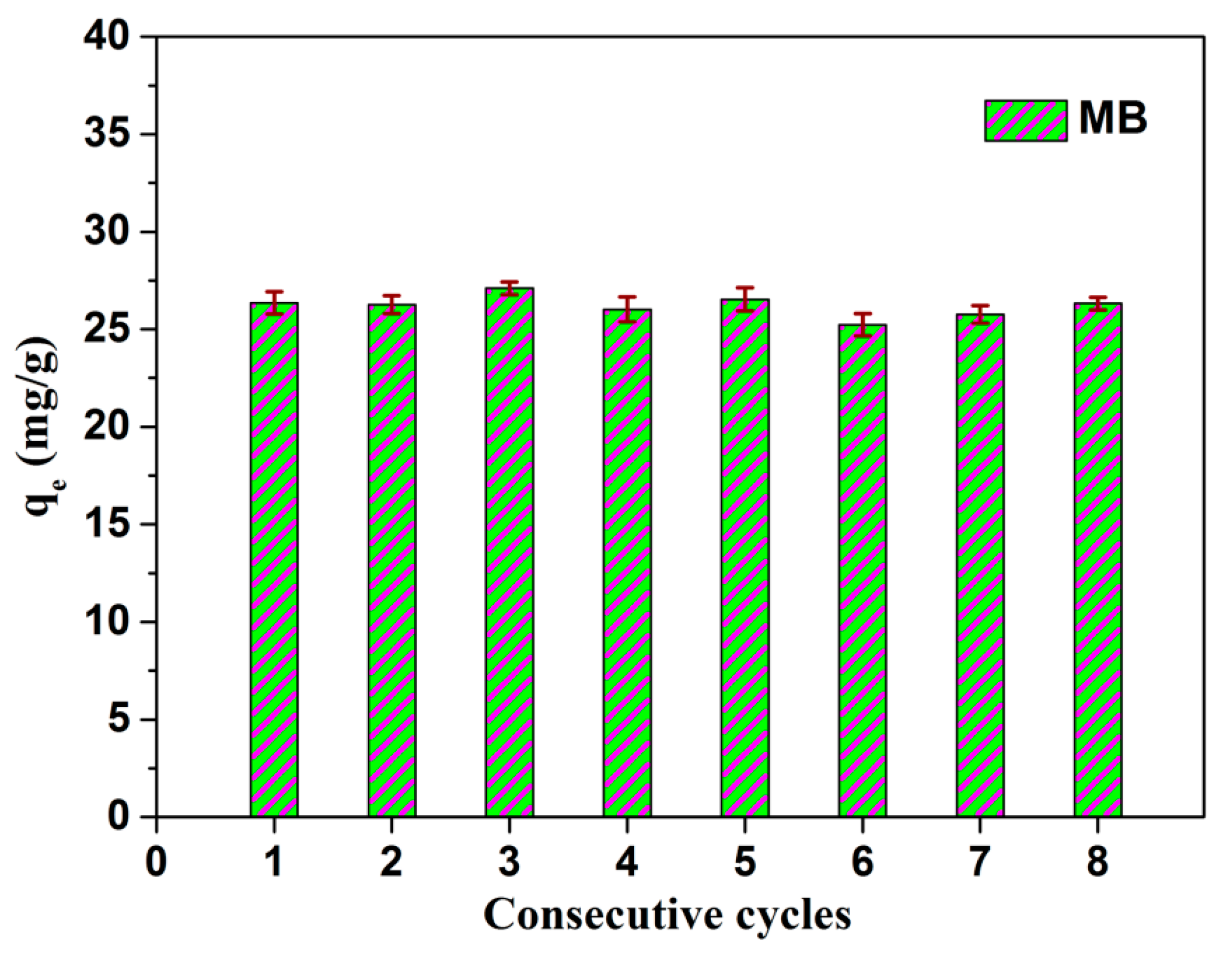
3.2. Catalytic Performances
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, Y.R.; Shen, S.L.; Wang, S.Q.; Huang, J.; Su, P.; Wang, Q.R.; Zhao, B.X. A dual function magnetic nanomaterial modified with lysine for removal of organic dyes from water solution. Chem. Eng. J. 2014, 239, 250–256. [Google Scholar] [CrossRef]
- Ding, Q.W.; Miao, Y.E.; Liu, T. Morphology and photocatalytic property of hierarchical polyimide/ZnO fibers prepared via a direct ion-exchange process. ACS Appl. Mater. Interfaces 2013, 5, 5617–5622. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Szlinder-Richert, J.; Usydus, Z.; Malesa-Ciecwierz, M.; Polak-Juszczak, L.; Ruczynska, W. Marine and farmed fish on the Polish market: Comparison of the nutritive value and human exposure to PCDD/Fs and other contaminants. Chemosphere 2011, 85, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- Lewis, N.S. Light work with water. Nature 2001, 414, 589–590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, C.; Ma, W.; Zhao, J. Visible-light-induced Aerobic oxidation of alcohols in a coupled photocatalytic system of dye-sensitized TiO2 and TEMPO & dagger. Angew. Chem. Int. Ed. 2008, 47, 9730–9733. [Google Scholar]
- Kalathil, S.; Khan, M.M.; Ansari, S.A.; Lee, J.; Cho, M.H. Band gap narrowing of titanium dioxide (TiO2) nanocrystals by electrochemically active biofilms and their visible light activity. Nanoscale 2013, 5, 6323–6326. [Google Scholar] [CrossRef] [PubMed]
- Litter, M.I. Heterogeneous photocatalysis: Transition metal ions in photocatalytic systems. Appl. Catal. B-Environ. 1999, 23, 89–114. [Google Scholar] [CrossRef]
- Gratzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.H.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J. Am. Chem. Soc. 2006, 128, 2115–2120. [Google Scholar] [CrossRef] [PubMed]
- Kiran, P.P.; Bhaktha, B.N.S.; Rao, D.N.; De, G. Nonlinear optical properties and surface-plasmon enhanced optical limiting in Ag–Cu nanoclusters co-doped in SiO2 Sol-Gel films. J. Appl. Phys. 2004, 96, 6717–6723. [Google Scholar] [CrossRef]
- Fei, J.; Li, J. Controlled preparation of porous TiO2-Ag nanostructures through supramolecular assembly for plasmon-enhanced photocatalysis. Adv. Mater. 2015, 27, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.F.; Wei, W.; Mao, D.J.; Chen, C.; Shi, Y.F.; Lv, X.M.; Xie, J.M. Silver-loaded nitrogen-doped yolk-shell mesoporous TiO2 hollow microspheres with enhanced visible light photocatalytic activity. Nanoscale 2015, 7, 784–797. [Google Scholar] [CrossRef] [PubMed]
- Atarod, M.; Nasrollahzadeh, M.; Sajadi, S.M. Euphorbia heterophylla leaf extract mediated green synthesis of Ag/TiO2 nanocomposite and investigation of its excellent catalytic activity for reduction of variety of dyes in water. J. Colloid Interface Sci. 2016, 462, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.Z.; Cao, C.Y.; Li, S.H.; Tang, Y.X.; Wang, L.N.; Qi, N.; Huang, J.Y.; Zhang, K.Q.; Al-Deyab, S.S.; Lai, Y.K. In situ plasmonic Ag nanoparticle anchored TiO2 nanotube arrays as visible-light-driven photocatalysts for enhanced water splitting. Nanoscale 2016, 8, 5226–5234. [Google Scholar] [CrossRef] [PubMed]
- Sung, D.I.; Kim, H.G.; Cha, S.K.; Kim, D.H.; Lee, H.B.R.; Kim, S.O.; Kim, D.W.; Yeom, G.Y. Photocatalytic effect of Ag/TiO2 nanotubes fabricated using 40 nm-scale BCP lithography. Nanosci. Nanotechnol. Lett. 2017, 9, 50–55. [Google Scholar] [CrossRef]
- Singh, J.; Satpati, B.; Mohapatra, S. Structural, Optical and Plasmonic Properties of Ag-TiO2 hybrid plasmonic nanostructures with enhanced photocatalytic activity. Plasmonics 2017, 12, 877–888. [Google Scholar] [CrossRef]
- Wang, T.; Wei, J.X.; Shi, H.M.; Zhou, M.; Zhang, Y.; Chen, Q.; Zhang, Z.M. Preparation of electrospun Ag/TiO2 nanotubes with enhanced photocatalytic activity based on water/oil phase separation. Physica E 2017, 86, 103–110. [Google Scholar] [CrossRef]
- Alsharaeh, E.H.; Bora, T.; Soliman, A.; Ahmed, F.; Bharath, G.; Ghoniem, M.G.; Abu-Salah, K.M.; Dutta, J. Sol-gel-assisted microwave-derived synthesis of anatase Ag/TiO2/GO nanohybrids toward efficient visible light phenol degradation. Catalysts 2017, 7, 133. [Google Scholar] [CrossRef]
- Prikrylova, K.; Polievkova, E.; Drbohlavova, J.; Vesela, M.; Hubalek, J. Nanostructured titania decorated with silver nanoparticles for photocatalytic water disinfection. Monatshefte für Chemie-Chemical Monthly 2017, 148, 1913–1919. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, Z.; Li, X.L.; Wang, H.Y.; Zhu, G. Novel Ag/TiO2 hierarchical hollow spheres composite with enhanced photocatalytic performance. J. Nanosci. Nanotechnol. 2017, 17, 2041–2046. [Google Scholar] [CrossRef]
- Misra, M.; Singh, N.; Gupta, R.K. Enhanced visible-light-driven photocatalytic activity of Au@Ag core-shell bimetallic nanoparticles immobilized on electrospun TiO2 nanofibers for degradation of organic compounds. Catal. Sci. Technol. 2017, 7, 570–580. [Google Scholar] [CrossRef]
- Jia, X.H.; Dai, R.R.; Lian, D.D.; Han, S.; Wu, X.Y.; Song, H.J. Facile synthesis and enhanced magnetic, photocatalytic properties of one-dimensional Ag@Fe3O4-TiO2. Appl. Surf. Sci. 2017, 392, 268–276. [Google Scholar] [CrossRef]
- Duan, Y.Y.; Zhang, M.; Wang, L.; Wang, F.; Yang, L.P.; Li, X.Y.; Wang, C.Y. Plasmonic Ag-TiO2−x nanocomposites for the photocatalytic removal of NO under visible light with high selectivity: The role of oxygen vacancies. Appl. Catal. B-Environ. 2017, 204, 67–77. [Google Scholar] [CrossRef]
- Choi, Y.; Koo, M.S.; Bokare, A.D.; Kim, D.H.; Bahnemann, D.W.; Choi, W. Sequential process combination of photocatalytic oxidation and dark reduction for the removal of organic pollutants and Cr(VI) using Ag/TiO2. Environ. Sci. Technol. 2017, 51, 3973–3981. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.X.; Ni, C.H.; Jiu, H.F.; Xie, C.M.; Yan, J.B.; Qi, G.S. One-pot synthesis of Ag-TiO2/reduced graphene oxide nanocomposite for high performance of adsorption and photocatalysis. Ceram. Int. 2017, 43, 5450–5456. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, T.; Zhou, M.; Wang, Y.; Zhang, Z.M. Hydrothermal preparation of Ag-TiO2 nanostructures with exposed {001}/{101} facets for enhancing visible light photocatalytic activity. Ceram. Int. 2017, 43, 3118–3126. [Google Scholar] [CrossRef]
- Greiner, A.; Wendorff, J.H. Electrospinning: A fascinating method for the preparation of ultrathin fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Patra, P.K.; Lovett, M.L.; Kaplan, D.L.; Bhowmick, S. Role of electrospun fibre diameter and corresponding specific surface area (SSA) on cell attachment. J. Tissue Eng. Regen. Med. 2009, 3, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Huang, Y.; Miao, Y.E.; Weng, W.T.; Liu, T. Polydopamine-coated electrospun poly(vinyl alcohol)/poly(acrylic acid) membranes as efficient dye adsorbent with good recyclability. J. Hazard. Mater. 2015, 283, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Wang, W.; Jiao, T.; Ma, K.; Zhang, Q.; Hong, W.; Qiu, H.; Zhou, J.; Zhang, L.; Peng, Q. Bioinspired polydopamine sheathed nanofibers containing carboxylate graphene oxide nanosheet for high-efficient dyes scavenger. ACS Sustain. Chem. Eng. 2017, 5, 4948–4956. [Google Scholar] [CrossRef]
- Villarreal-Gómez, L.J.; Cornejo-Bravo, J.M.; Vera-Graziano, R.; Grande, D. Electrospinning as a powerful technique for biomedical applications: A critically selected survey. J. Biomater. Sci. Polym. Ed. 2016, 27, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Ma, K.; Jiao, T.; Xing, R.; Li, K.; Zhou, J.; Zhang, L. Preparation and dye removal capacities of porous silver nanoparticle-containing composite hydrogels via poly(acrylic acid) and silver ions. RSC Adv. 2016, 6, 110799–110807. [Google Scholar] [CrossRef]
- Narayan, R.; Kim, J.E.; Kim, J.Y.; Lee, K.E.; Kim, S.O. Graphene oxide liquid crystals: Discovery, evolution and applications. Adv. Mater. 2016, 28, 3045–3068. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Hou, H.Q.; Wendorff, J.H.; Greiner, A. Electrospun poly(vinyl alcohol)/poly(acrylic acid) fibres with excellent water-stability. E-Polymers 2004, 4, 899–906. [Google Scholar] [CrossRef]
- Li, D.; Mueller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Orth, E.S.; Fonsaca, J.E.S.; Domingues, S.H.; Mehl, H.; Oliveira, M.M.; Zarbin, A.J.G. Targeted thiolation of graphene oxide and its utilization as precursor for graphene/silver nanoparticles composites. Carbon 2013, 61, 543–550. [Google Scholar] [CrossRef]
- Basturk, E.; Demir, S.; Danis, O.; Kahraman, M.V. Covalent immobilization of a-amylase onto thermally crosslinked electrospun PVA/PAA nanofibrous hybrid membranes. J. Appl. Polym. Sci. 2013, 127, 349–355. [Google Scholar] [CrossRef]
- Xu, X.; Yang, Q.; Wang, Y.; Yu, H.; Chen, X.; Jing, X. Biodegradable electrospun poly(l-lactide) fibers containing antibacterial silver nanoparticles. Eur. Polym. J. 2006, 42, 2081–2087. [Google Scholar] [CrossRef]
- Konwer, S.; Boruah, R.; Dolui, S.K. Studies on conducting polypyrrole/graphene oxide composites as supercapacitor electrode. J. Electron. Mater. 2011, 40, 2248–2255. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, S.; Tripathi, B.; Singh, M.; Vijay, Y.K. Aligned CNT/polymer nanocomposite membranes for hydrogen separation. Int. J. Hydrogen Energy 2009, 34, 3977–3982. [Google Scholar] [CrossRef]
- Xue, P.; Lu, R.; Chen, G.; Zhang, Y.; Nomoto, H.; Takafuji, M.; Ihara, H. Functional organogel based on a salicylideneaniline derivative with enhanced fluorescence emission and photochromism. Chem. Eur. J. 2007, 13, 8231–8239. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Jiao, T.; Liu, Y.; Ma, K.; Zou, Q.; Ma, G.; Yan, X. Co-assembly of graphene oxide and albumin/photosensitizer nanohybrids towards enhanced photodynamic therapy. Polymers 2016, 8, 181. [Google Scholar] [CrossRef]
- Luo, X.; Ma, K.; Jiao, T.; Xing, R.; Zhang, L.; Zhou, J.; Li, B. Graphene oxide-polymer composite Langmuir films constructed by interfacial thiol-ene photopolymerization. Nanoscale Res. Lett. 2017, 12, 99. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Jiao, T.; Xing, R.; Chen, Y.; Guo, W.; Zhou, J.; Zhang, L.; Peng, Q. Hierarchical AuNPs-loaded Fe3O4/polymers nanocomposites constructed by electrospinning with enhanced and magnetically recyclable catalytic capacities. Nanomaterials 2017, 7, 317. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Park, M.; Shin, H.K.; Pant, B.; Choi, J.; Park, Y.W.; Lee, J.Y.; Park, S.J.; Kim, H.-Y. Facile preparation and characterization of poly(vinyl alcohol)/chitosan/graphene oxide biocomposite nanofibers. J. Ind. Eng. Chem. 2014, 20, 4415–4420. [Google Scholar] [CrossRef]
- Kudin, K.N.; Ozbas, B.; Schniepp, H.C.; Prudhomme, R.K.; Aksay, I.A.; Car, R. Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett. 2008, 8, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.H.; Kim, P.; Choi, J.Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O. Bacteriorhodopsin as a superior substitute for hydrazine in chemical reduction of single-layer graphene oxide sheets. Carbon 2015, 81, 158–166. [Google Scholar] [CrossRef]
- Guo, R.; Jiao, T.; Li, R.; Chen, Y.; Guo, W.; Zhang, L.; Zhou, J.; Zhang, Q.; Peng, Q. Sandwiched Fe3O4/carboxylate graphene oxide nanostructures constructed by layer-by-layer assembly for highly efficient and magnetically recyclable dye removal. ACS Sustain. Chem. Eng. 2018, 6, 1279–1288. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Y.; Jiao, T.; Xing, R.; Yang, Z.; Fan, J.; Liu, J.; Li, B.; Peng, Q. Preparation and enhanced structural integrity of electrospun poly(ε-caprolactone)-based fibers by freezing amorphous chains through thiol-ene click reaction. Colloid Surf. A-Physicochem. Eng. Asp. 2018, 538, 7–13. [Google Scholar] [CrossRef]
- Zhang, R.; Xing, R.; Jiao, T.; Ma, K.; Chen, C.; Ma, G.; Yan, X. Carrier-free, chemo-photodynamic dual nanodrugs via self-assembly for synergistic antitumor therapy. ACS Appl. Mater. Interfaces 2016, 8, 13262–13269. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.; Duan, P.; Jiao, T.; Peng, Q.; Liu, M. Self-assembled luminescent quantum dots to generate full-color and white circularly polarized light. Angew. Chem. Int. Ed. 2017, 56, 12174–12178. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ma, K.; Jiao, T.; Xing, R.; Ma, X.; Hu, J.; Huang, H.; Zhang, L.; Yan, X. Fabrication of hierarchical layer-by-layer assembled diamond based core-shell nanocomposites as highly efficient dye absorbents for wastewater treatment. Sci. Rep. 2017, 7, 44076. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Jiao, T.; Zhang, Q.; Guo, W.; Peng, Q.; Yan, X. Preparation of graphene oxide-based hydrogels as efficient dye adsorbents for wastewater treatment. Nanoscale Res. Lett. 2015, 10, 272. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Jiao, T.; Xing, R.; Zou, G.; Zhou, J.; Zhang, L.; Peng, Q. Fabrication of tunable hierarchical MXene@AuNPs nanocomposites constructed by self-reduction reactions with enhanced catalytic performances. Sci. China Mater. 2018. [Google Scholar] [CrossRef]
- Jiao, T.; Ma, K.; Xing, R.; Zhang, L.; Zhou, J. Recent progress on peptide-regulated self-assembly of chromophores nanoarchitectonics and applications. J. YanShan Univ. 2017, 41, 1–12. [Google Scholar]
- Jiao, T.; Chen, K.; Zhang, L. Research progress on preparation and application of self-assembled nanofilms. J. YanShan Univ. 2017, 41, 377–386. [Google Scholar]
- Jiao, T.; Huang, X.; Zhang, L.; Zhou, J. Research progress on syntheses of nanomaterials based on photothermal agent/photosensitizer and applications. J. YanShan Univ. 2017, 41, 189–203. [Google Scholar]
- Xing, R.; Liu, K.; Jiao, T.; Zhang, N.; Ma, K.; Zhang, R.; Zou, Q.; Ma, G.; Yan, X. An injectable self-assembling collagen-gold hybrid hydrogel for combinatorial antitumor photothermal/photodynamic therapy. Adv. Mater. 2016, 28, 3669–3676. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yang, W.; Kong, L.; Song, A.; Qin, X.; Shao, G. Phosphorus-doped 3D hierarchical porous carbon for high-performance supercapacitors: A balanced strategy for pore structure and chemical composition. Carbon 2018, 127, 557–567. [Google Scholar] [CrossRef]
- Yang, W.; Yang, W.; Song, A.; Sun, G.; Shao, G. 3D interconnected porous carbon nanosheets/carbon nanotubes as a polysulfide reservoir for high performance lithium–sulfur batteries. Nanoscale 2018, 10, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yang, W.; Song, A.; Gao, L.; Sun, G.; Shao, G. Pyrrole as a promising electrolyte additive to trap polysulfides for lithium-sulfur batteries. J. Power Sources 2017, 348, 175–182. [Google Scholar] [CrossRef]
- Lopez-Salido, I.; Lim, D.C.; Kim, Y.D. Ag nanoparticles on highly ordered pyrolytic graphite (HOPG) surfaces studied using STM and XPS. Surf. Sci. 2005, 588, 6–18. [Google Scholar] [CrossRef]
- Wang, P.; Huang, B.; Qin, X.; Zhang, X.; Dai, Y.; Whangbo, M.H. Ag/AgBr/WO3 center dot H2O: Visible-light Photocatalyst for Bacteria Destruction. Inorg. Chem. 2009, 48, 10697–10702. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Huang, B.; Lou, Z.; Zhang, X.; Qin, X.; Dai, Y.; Zheng, Z.; Wang, X. Synthesis of highly efficient Ag@AgCl plasmonic photocatalysts with various structures. Chem.-Eur. J. 2010, 16, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Huang, B.; Zhang, Q.; Zhang, X.; Qin, X.; Dai, Y.; Zhan, J.; Yu, J.; Liu, H.; Lou, Z. Highly efficient visible light plasmonic photocatalyst Ag@Ag(Br,I). Chem.-Eur. J. 2010, 16, 10042–10047. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Xing, R.; Jiao, T.; Peng, Q.; Yuan, C.; Möhwald, H.; Yan, X. Crystalline dipeptide nanobelts based on solid-solid phase transformation self-assembly and their polarization imaging of cells. ACS Appl. Mater. Interfaces 2018. [Google Scholar] [CrossRef] [PubMed]

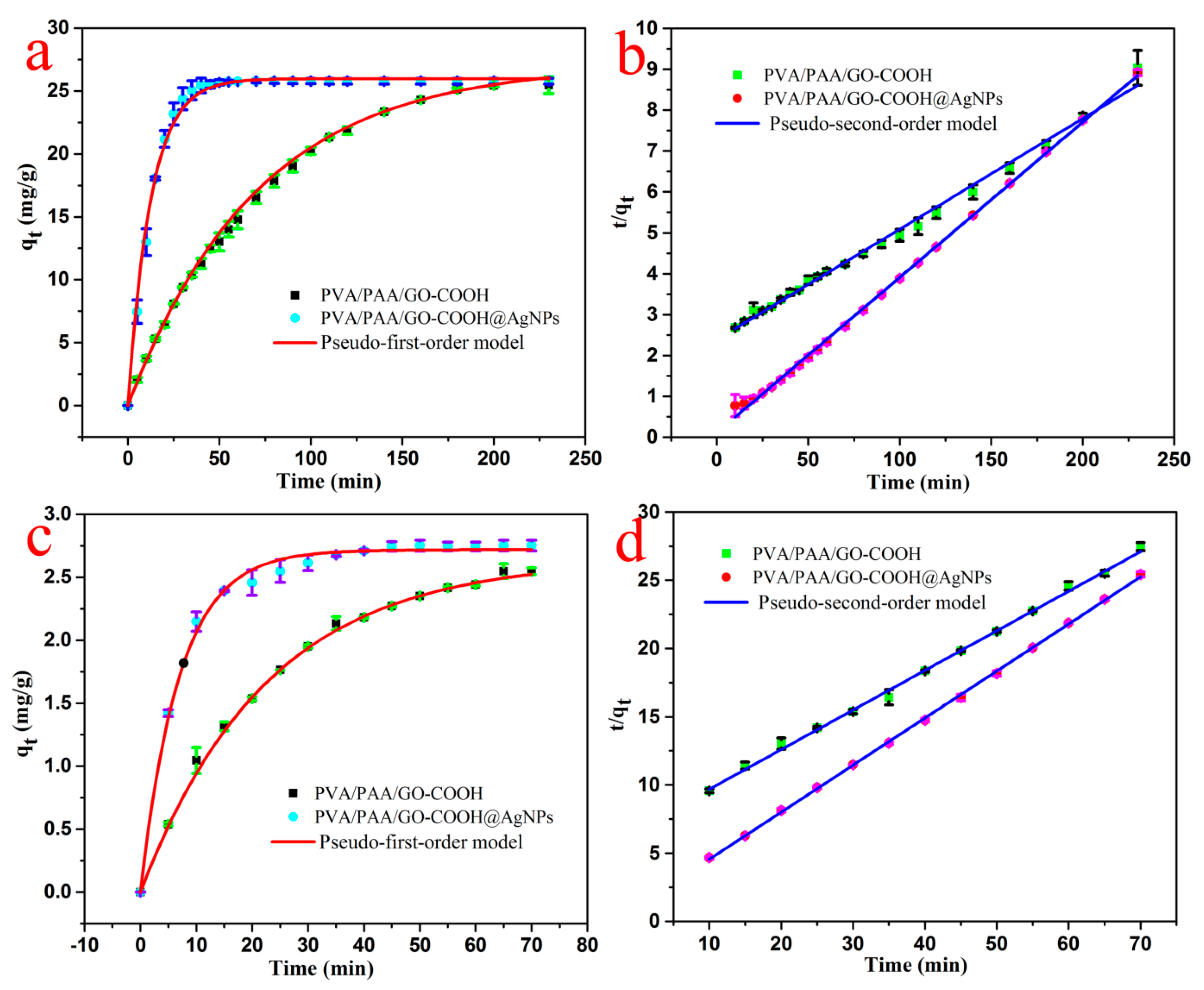
| RhB | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
| qe (mg/g) | R2 | K1 (min−1) | qe (mg/g) | R2 | K2 (g/mg·min) | |
| PVA/PAA/GO-COOH | 2.64 | 0.9981 | 0.0442 | 3.43 | 0.9976 | 0.0125 |
| PVA/PAA/GO-COOH@AgNPs | 2.71 | 0.9972 | 0.1445 | 2.90 | 0.9996 | 0.1072 |
| MB | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
| qe (mg/g) | R2 | K1 (min−1) | qe (mg/g) | R2 | K2 (g/mg·min) | |
| PVA/PAA/GO-COOH | 27.19 | 0.9989 | 0.01405 | 36.97 | 0.9932 | 2.07 × 10−4 |
| PVA/PAA/GO-COOH@AgNPs | 26.01 | 0.9945 | 0.07784 | 26.36 | 0.9988 | 1.20 × 10−2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Hou, C.; Jiao, T.; Song, J.; Zhang, X.; Xing, R.; Zhou, J.; Zhang, L.; Peng, Q. Self-Assembled AgNP-Containing Nanocomposites Constructed by Electrospinning as Efficient Dye Photocatalyst Materials for Wastewater Treatment. Nanomaterials 2018, 8, 35. https://doi.org/10.3390/nano8010035
Liu Y, Hou C, Jiao T, Song J, Zhang X, Xing R, Zhou J, Zhang L, Peng Q. Self-Assembled AgNP-Containing Nanocomposites Constructed by Electrospinning as Efficient Dye Photocatalyst Materials for Wastewater Treatment. Nanomaterials. 2018; 8(1):35. https://doi.org/10.3390/nano8010035
Chicago/Turabian StyleLiu, Yamei, Caili Hou, Tifeng Jiao, Jingwen Song, Xu Zhang, Ruirui Xing, Jingxin Zhou, Lexin Zhang, and Qiuming Peng. 2018. "Self-Assembled AgNP-Containing Nanocomposites Constructed by Electrospinning as Efficient Dye Photocatalyst Materials for Wastewater Treatment" Nanomaterials 8, no. 1: 35. https://doi.org/10.3390/nano8010035
APA StyleLiu, Y., Hou, C., Jiao, T., Song, J., Zhang, X., Xing, R., Zhou, J., Zhang, L., & Peng, Q. (2018). Self-Assembled AgNP-Containing Nanocomposites Constructed by Electrospinning as Efficient Dye Photocatalyst Materials for Wastewater Treatment. Nanomaterials, 8(1), 35. https://doi.org/10.3390/nano8010035