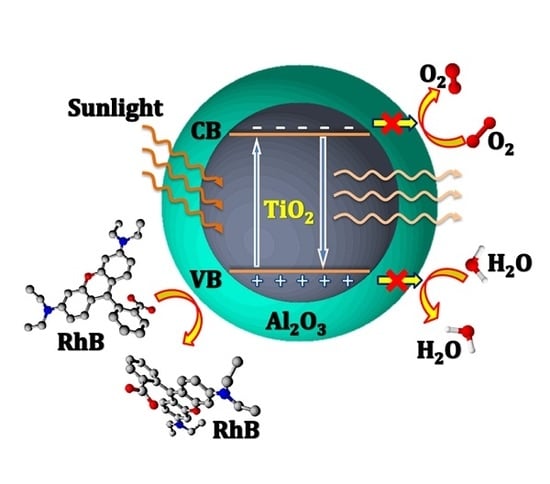
Suppressing the Photocatalytic Activity of TiO2 Nanoparticles by Extremely Thin Al2O3 Films Grown by Gas-Phase Deposition at Ambient Conditions
Abstract
1. Introduction
2. Reaction Mechanism of Al2O3 ALD Using TMA and H2O: A Brief Overview
3. Results and Discussion
3.1. Properties of the Al2O3 Coating Layers
3.1.1. Morphology of Al2O3-Coated TiO2
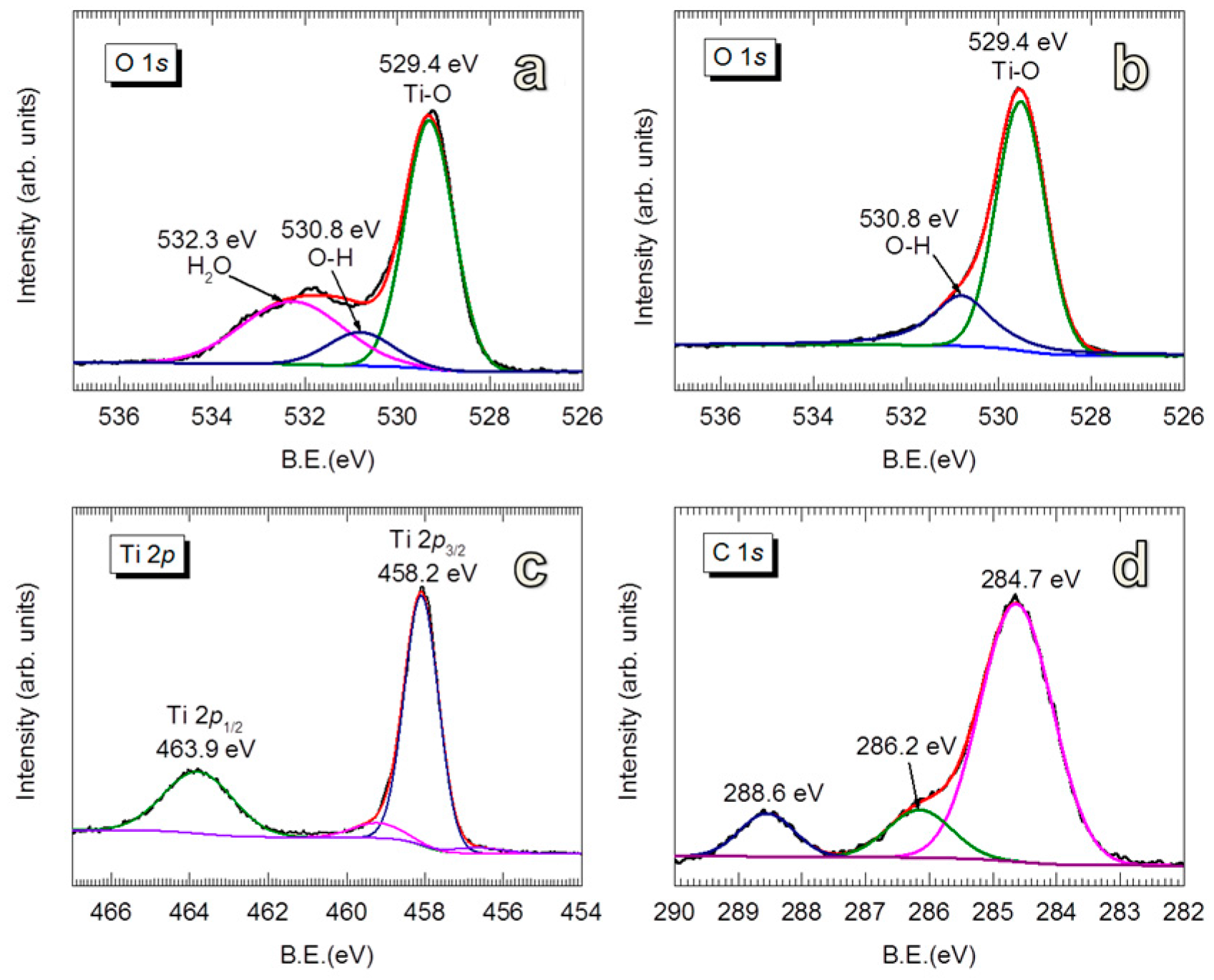
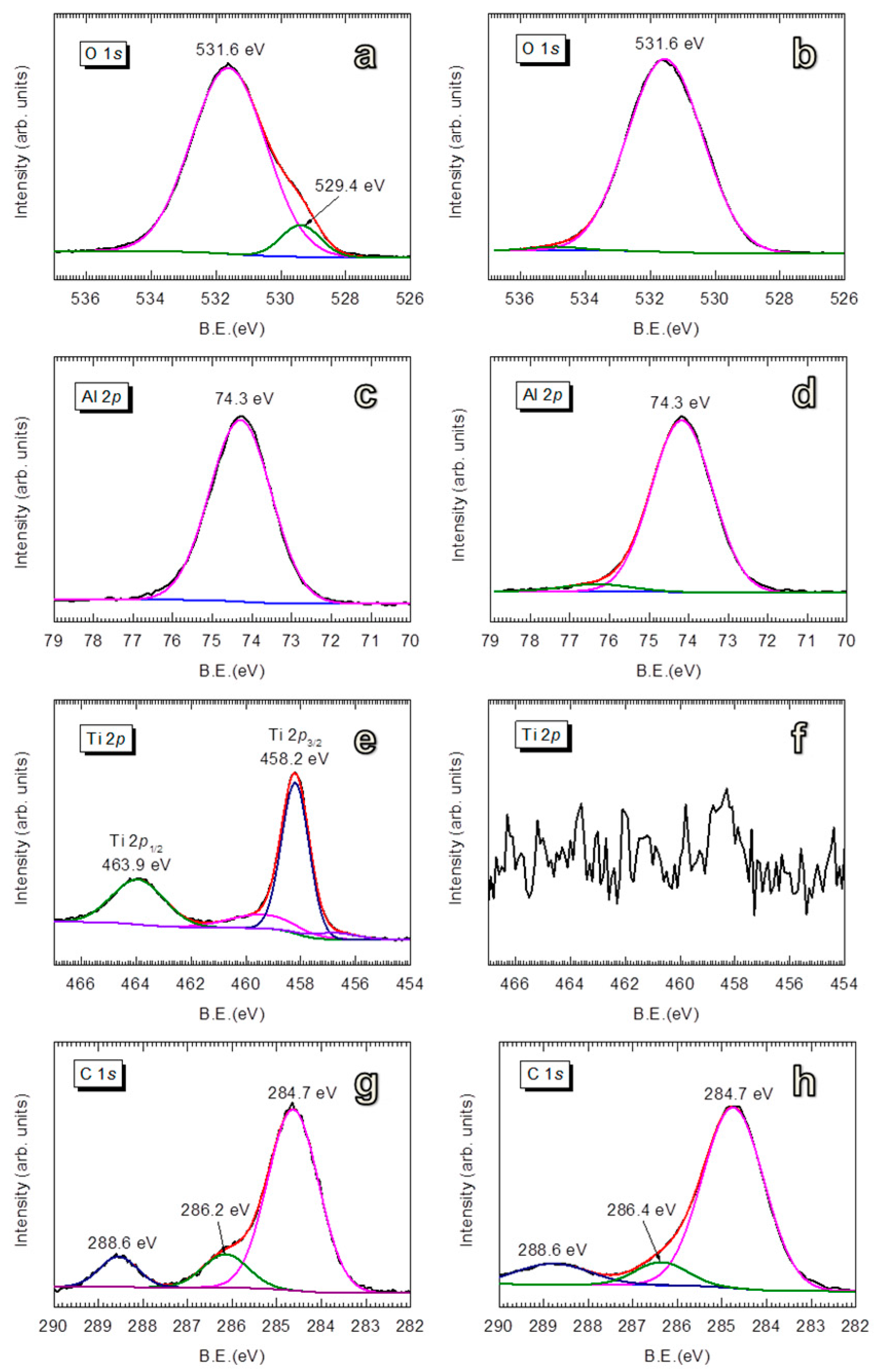
3.1.2. Structural and Optical Properties of Room-Temperature-Grown Al2O3
3.1.3. Thermogravimetric Analysis
3.2. Photocatalytic Activity of Al2O3-Coated TiO2
3.2.1. Dependence of Photocatalytic Suppression Ability of Al2O3 on Film Thickness
3.2.2. Influence of High-Temperature Calcination on Photocatalytic Suppression Ability of Al2O3
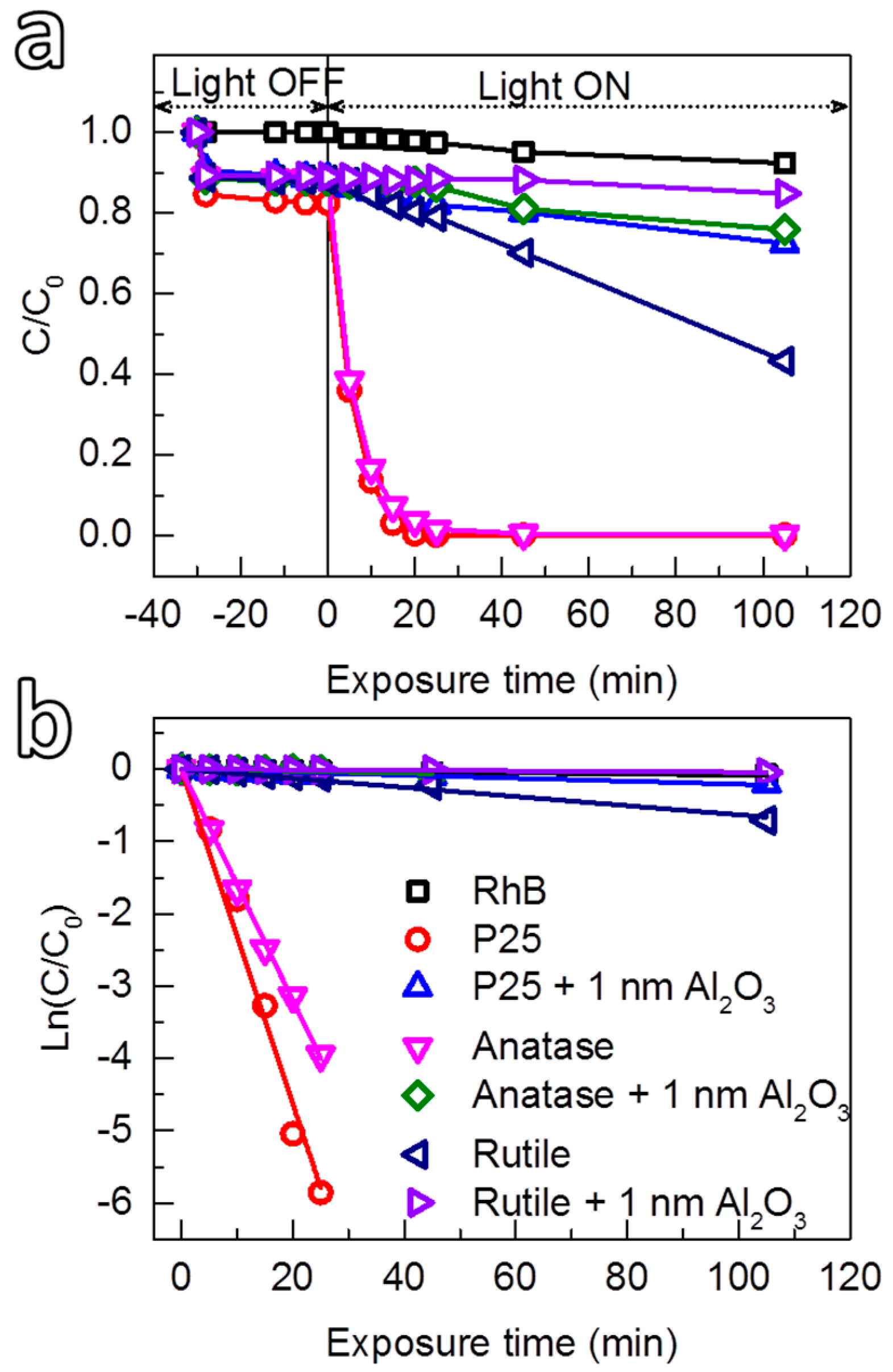
3.2.3. Photocatalytic Suppression Ability of Al2O3 on P25 and Rutile TiO2
4. Experimental Section
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shi, H.; Magaye, R.; Castranova, V.; Zhao, J. Titanium dioxide nanoparticles: A review of current toxicological data. Part. Fibre Toxicol. 2013, 10, 15–47. [Google Scholar] [CrossRef] [PubMed]
- Gettens, R.J.; Kühn, H.; Chase, W.T. Lead white. Stud. Conserv. 1967, 12, 125–139. [Google Scholar]
- Van Driel, B.A.; Wezendonk, T.A.; van den Berg, K.J.; Kooyman, P.J.; Gascon, J.; Dik, J. Determination of early warning signs for photocatalytic degradation of titanium white oil paints by means of surface analysis. Spectrochim. Acta Part A 2017, 172, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Yousif, E.; Haddad, R. Photodegradation and photostabilization of polymers, especially polystyrene: Review. SpringerPlus 2013, 2, 398. [Google Scholar] [CrossRef] [PubMed]
- Völz Hans, G.; Kaempf, G.; Fitzky, H.G.; Klaeren, A. The chemical nature of chalking in the presence of titanium dioxide pigments. In Photodegradation and Photostabilization of Coatings; Winslow, F.H., Ed.; ACS Symposium Series; ACS Publiising: Washington, DC, USA, 1981; Volume 151, pp. 163–182. [Google Scholar]
- Samain, L.; Silversmit, G.; Sanyova, J.; Vekemans, B.; Salomon, H.; Gilbert, B.; Grandjean, F.; Long, G.J.; Hermann, R.P.; Vincze, L.; et al. Fading of modern prussian blue pigments in linseed oil medium. J. Anal. At. Spectrom. 2011, 26, 930–941. [Google Scholar] [CrossRef]
- Van Driel, B.A.; Kooyman, P.J.; van den Berg, K.J.; Schmidt-Ott, A.; Dik, J. A quick assessment of the photocatalytic activity of TiO2 pigments—From lab to conservation studio! Microchem. J. 2016, 126, 162–171. [Google Scholar] [CrossRef]
- Lee, H.; Koo, S.; Yoo, J. TiO2–SiO2 nanoparticles for suppressing photocatalytic activities and improving hydrophilicity. J. Ceram. Process. Res. 2012, 13, S300–S303. [Google Scholar]
- Park, O.K.; Kang, Y.S. Preparation and characterization of silica-coated TiO2 nanoparticle. Colloids Surf. A 2005, 257, 261–265. [Google Scholar] [CrossRef]
- Ren, Y.; Chen, M.; Zhang, Y.; Wu, L. Fabrication of rattle-type TiO2/SiO2 core/shell particles with both high photoactivity and UV-shielding property. Langmuir 2010, 26, 11391–11396. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ge, C.; Ren, M.; Yin, H.; Wang, A.; Zhang, D.; Liu, C.; Chen, J.; Feng, H.; Yao, H.; et al. Effects of coating parameters on the morphology of SiO2-coated TiO2 and the pigmentary properties. Appl. Surf. Sci. 2008, 254, 2809–2819. [Google Scholar] [CrossRef]
- Wu, H.-X.; Wang, T.-J.; Jin, Y. Morphology “phase diagram” of the hydrous alumina coating on TiO2 particles during aqueous precipitation. Ind. Eng. Chem. Res. 2006, 45, 5274–5278. [Google Scholar] [CrossRef]
- Simpson, D.J.; Thilagam, A.; Cavallaro, G.P.; Kaplun, K.; Gerson, A.R. SiO2 coated pure and doped titania pigments: Low temperature CVD deposition and quantum chemical study. Phys. Chem. Chem. Phys. 2011, 13, 21132–21138. [Google Scholar] [CrossRef] [PubMed]
- King, D.M.; Liang, X.; Burton, B.B.; Kamal Akhtar, M.; Weimer, A.W. Passivation of pigment-grade TiO2 particles by nanothick atomic layer deposited SiO2 films. Nanotechnology 2008, 19, 255604. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Barrett, K.S.; Jiang, Y.-B.; Weimer, A.W. Rapid silica atomic layer deposition on large quantities of cohesive nanoparticles. ACS Appl. Mater. Interfaces 2010, 2, 2248–2253. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yuan, S.; Yu, Y.; van Ommen, J.R.; Van Bui, H.; Liang, B. Room-temperature pulsed CVD-grown SiO2 protective layer on TiO2 particles for photocatalytic activity suppression. RSC Adv. 2017, 7, 4547–4554. [Google Scholar] [CrossRef]
- Seidel, G.R. Production of Improved Titanium Pigments. U.S. Patent 2,387,534, 23 October 1945. [Google Scholar]
- Wei, B.-X.; Zhao, L.; Wang, T.-J.; Jin, Y. Detrimental thixotropic thinning of filter cake of SiO2–Al2O3 composite coated TiO2 particles and its control. Ind. Eng. Chem. Res. 2011, 50, 13799–13804. [Google Scholar] [CrossRef]
- Wei, B.-X.; Zhao, L.; Wang, T.-J.; Gao, H.; Wu, H.-X.; Jin, Y. Photo-stability of TiO2 particles coated with several transition metal oxides and its measurement by Rhodamine-B degradation. Adv. Powder Technol. 2013, 24, 708–713. [Google Scholar] [CrossRef]
- Van Bui, H.; Grillo, F.; van Ommen, J.R. Atomic and molecular layer deposition: Off the beaten track. Chem. Commun. 2017, 53, 45–71. [Google Scholar] [CrossRef] [PubMed]
- Puurunen, R.L. Surface chemistry of atomic layer deposition: A case study for the trimethylaluminum/water process. J. Appl. Phys. 2005, 97, 121301. [Google Scholar] [CrossRef]
- George, S.M. Atomic layer deposition: An overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Puurunen, R.L. A short history of atomic layer deposition: Tuomo suntola’s atomic layer epitaxy. Chem. Vap. Depos. 2014, 20, 332–344. [Google Scholar] [CrossRef]
- Malygin, A.A.; Drozd, V.E.; Malkov, A.A.; Smirnov, V.M. From V. B. Aleskovskii’s “framework” hypothesis to the method of molecular layering/atomic layer deposition. Chem. Vap. Depos. 2015, 21, 216–240. [Google Scholar] [CrossRef]
- Lu, J.; Elam, J.W.; Stair, P.C. Atomic layer deposition—Sequential self-limiting surface reactions for advanced catalyst “bottom-up” synthesis. Surf. Sci. Rep. 2016, 71, 410–472. [Google Scholar] [CrossRef]
- Johnson, R.W.; Hultqvist, A.; Bent, S.F. A brief review of atomic layer deposition: From fundamentals to applications. Mater. Today 2014, 17, 236–246. [Google Scholar] [CrossRef]
- Ferguson, J.; Weimer, A.; George, S. Atomic layer deposition of ultrathin and conformal Al2O3 films on BN particles. Thin Solid Films 2000, 371, 95–104. [Google Scholar] [CrossRef]
- Ferguson, J.D.; Weimer, A.W.; George, S.M. Atomic layer deposition of SiO2 films on BN particles using sequential surface reactions. Chem. Mater. 2000, 12, 3472–3480. [Google Scholar] [CrossRef]
- Ferguson, J.; Weimer, A.; George, S. Atomic layer deposition of Al2O3 films on polyethylene particles. Chem. Mater. 2004, 16, 5602–5609. [Google Scholar] [CrossRef]
- Ferguson, J.; Smith, E.; Weimer, A.; George, S. ALD of SiO2 at room temperature using TEOS and H2O with NH3 as the catalyst. J. Electrochem. Soc. 2004, 151, G528–G535. [Google Scholar] [CrossRef]
- Wank, J.R.; George, S.M.; Weimer, A.W. Coating fine nickel particles with Al2O3 utilizing an atomic layer deposition-fluidized bed reactor (ALD–FBR). J. Am. Ceram. Soc. 2004, 87, 762–765. [Google Scholar] [CrossRef]
- McCormick, J.A.; Rice, K.P.; Paul, D.F.; Weimer, A.W.; George, S.M. Analysis of Al2O3 atomic layer deposition on ZrO2 nanoparticles in a rotary reactor. Chem. Vap. Depos. 2007, 13, 491–498. [Google Scholar] [CrossRef]
- Hakim, L.F.; George, S.M.; Weimer, A.W. Conformal nanocoating of zirconia nanoparticles by atomic layer deposition in a fluidized bed reactor. Nanotechnology 2005, 16, S375–S381. [Google Scholar] [CrossRef] [PubMed]
- Valdesueiro, D.; Meesters, G.; Kreutzer, M.; van Ommen, J. Gas-phase deposition of ultrathin aluminium oxide films on nanoparticles at ambient conditions. Materials 2015, 8, 1249–1263. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.-L.; Deng, Z.; Cao, K.; Yin, H.-F.; Shan, B.; Chen, R. Surface passivation of Fe3O4 nanoparticles with Al2O3 via atomic layer deposition in a rotating fluidized bed reactor. J. Vac. Sci. Technol. A 2016, 34, 04C103. [Google Scholar] [CrossRef]
- Manandhar, K.; Wollmershauser, J.A.; Boercker, J.E.; Feigelson, B.N. Growth per cycle of alumina atomic layer deposition on nano- and micro-powders. J. Vac. Sci. Technol. A 2016, 34, 021519. [Google Scholar] [CrossRef]
- McCormick, J.A.; Cloutier, B.L.; Weimer, A.W.; George, S.M. Rotary reactor for atomic layer deposition on large quantities of nanoparticles. J. Vac. Sci. Technol. A 2007, 25, 67–74. [Google Scholar] [CrossRef]
- King, D.M.; Spencer, J.A.; Liang, X.; Hakim, L.F.; Weimer, A.W. Atomic layer deposition on particles using a fluidized bed reactor with in situ mass spectrometry. Surf. Coat. Technol. 2007, 201, 9163–9171. [Google Scholar] [CrossRef]
- Beetstra, R.; Lafont, U.; Nijenhuis, J.; Kelder, E.M.; van Ommen, J.R. Atmospheric pressure process for coating particles using atomic layer deposition. Chem. Vap. Depos. 2009, 15, 227–233. [Google Scholar] [CrossRef]
- Hakim, L.F.; King, D.M.; Zhou, Y.; Gump, C.J.; George, S.M.; Weimer, A.W. Nanoparticle coating for advanced optical, mechanical and rheological properties. Adv. Funct. Mater. 2007, 17, 3175–3181. [Google Scholar] [CrossRef]
- Ritala, M.; Leskelä, M.; Dekker, J.P.; Mutsaers, C.; Soininen, P.J.; Skarp, J. Perfectly conformal tin and Al2O3 films deposited by atomic layer deposition. Chem. Vap. Depos. 1999, 5, 7–9. [Google Scholar] [CrossRef]
- Elam, J.W.; Routkevitch, D.; Mardilovich, P.P.; George, S.M. Conformal coating on ultrahigh-aspect-ratio nanopores of anodic alumina by atomic layer deposition. Chem. Mater. 2003, 15, 3507–3517. [Google Scholar] [CrossRef]
- Dendooven, J.; Deduytsche, D.; Musschoot, J.; Vanmeirhaeghe, R.L.; Detavernier, C. Conformality of Al2O3 and AlN deposited by plasma-enhanced atomic layer deposition. J. Electrochem. Soc. 2010, 157, G111–G116. [Google Scholar] [CrossRef]
- Berland, B.S.; Gartland, I.P.; Ott, A.W.; George, S.M. In situ monitoring of atomic layer controlled pore reduction in alumina tubular membranes using sequential surface reactions. Chem. Mater. 1998, 10, 3941–3950. [Google Scholar] [CrossRef]
- Elam, J.W.; Xiong, G.; Han, C.Y.; Wang, H.H.; Birrell, J.P.; Welp, U.; Hryn, J.N.; Pellin, M.J.; Baumann, T.F.; Poco, J.F.; et al. Atomic layer deposition for the conformal coating of nanoporous materials. J. Nanomater. 2006, 2006, 64501. [Google Scholar] [CrossRef]
- Levrau, E.; Van de Kerckhove, K.; Devloo-Casier, K.; Pulinthanathu Sree, S.; Martens, J.A.; Detavernier, C.; Dendooven, J. In situ IR spectroscopic investigation of alumina ALD on porous silica films: Thermal versus plasma-enhanced ALD. J. Phys. Chem. C 2014, 118, 29854–29859. [Google Scholar] [CrossRef]
- Valdesueiro, D.; Prabhu, M.K.; Guerra-Nunez, C.; Sandeep, C.S.S.; Kinge, S.; Siebbeles, L.D.A.; de Smet, L.C.P.M.; Meesters, G.M.H.; Kreutzer, M.T.; Houtepen, A.J.; et al. Deposition mechanism of aluminum oxide on quantum dot films at atmospheric pressure and room temperature. J. Phys. Chem. C 2016, 120, 4266–4275. [Google Scholar] [CrossRef]
- Mirvakili, M.N.; Van Bui, H.; van Ommen, J.R.; Hatzikiriakos, S.G.; Englezos, P. Enhanced barrier performance of engineered paper by atomic layer deposited Al2O3 thin films. ACS Appl. Mater. Interfaces 2016, 8, 13590–13600. [Google Scholar] [CrossRef] [PubMed]
- Farmer, D.B.; Gordon, R.G. Atomic layer deposition on suspended single-walled carbon nanotubes via gas-phase noncovalent functionalization. Nano Lett. 2006, 6, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, A.S.; Wilson, C.A.; Weimer, A.W.; George, S.M. Atomic layer deposition on gram quantities of multi-walled carbon nanotubes. Nanotechnology 2009, 20, 255602. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Park, S.-Y.; Kim, H.-C.; Cho, K.; Vogel, E.M.; Kim, M.J.; Wallace, R.M.; Kim, J. Conformal Al2O3 dielectric layer deposited by atomic layer deposition for graphene-based nanoelectronics. Appl. Phys. Lett. 2008, 92, 203102. [Google Scholar] [CrossRef]
- Young, M.J.; Musgrave, C.B.; George, S.M. Growth and characterization of Al2O3 atomic layer deposition films on sp2-graphitic carbon substrates using NO2/trimethylaluminum pretreatment. ACS Appl. Mater. Interfaces 2015, 7, 12030–12037. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Cheng, X.; Cao, D.; Wang, G.; Wang, Z.; Xu, D.; Xia, C.; Shen, L.; Yu, Y.; Shen, D. Improvement of Al2O3 films on graphene grown by atomic layer deposition with pre-H2O treatment. ACS Appl. Mater. Interfaces 2014, 6, 7014–7019. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.; Grubbs, R.; George, S. Nucleation and growth during Al2O3 atomic layer deposition on polymers. Chem. Mater. 2005, 17, 5625–5634. [Google Scholar] [CrossRef]
- Knez, M.; Kadri, A.; Wege, C.; Gösele, U.; Jeske, H.; Nielsch, K. Atomic layer deposition on biological macromolecules: Metal oxide coating of tobacco mosaic virus and ferritin. Nano Lett. 2006, 6, 1172–1177. [Google Scholar] [CrossRef] [PubMed]
- Dillon, A.; Ott, A.; Way, J.; George, S. Surface chemistry of Al2O3 deposition using Al(CH3)3 and H2O in a binary reaction sequence. Surf. Sci. 1995, 322, 230–242. [Google Scholar] [CrossRef]
- Rahtu, A.; Alaranta, T.; Ritala, M. In situ quartz crystal microbalance and quadrupole mass spectrometry studies of atomic layer deposition of aluminum oxide from trimethylaluminum and water. Langmuir 2001, 17, 6506–6509. [Google Scholar] [CrossRef]
- Widjaja, Y.; Musgrave, C.B. Quantum chemical study of the mechanism of aluminum oxide atomic layer deposition. Appl. Phys. Lett. 2002, 80, 3304–3306. [Google Scholar] [CrossRef]
- Elliott, S.D.; Greer, J.C. Simulating the atomic layer deposition of alumina from first principles. J. Mater. Chem. 2004, 14, 3246–3250. [Google Scholar] [CrossRef]
- Puurunen, R.L.; Vandervorst, W.; Besling, W.F.A.; Richard, O.; Bender, H.; Conard, T.; Zhao, C.; Delabie, A.; Caymax, M.; De Gendt, S.; et al. Island growth in the atomic layer deposition of zirconium oxide and aluminum oxide on hydrogen-terminated silicon: Growth mode modeling and transmission electron microscopy. J. Appl. Phys. 2004, 96, 4878–4889. [Google Scholar] [CrossRef]
- Groner, M.; Fabreguette, F.; Elam, J.; George, S. Low-temperature Al2O3 atomic layer deposition. Chem. Mater. 2004, 16, 639–645. [Google Scholar] [CrossRef]
- Wind, R.A.; George, S.M. Quartz crystal microbalance studies of Al2O3 atomic layer deposition using trimethylaluminum and water at 125 °C. J. Phys. Chem. A 2009, 114, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- Potts, S.E.; Keuning, W.; Langereis, E.; Dingemans, G.; van de Sanden, M.C.M.; Kessels, W.M.M. Low temperature plasma-enhanced atomic layer deposition of metal oxide thin films. J. Electrochem. Soc. 2010, 157, P66–P74. [Google Scholar] [CrossRef]
- Shirazi, M.; Elliott, S.D. Cooperation between adsorbates accounts for the activation of atomic layer deposition reactions. Nanoscale 2015, 7, 6311–6318. [Google Scholar] [CrossRef] [PubMed]
- Vandalon, V.; Kessels, W.M.M. What is limiting low-temperature atomic layer deposition of Al2O3? A vibrational sum-frequency generation study. Appl. Phys. Lett. 2016, 108, 011607. [Google Scholar] [CrossRef]
- Guerra-Nuñez, C.; Döbeli, M.; Michler, J.; Utke, I. Reaction and growth mechanisms in Al2O3 deposited via atomic layer deposition: Elucidating the hydrogen source. Chem. Mater. 2017, 29, 8690–8703. [Google Scholar] [CrossRef]
- Lownsbury, J.M.; Gladden, J.A.; Campbell, C.T.; Kim, I.S.; Martinson, A.B.F. Direct measurements of half-cycle reaction heats during atomic layer deposition by calorimetry. Chem. Mater. 2017, 29, 8566–8577. [Google Scholar] [CrossRef]
- Puurunen, R.L.; Lindblad, M.; Root, A.; Outi, I.; Krause, A. Successive reactions of gaseous trimethylaluminium and ammonia on porous alumina. Phys. Chem. Chem. Phys. 2001, 3, 1093–1102. [Google Scholar] [CrossRef]
- Ishida, M.; Eto, A.; Nakamura, T.; Suzaki, T. Decomposition of trimethylaluminum and N2O on Si surfaces using ultraviolet laser photolysis to produce Al2O3 films. J. Vac. Sci. Technol. A 1989, 7, 2931–2934. [Google Scholar] [CrossRef]
- Gow, T.; Lin, R.; Cadwell, L.; Lee, F.; Backman, A.; Masel, R.I. Decomposition of trimethylaluminum on silicon (100). Chem. Mater. 1989, 1, 406–411. [Google Scholar] [CrossRef]
- Mayer, T.M.; Rogers, J., Jr.; Michalske, T. Mechanism of nucleation and atomic layer growth of aluminum nitride on silicon. Chem. Mater. 1991, 3, 641–646. [Google Scholar] [CrossRef]
- Van Bui, H.; Wiggers, F.; Friedlein, R.; Yamada-Takamura, Y.; Kovalgin, A.; de Jong, M. On the feasibility of silicene encapsulation by AlN deposited using an atomic layer deposition process. J. Chem. Phys. 2015, 142, 064702. [Google Scholar] [CrossRef] [PubMed]
- Juppo, M.; Rahtu, A.; Ritala, M.; Leskelä, M. In situ mass spectrometry study on surface reactions in atomic layer deposition of Al2O3 thin films from trimethylaluminum and water. Langmuir 2000, 16, 4034–4039. [Google Scholar] [CrossRef]
- Ylivaara, O.M.E.; Liu, X.; Kilpi, L.; Lyytinen, J.; Schneider, D.; Laitinen, M.; Julin, J.; Ali, S.; Sintonen, S.; Berdova, M.; et al. Aluminum oxide from trimethylaluminum and water by atomic layer deposition: The temperature dependence of residual stress, elastic modulus, hardness and adhesion. Thin Solid Films 2014, 552, 124–135. [Google Scholar] [CrossRef]
- Liang, X.; Hakim, L.F.; Zhan, G.-D.; McCormick, J.A.; George, S.M.; Weimer, A.W.; Spencer, J.A.; Buechler, K.J.; Blackson, J.; Wood, C.J.; et al. Novel processing to produce polymer/ceramic nanocomposites by atomic layer deposition. J. Am. Ceram. Soc. 2007, 90, 57–63. [Google Scholar] [CrossRef]
- Jensen, H.; Soloviev, A.; Li, Z.; Søgaard, E.G. XPS and FTIR investigation of the surface properties of different prepared titania nano-powders. Appl. Surf. Sci. 2005, 246, 239–249. [Google Scholar] [CrossRef]
- Benkoula, S.; Sublemontier, O.; Patanen, M.; Nicolas, C.; Sirotti, F.; Naitabdi, A.; Gaie-Levrel, F.; Antonsson, E.; Aureau, D.; Ouf, F.X.; et al. Water adsorption on TiO2 surfaces probed by soft X-ray spectroscopies: Bulk materials vs. Isolated nanoparticles. Sci. Rep. 2015, 5, 15088. [Google Scholar] [CrossRef] [PubMed]
- Jankovský, O.; Šimek, P.; Sedmidubský, D.; Huber, Š.; Pumera, M.; Sofer, Z. Towards highly electrically conductive and thermally insulating graphene nanocomposites: Al2O3–graphene. RSC Adv. 2014, 4, 7418–7424. [Google Scholar] [CrossRef]
- Chen, A.H.; Liang, L.Y.; Zhang, H.Z.; Liu, Z.M.; Ye, X.J.; Yu, Z.; Cao, H.T. Enhancement of a-IZO TTFT performance by using Y2O3/Al2O3 bilayer dielectrics. Electrochem. Solid-State Lett. 2011, 14, H88–H92. [Google Scholar] [CrossRef]
- Iatsunskyi, I.; Kempiński, M.; Jancelewicz, M.; Załęski, K.; Jurga, S.; Smyntyna, V. Structural and XPS characterization of ALD Al2O3 coated porous silicon. Vacuum 2015, 113, 52–58. [Google Scholar] [CrossRef]
- Reddy, K.M.; Manorama, S.V.; Reddy, A.R. Bandgap studies on anatase titanium dioxide nanoparticles. Mater. Chem. Phys. 2003, 78, 239–245. [Google Scholar] [CrossRef]
- Spicer, P.T.; Artelt, C.; Sanders, S.; Pratsinis, S.E. Flame synthesis of composite carbon black-fumed silica nanostructured particles. J. Aerosol Sci. 1998, 29, 647–659. [Google Scholar] [CrossRef]
- Mueller, R.; Kammler, H.K.; Wegner, K.; Pratsinis, S.E. OH surface density of SiO2 and TiO2 by thermogravimetric analysis. Langmuir 2003, 19, 160–165. [Google Scholar] [CrossRef]
- Moniz, S.J.A.; Tang, J. Charge transfer and photocatalytic activity in CuO/TiO2 nanoparticle heterojunctions synthesised through a rapid, one-pot, microwave solvothermal route. ChemCatChem 2015, 7, 1659–1667. [Google Scholar] [CrossRef]
- Jang, E.; Sridharan, K.; Park, Y.M.; Park, T.J. Eliminated phototoxicity of TiO2 particles by an atomic-layer-deposited Al2O3 coating layer for UV-protection applications. Chem. Eur. J. 2016, 22, 12022–12026. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Weimer, A.W. Photoactivity passivation of TiO2 nanoparticles using molecular layer deposited (MLD) polymer films. J. Nanopart. Res. 2009, 12, 135–142. [Google Scholar] [CrossRef]
- Gao, H.; Qiao, B.; Wang, T.-J.; Wang, D.; Jin, Y. Cerium oxide coating of titanium dioxide pigment to decrease its photocatalytic activity. Ind. Eng. Chem. Res. 2014, 53, 189–197. [Google Scholar] [CrossRef]
- Simonsen, M.E.; Li, Z.; Søgaard, E.G. Influence of the OH groups on the photocatalytic activity and photoinduced hydrophilicity of microwave assisted sol–gel TiO2 film. Appl. Surf. Sci. 2009, 255, 8054–8062. [Google Scholar] [CrossRef]
- Di Paola, A.; Bellardita, M.; Palmisano, L.; Barbieriková, Z.; Brezová, V. Influence of crystallinity and OH surface density on the photocatalytic activity of TiO2 powders. J. Photochem. Photobiol. A Chem. 2014, 273, 59–67. [Google Scholar] [CrossRef]
- Sclafani, A.; Palmisano, L.; Schiavello, M. Influence of the preparation methods of titanium dioxide on the photocatalytic degradation of phenol in aqueous dispersion. J. Phys. Chem. 1990, 94, 829–832. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, P.; Liu, J.; Yu, J. New understanding of the difference of photocatalytic activity among anatase, rutile and brookite TiO2. Phys. Chem. Chem. Phys. 2014, 16, 20382–20386. [Google Scholar] [CrossRef] [PubMed]



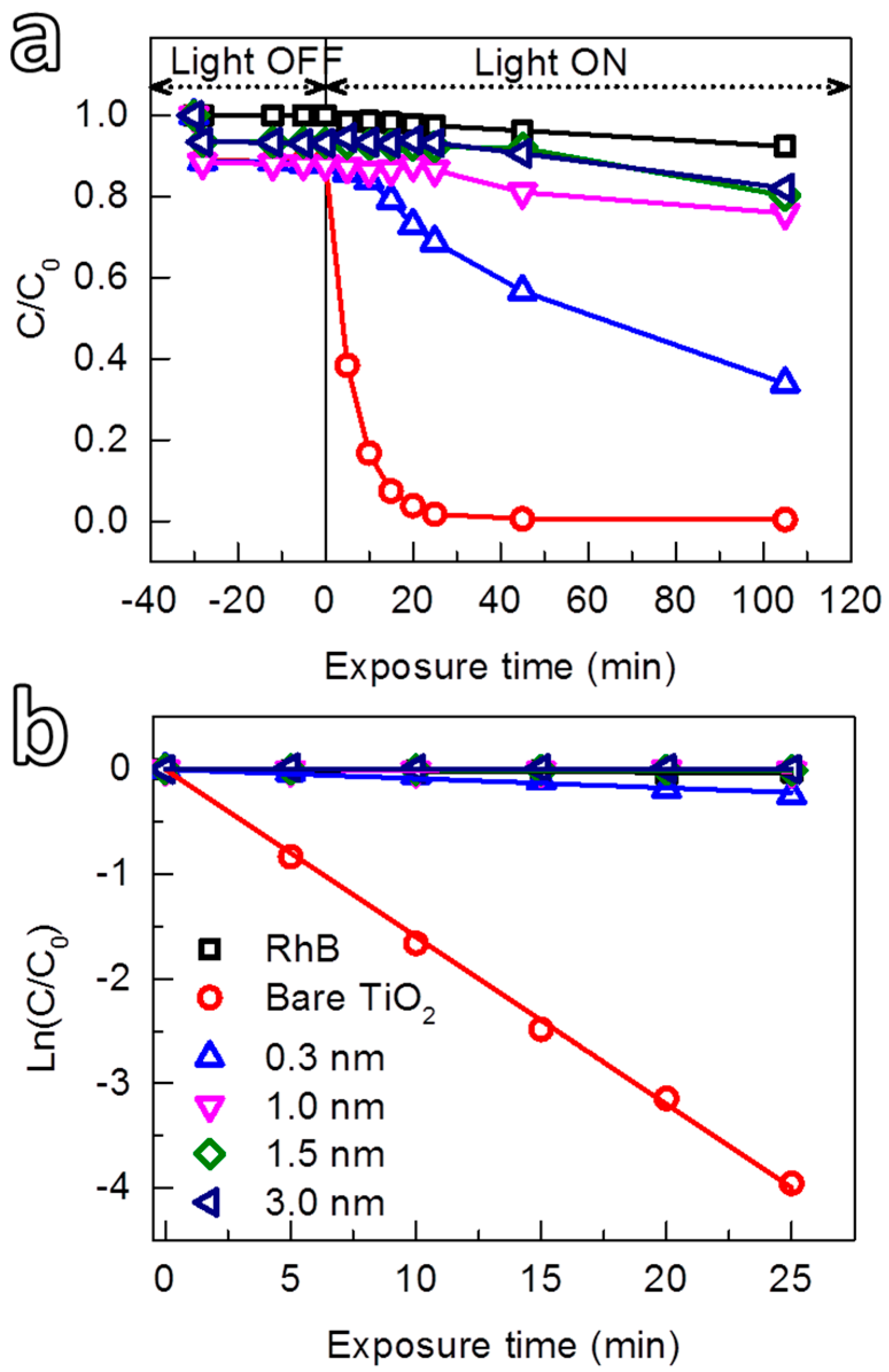
| Sample | kapp × 103/min−1 | R2 of Fitting | ||
|---|---|---|---|---|
| As-Deposited | Calcined | As-Deposited | Calcined | |
| RhB | 0.52 ± 0.03 | 0.52 ± 0.03 | 0.99 | 0.99 |
| Uncoated TiO2 | 160 ± 1.60 | 100 ± 4.12 | 1.00 | 0.99 |
| 0.3 nm Al2O3 coated TiO2 | 8.87 ± 0.71 | 19.3 ± 0.03 | 0.96 | 0.99 |
| 1.0 nm Al2O3 coated TiO2 | 0.64 ± 0.21 | 7.07 ± 0.53 | 0.90 | 0.96 |
| 1.5 nm Al2O3 coated TiO2 | 0.41 ± 0.02 | 4.77 ± 0.68 | 0.99 | 0.89 |
| 3.0 nm Al2O3 coated TiO2 | 0.35 ± 0.03 | 3.27 ± 0.80 | 0.98 | 0.72 |
| TiO2 Material | Average Diameter (nm) | Coating Method | Coating Material | Coating Thickness (nm) | Deposition Temperature (°C) | Photocat. Reaction | Ref. |
|---|---|---|---|---|---|---|---|
| P25 | 21 | ALD | Al2O3 | 6.0 | 177 | Methylene blue | [40] |
| Anatase Rutile | 160 280 | ALD | SiO2 | 2.0 | 500 for SiO2 177 for Al2O3 | IPA to acetone | [14] |
| SiO2/Al2O3 | 1.0/1.0 | ||||||
| SiO2/Al2O3/SiO2/Al2O3 | 0.5/0.5/0.5/0.5 | ||||||
| P25 | 21 | ALD | Al2O3 | 3.8 | 150 | RhB | [85] |
| Anatase | 160 | ALD | SiO2 | 6.0 | 175 | Methylene blue | [15] |
| P25 | 18 | 9.0 | |||||
| Anatase | 160 | MLD | Alucone | 7–10 | 100–160 | Methylene blue | [86] |
| Rutile | Not reported | CVD | SiO2 | 1–2 | 900–1000 | Methylene blue | [13] |
| Rutile | 300 | Wet-chemistry | ZrO2 | 5.0 | 40 | RhB | [19] |
| CeO2 | 1–2 | 60 | |||||
| Rutile | 300 | Wet-chemistry | CeO2 | 1–2 | 60 | RhB | [87] |
| ST-21 | 20 | Wet-chemistry | SiO2 | 4.0 | 40 | Methylene blue | [8] |
| P25 | 21 | Wet-chemistry | Porous SiO2 | 20.0 | Room temperature | RhB | [10] |
| Anatase Rutile P25 | 270 300 21 | ALD-like | Al2O3 | 1.0 | Room temperature | RhB | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Van Bui, H.; Valdesueiro, D.; Yuan, S.; Liang, B.; Van Ommen, J.R. Suppressing the Photocatalytic Activity of TiO2 Nanoparticles by Extremely Thin Al2O3 Films Grown by Gas-Phase Deposition at Ambient Conditions. Nanomaterials 2018, 8, 61. https://doi.org/10.3390/nano8020061
Guo J, Van Bui H, Valdesueiro D, Yuan S, Liang B, Van Ommen JR. Suppressing the Photocatalytic Activity of TiO2 Nanoparticles by Extremely Thin Al2O3 Films Grown by Gas-Phase Deposition at Ambient Conditions. Nanomaterials. 2018; 8(2):61. https://doi.org/10.3390/nano8020061
Chicago/Turabian StyleGuo, Jing, Hao Van Bui, David Valdesueiro, Shaojun Yuan, Bin Liang, and J. Ruud Van Ommen. 2018. "Suppressing the Photocatalytic Activity of TiO2 Nanoparticles by Extremely Thin Al2O3 Films Grown by Gas-Phase Deposition at Ambient Conditions" Nanomaterials 8, no. 2: 61. https://doi.org/10.3390/nano8020061
APA StyleGuo, J., Van Bui, H., Valdesueiro, D., Yuan, S., Liang, B., & Van Ommen, J. R. (2018). Suppressing the Photocatalytic Activity of TiO2 Nanoparticles by Extremely Thin Al2O3 Films Grown by Gas-Phase Deposition at Ambient Conditions. Nanomaterials, 8(2), 61. https://doi.org/10.3390/nano8020061