Preparation of Cuprous Oxide Mesoporous Spheres with Different Pore Sizes for Non-Enzymatic Glucose Detection
Abstract
:1. Introduction
2. Materials and Methods
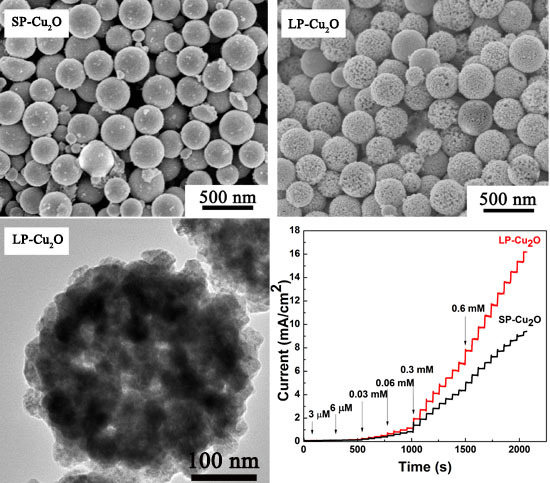
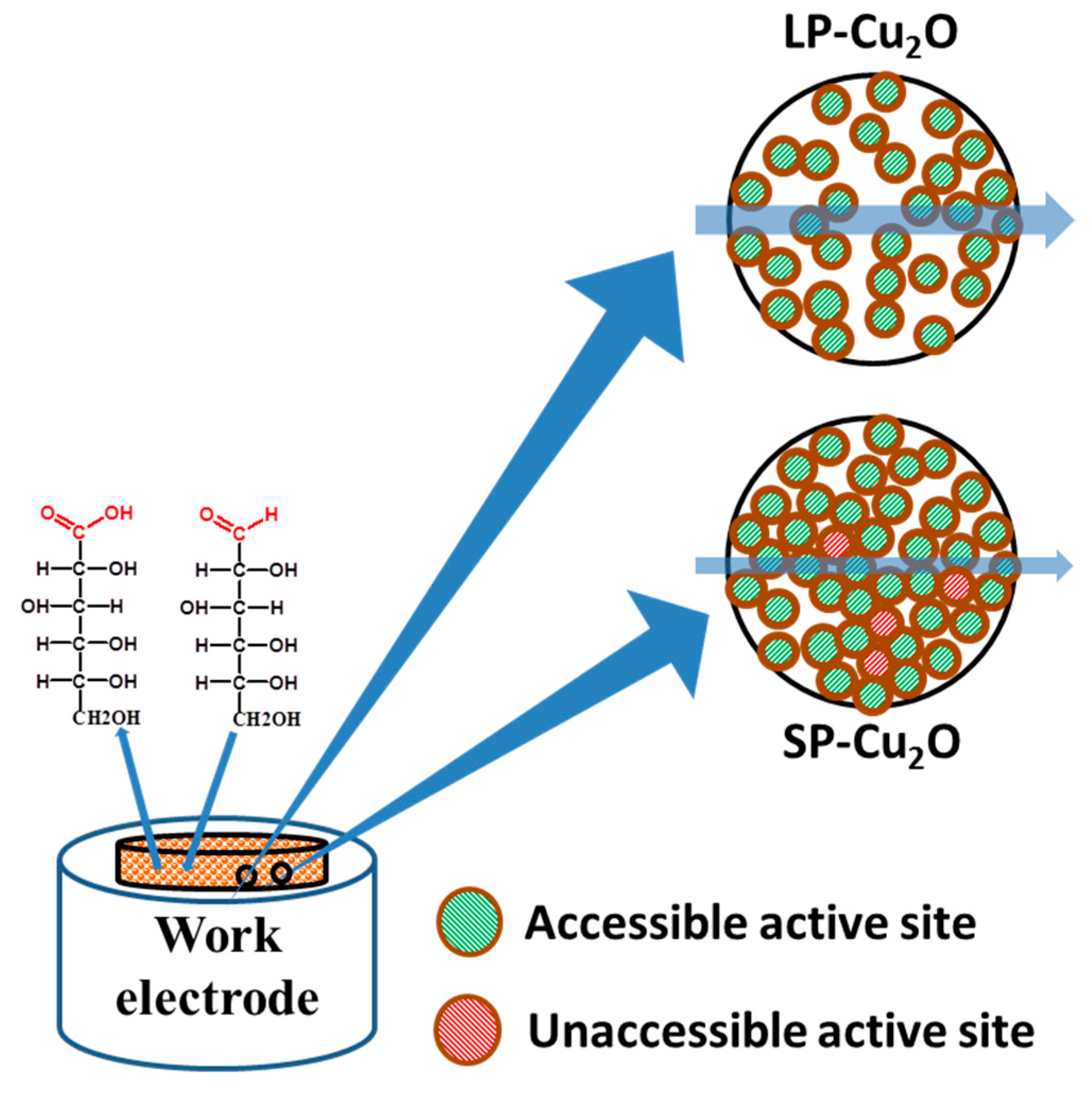
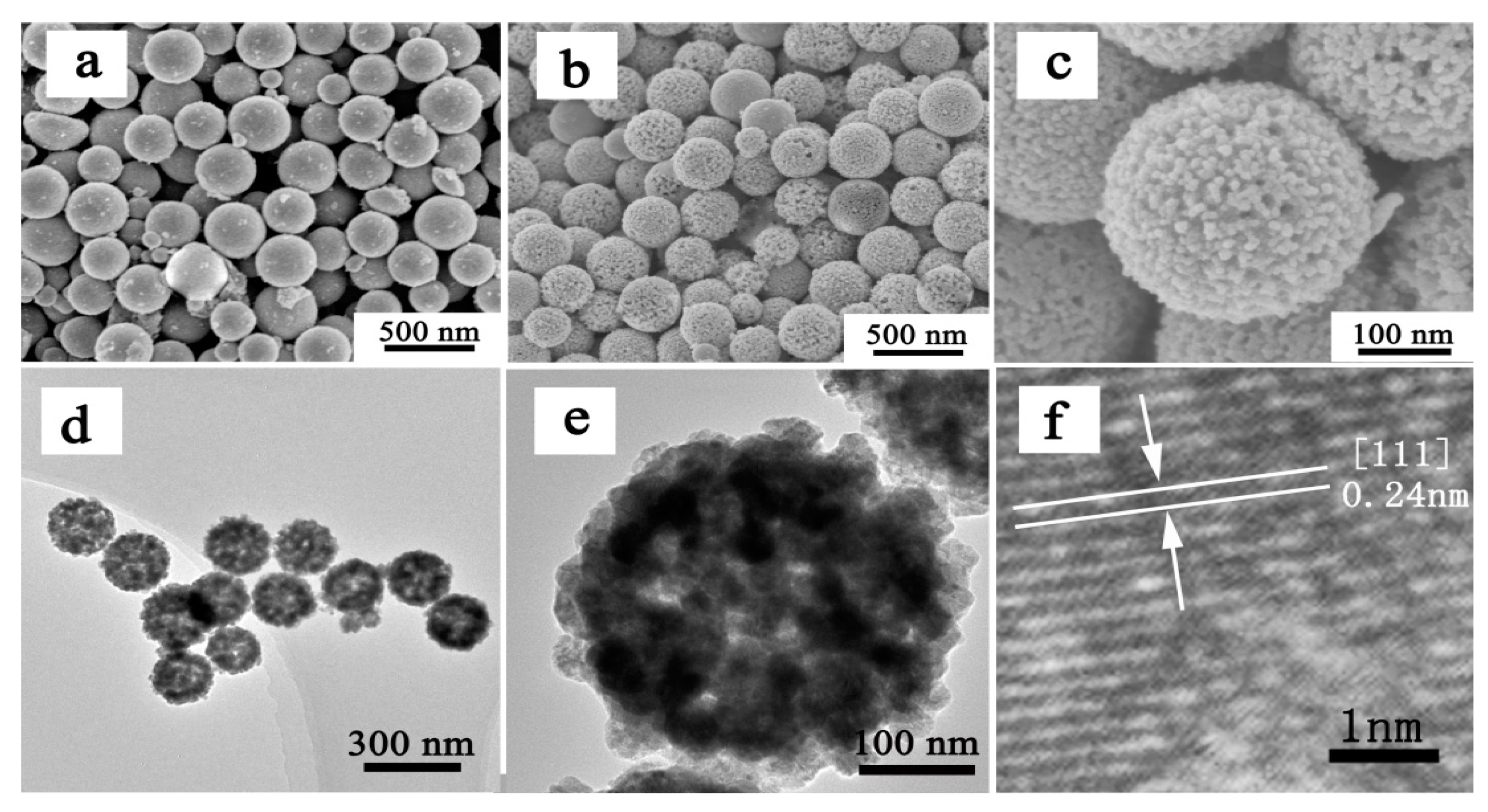
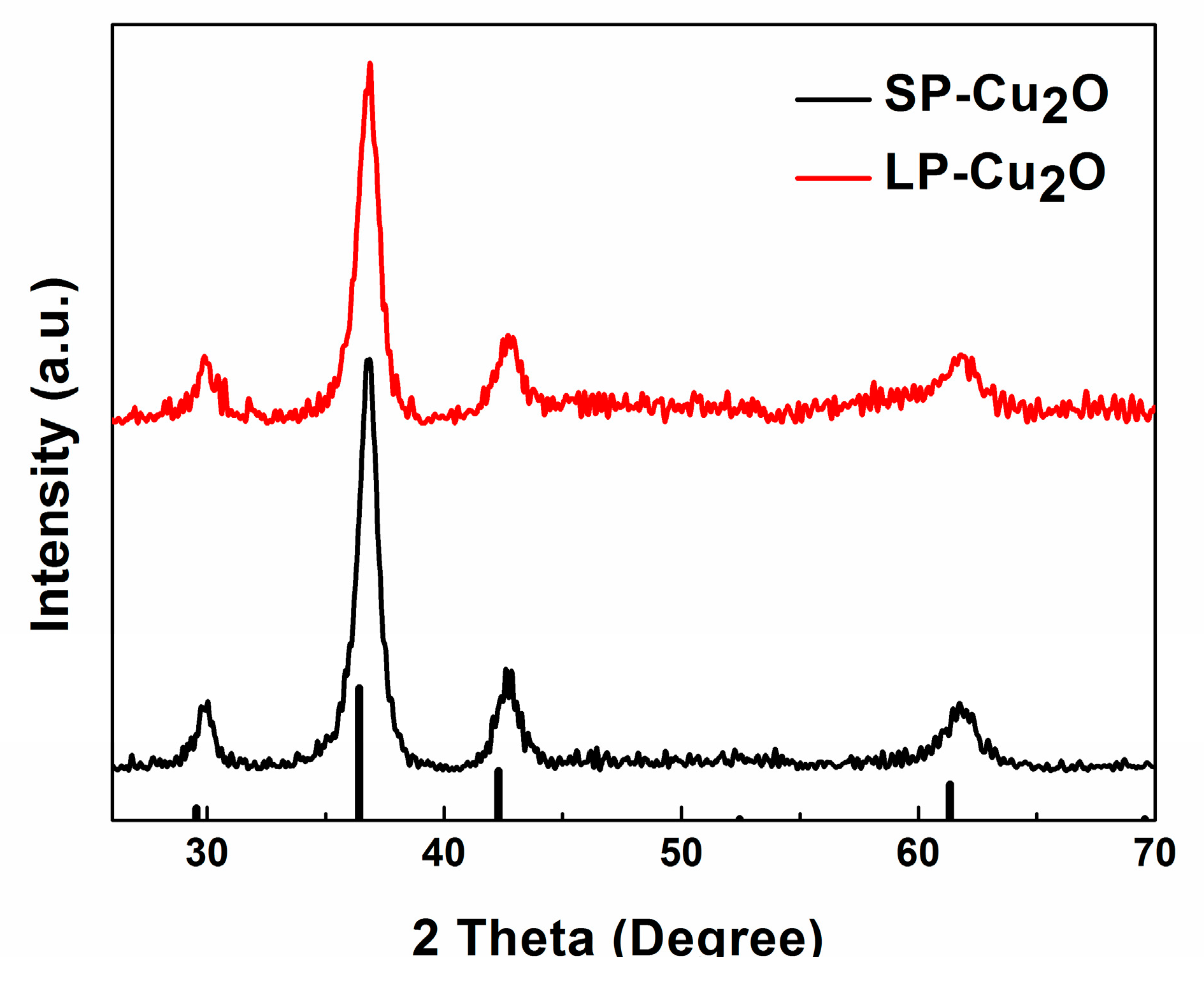
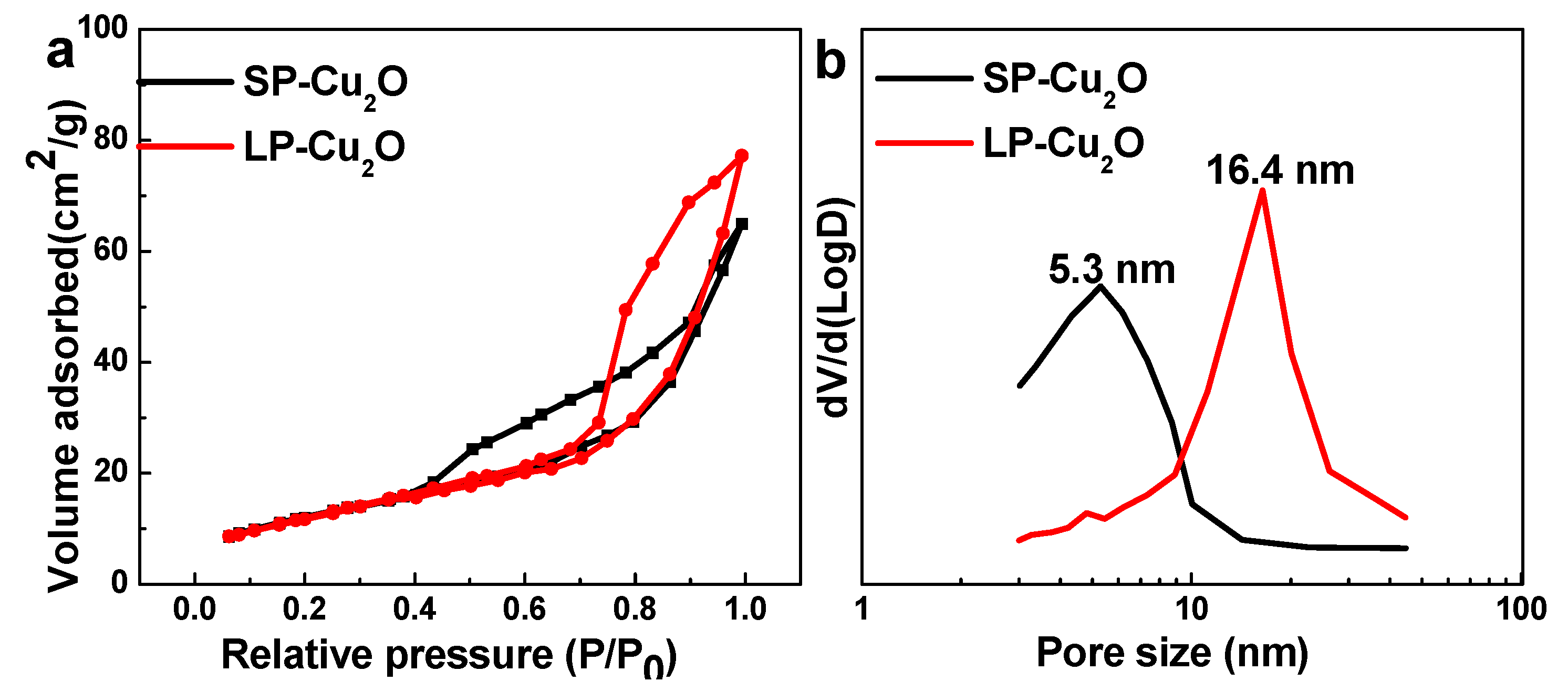
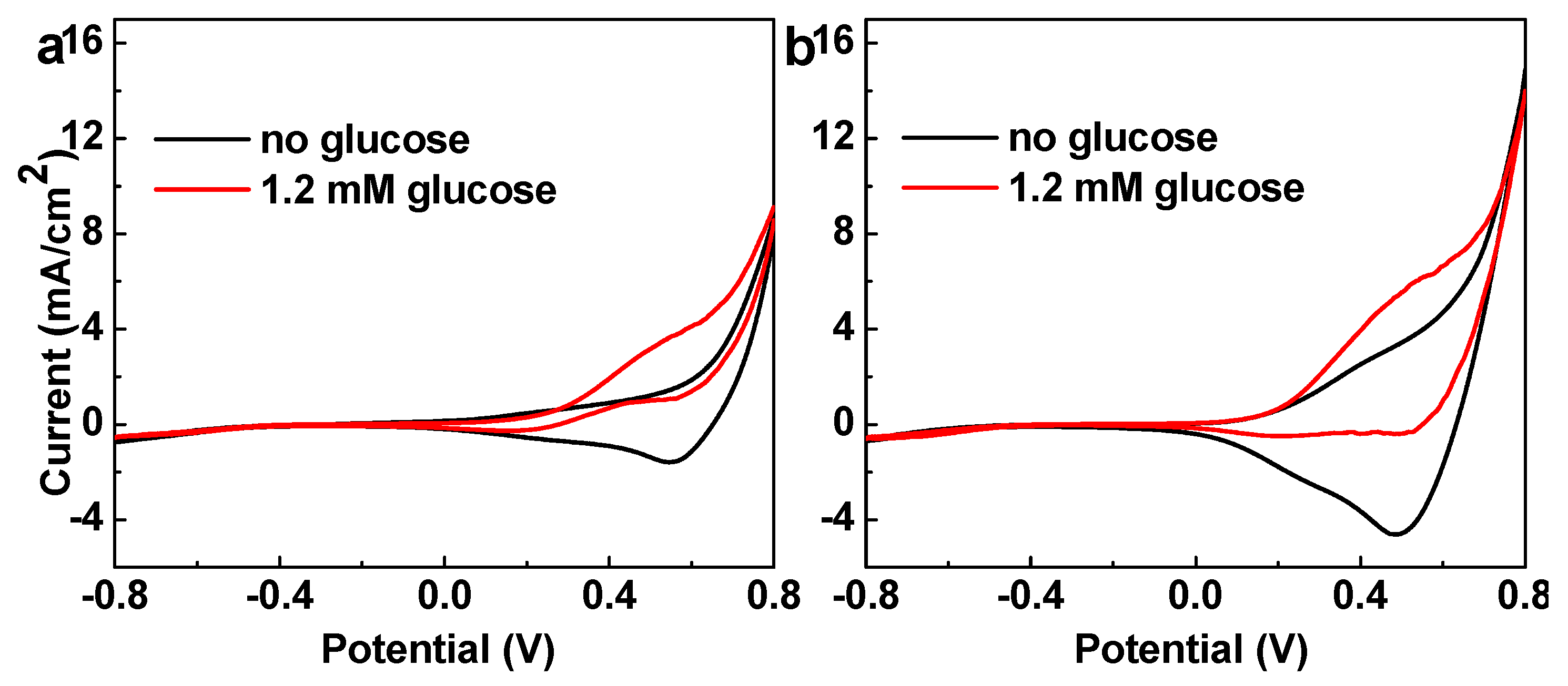
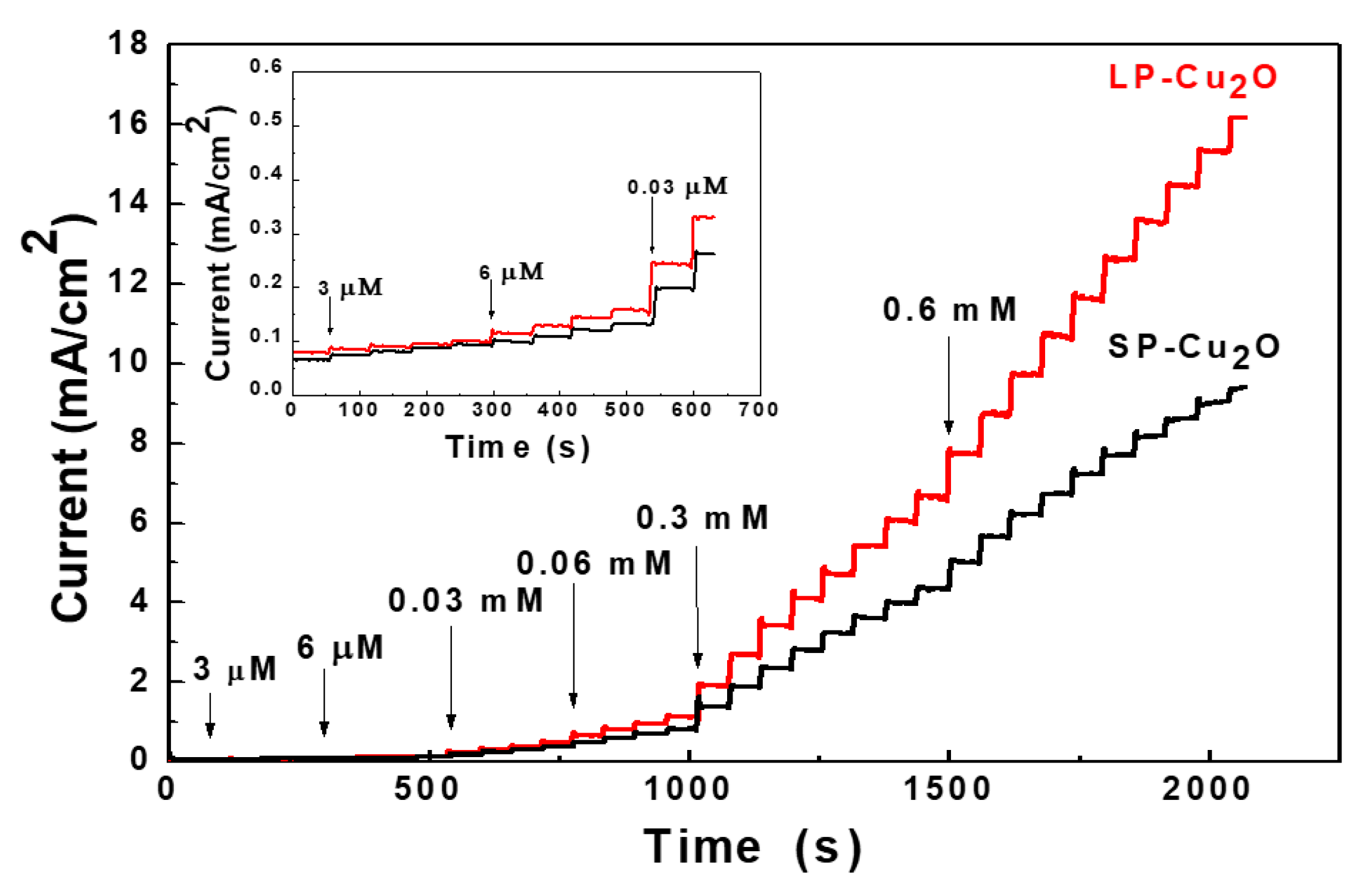
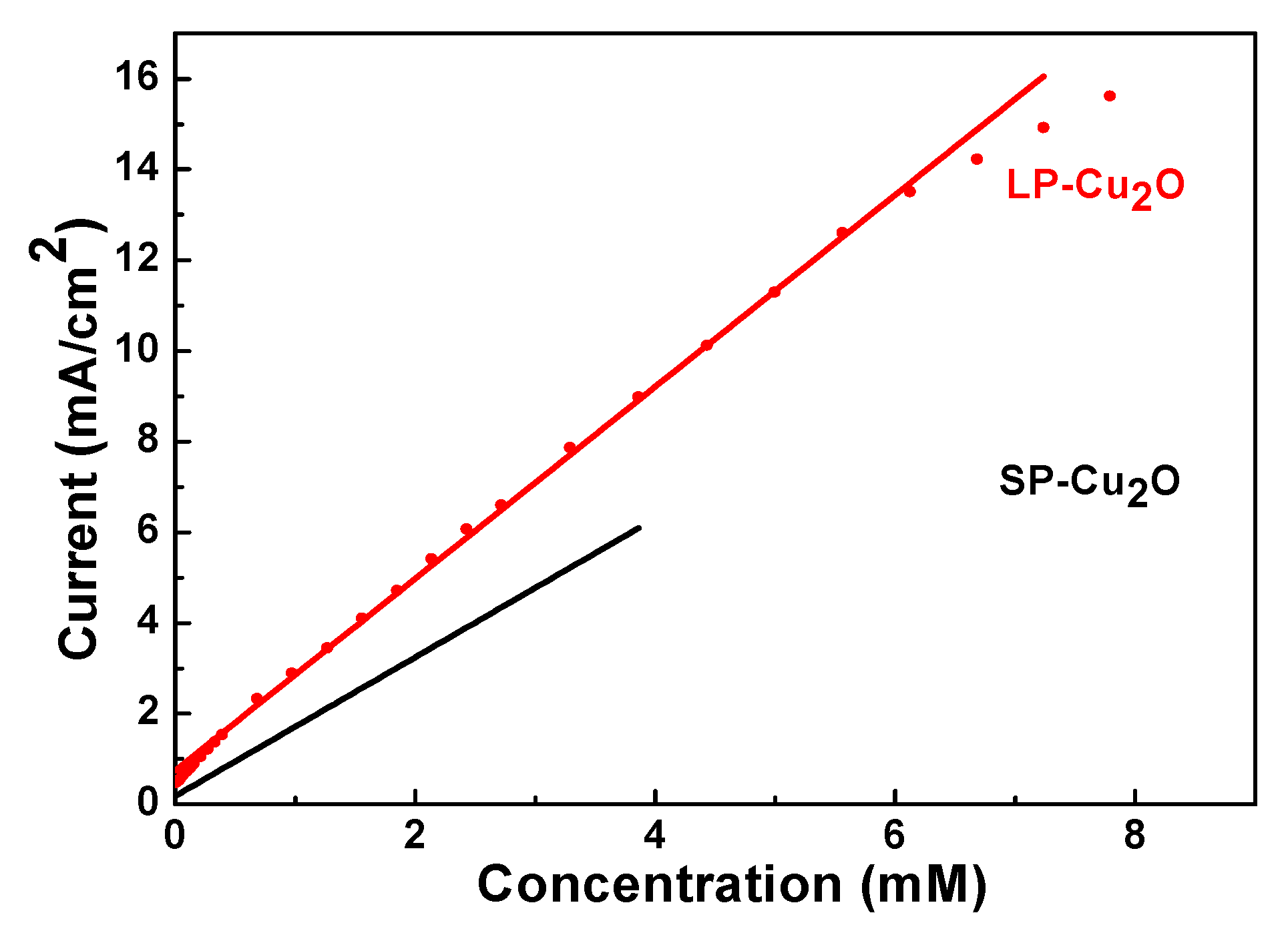
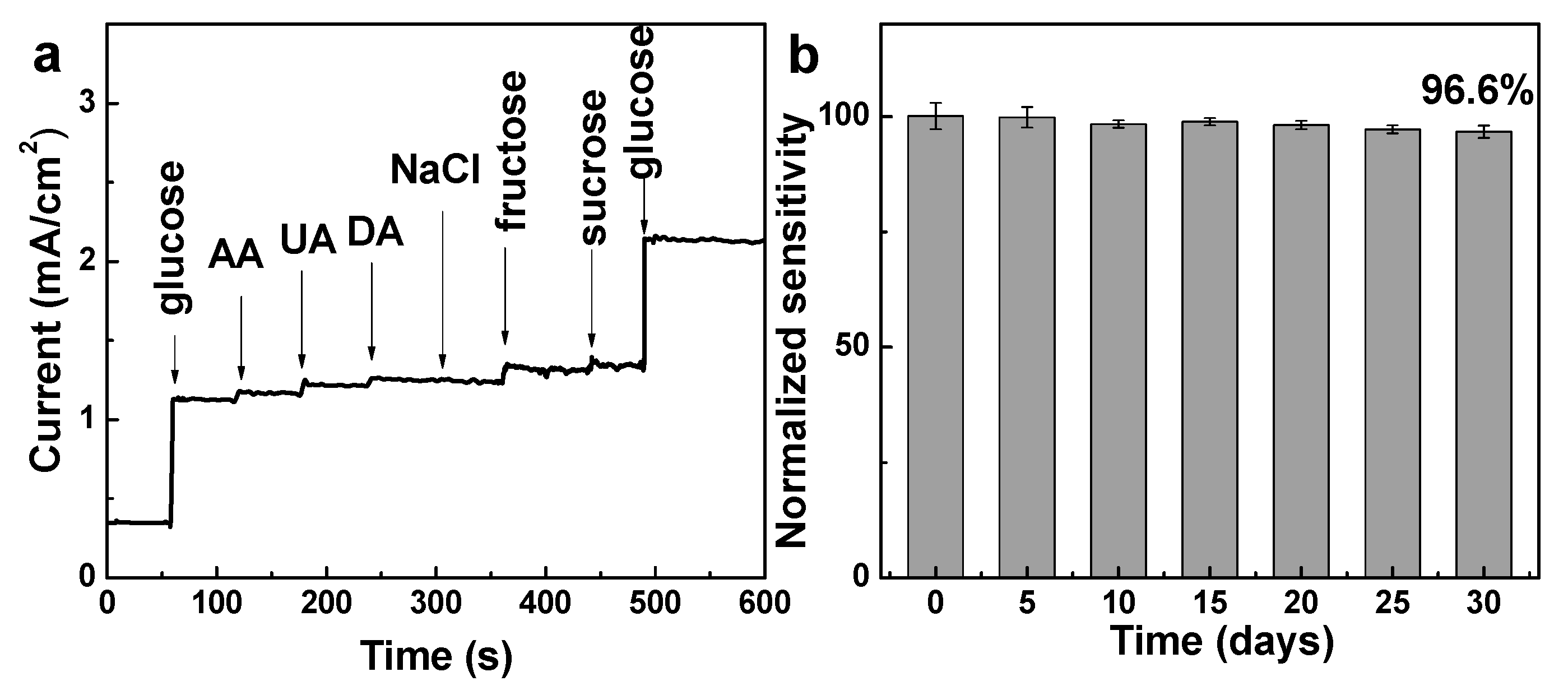
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Galant, A.L.; Kaufman, R.C.; Wilson, J.D. Glucose: Detection and analysis. Food Chem. 2015, 188, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, L.; Zhou, W.; Shao, Z.; Chen, X. Advances in non-enzymatic glucose sensors based on metal oxides. J. Mater. Chem. B 2016, 4, 7333–7349. [Google Scholar] [CrossRef]
- Chen, A.; Ding, Y.; Yang, Z.; Yang, S. Constructing heterostructure on highly roughened caterpillar-like gold nanotubes with cuprous oxide grains for ultrasensitive and stable nonenzymatic glucose sensor. Biosens. Bioelectron. 2015, 74, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Chung, R.J.; Wang, A.N.; Liao, Q.L.; Chuang, K.Y. Non-enzymatic glucose sensor composed of carbon-coated nano-zinc oxide. Nanomaterials 2017, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.-M.; Li, H.-J.; Yin, X.-M.; Lu, J.-H.; Zhang, L.-L. 3D CuO nanosheet wrapped nanofilm grown on Cu foil for high-performance non-enzymatic glucose biosensor electrode. Talanta 2017, 174, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Xie, Y.; Ci, S.; Jia, J.; Wen, Z. Porous Co3O4 hollow nanododecahedra for nonenzymatic glucose biosensor and biofuel cell. Biosens. Bioelectron. 2016, 81, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Tripathy, N.; Ahn, M.S.; Bhat, K.S.; Mahmoudi, T.; Wang, Y.; Yoo, J.Y.; Kwon, D.W.; Yang, H.Y.; Hahn, Y.B. Highly Efficient Non-Enzymatic Glucose Sensor Based on CuO Modified Vertically-Grown ZnO Nanorods on Electrode. Sci. Rep. 2017, 7, 5715. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gao, F.; Gu, Z. Vertically aligned pt nanowire array/au nanoparticle hybrid structure as highly sensitive amperometric biosensors. Sens. Actuators B Chem. 2017, 243, 1092–1101. [Google Scholar] [CrossRef]
- Wen, D.; Herrmann, A.-K.; Borchardt, L.; Simon, F.; Liu, W.; Kaskel, S.; Eychmüller, A. Controlling the growth of palladium aerogels with high-performance toward bioelectrocatalytic oxidation of glucose. J. Am. Chem. Soc. 2014, 136, 2727–2730. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Hai, X.; Chen, X.-W.; Wang, J.-H. In situ growth of silver nanoparticles on graphene quantum dots for ultrasensitive colorimetric detection of H2O2 and glucose. Anal. Chem. 2014, 86, 6689–6694. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wu, Y.; Chen, Y.; Weng, B.; Li, C. Flexible paper sensor fabricated via in situ growth of cu nanoflower on rgo sheets towards amperometrically non-enzymatic detection of glucose. Sens. Actuators B Chem. 2017, 238, 802–808. [Google Scholar] [CrossRef]
- Wu, H.; Yu, Y.; Gao, W.; Gao, A.; Qasim, A.M.; Zhang, F.; Wang, J.; Ding, K.; Wu, G.; Chu, P.K. Nickel plasma modification of graphene for high-performance non-enzymatic glucose sensing. Sens. Actuators B Chem. 2017, 251, 842–850. [Google Scholar] [CrossRef]
- Shu, Y.; Yan, Y.; Chen, J.; Xu, Q.; Pang, H.; Hu, X. Ni and nio nanoparticles decorated metal–organic framework nanosheets: Facile synthesis and high-performance nonenzymatic glucose detection in human serum. ACS Appl. Mater. Interfaces 2017, 9, 22342–22349. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Shi, J.; Liu, A. Novel biotemplated MnO2 1D nanozyme with controllable peroxidase-like activity and unique catalytic mechanism and its application for glucose sensing. Sens. Actuators B Chem. 2017, 252, 919–926. [Google Scholar] [CrossRef]
- Kannan, P.; Maiyalagan, T.; Marsili, E.; Ghosh, S.; Guo, L.; Huang, Y.; Rather, J.A.; Thiruppathi, D.; Niedziolka-Jonsson, J.; Jonsson-Niedziolka, M. Highly active 3-dimensional cobalt oxide nanostructures on the flexible carbon substrates for enzymeless glucose sensing. Analyst 2017, 142, 4299–4307. [Google Scholar] [CrossRef] [PubMed]
- Comini, E.; Sberveglieri, G. Metal oxide nanowires as chemical sensors. Mater. Today 2010, 13, 36–44. [Google Scholar] [CrossRef]
- Lai, J.; Yi, Y.; Zhu, P.; Shen, J.; Wu, K.; Zhang, L.; Liu, J. Polyaniline-based glucose biosensor: A review. J. Electroanal. Chem. 2016, 782, 138–153. [Google Scholar] [CrossRef]
- Wang, J.; Ma, J.; Li, X.; Li, Y.; Zhang, G.; Zhang, F.; Fan, X. Cu2O mesoporous spheres with a high internal diffusion capacity and improved catalytic ability for the aza-Henry reaction driven by visible light. Chem. Commun. 2014, 50, 14237–14240. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Liu, J.; Liu, X.; Yuen, M.M.F.; Fu, X.-Z.; Yang, Y.; Sun, R.; Wong, C.-P. 3D porous Cu@Cu2O films supported Pd nanoparticles for glucose electrocatalytic oxidation. Electrochim. Acta 2017, 248, 299–306. [Google Scholar] [CrossRef]
- Liu, M.; Liu, R.; Chen, W. Graphene wrapped Cu2O nanocubes: Non-enzymatic electrochemical sensors for the detection of glucose and hydrogen peroxide with enhanced stability. Biosens. Bioelectron. 2013, 45, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, Y.; Li, P.; Sang, S.; Zhang, W.; Hu, J.; Lian, K. A highly sensitive electrochemical sensor based on Cu/Cu2O@carbon nanocomposite structures for hydrazine detection. Anal. Methods 2015, 7, 9040–9046. [Google Scholar] [CrossRef]
- Cai, C.; Zhu, T.; Li, D.; Ran, Y.; Dong, H.; Zhao, N.; Xu, J. Spherically aggregated Cu2O–Ta hybrid sub-microparticles with modulated size and improved chemical stability. CrystEngComm 2017, 19, 1888–1895. [Google Scholar] [CrossRef]
- Xie, H.; Ke, Q.; Xiong, X. Preparation of a Cu2O/rGO porous composite through a double-sacrificial-template method for non-enzymatic glucose detection. J. Mater. Sci. 2017, 52, 5652–5660. [Google Scholar] [CrossRef]
- Li, S.; Zheng, Y.; Qin, G.W.; Ren, Y.; Pei, W.; Zuo, L. Enzyme-free amperometric sensing of hydrogen peroxide and glucose at a hierarchical Cu2O modified electrode. Talanta 2011, 85, 1260–1264. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Liu, J.; Chang, J.; Wu, D.; He, J.; Wang, K.; Xu, F.; Jiang, K. Mesocrystalline Cu2O hollow nanocubes: Synthesis and application in non-enzymatic amperometric detection of hydrogen peroxide and glucose. CrystEngComm 2012, 14, 6639. [Google Scholar] [CrossRef]
- Zhou, D.-L.; Feng, J.-J.; Cai, L.-Y.; Fang, Q.-X.; Chen, J.-R.; Wang, A.-J. Facile synthesis of monodisperse porous Cu2O nanospheres on reduced graphene oxide for non-enzymatic amperometric glucose sensing. Electrochim. Acta 2014, 115, 103–108. [Google Scholar] [CrossRef]
- Zhang, L.; Mao, Z.; Thomason, J.D.; Wang, S.; Huang, K.; Butt, D. Synthesis of a homogeneously porous solid oxide matrix with tunable porosity and pore size. J. Am. Ceram. Soc. 2012, 95, 1832–1837. [Google Scholar] [CrossRef]
- Du, J.; Lai, X.; Yang, N.; Zhai, J.; Kisailus, D.; Su, F.; Wang, D.; Jiang, L. Hierarchically ordered macro−mesoporous TiO2−graphene composite films: Improved mass transfer, reduced charge recombination, and their enhanced photocatalytic activities. ACS Nano 2011, 5, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Klaewkla, R.; Arend, M.; Hoelderich, W.F. Mass Transfer—Advanced Aspects; InTech: London, UK, 2011. [Google Scholar]
- Zhao, Q.; Li, Y.; Liu, R.; Chen, A.; Zhang, G.; Zhang, F.; Fan, X. Enhanced hydrogenation of olefins and ketones with a ruthenium complex covalently anchored on graphene oxide. J. Mater. Chem. A 2013, 1, 15039–15045. [Google Scholar] [CrossRef]
- Lv, J.; Kong, C.; Hu, X.; Zhang, X.; Liu, K.; Yang, S.; Bi, J.; Liu, X.; Meng, G.; Li, J.; Yang, Z.; Yang, S. Zinc ion mediated synthesis of cuprous oxide crystals for non-enzymatic glucose detection. J. Mater. Chem. B 2017, 5, 8686–8694. [Google Scholar] [CrossRef]
- Zhuang, W.; Zhang, Y.; Zhu, J.; An, R.; Li, B.; Mu, L.; Ying, H.; Wu, J.; Zhou, J.; Chen, Y.; et al. Influences of geometrical topography and surface chemistry on the stable immobilization of adenosine deaminase on mesoporous TiO2. Chem. Eng. Sci. 2016, 139, 142–151. [Google Scholar] [CrossRef]
- Bird, R.B.; Stewart, W.E.; Lightfoot, E.N. Transport Phenomena; John Wiley & Son: New York, NY, USA, 2002. [Google Scholar]
- Li, Y.; Guan, P.; Yu, F.; Li, W.; Xie, X. CeO2 nanorods embedded in Ni(OH)2 matrix for the non-enzymatic detection of glucose. Nanomaterials 2017, 7, 205. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Wang, J.; Wang, M.; Zhang, G.; Peng, W.; Li, Y.; Fan, X.; Zhang, F. Preparation of Cuprous Oxide Mesoporous Spheres with Different Pore Sizes for Non-Enzymatic Glucose Detection. Nanomaterials 2018, 8, 73. https://doi.org/10.3390/nano8020073
Ma J, Wang J, Wang M, Zhang G, Peng W, Li Y, Fan X, Zhang F. Preparation of Cuprous Oxide Mesoporous Spheres with Different Pore Sizes for Non-Enzymatic Glucose Detection. Nanomaterials. 2018; 8(2):73. https://doi.org/10.3390/nano8020073
Chicago/Turabian StyleMa, Jingwen, Jun Wang, Min Wang, Guoliang Zhang, Wenchao Peng, Yang Li, Xiaobin Fan, and Fengbao Zhang. 2018. "Preparation of Cuprous Oxide Mesoporous Spheres with Different Pore Sizes for Non-Enzymatic Glucose Detection" Nanomaterials 8, no. 2: 73. https://doi.org/10.3390/nano8020073