Nanotherapeutics Containing Lithocholic Acid-Based Amphiphilic Scorpion-Like Macromolecules Reduce In Vitro Inflammation in Macrophages: Implications for Atherosclerosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Synthesis
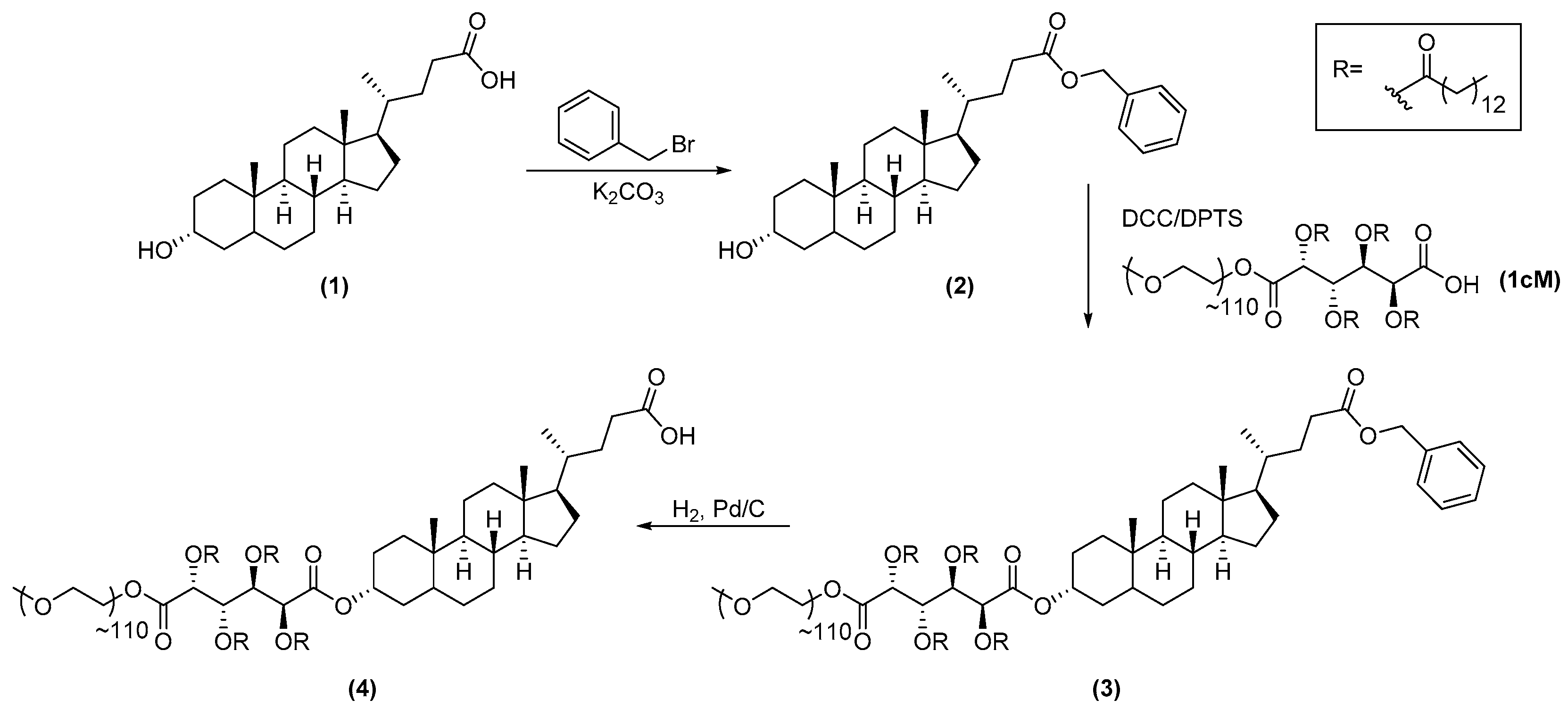
2.3.1. Synthesis of 1cMLCA (4)
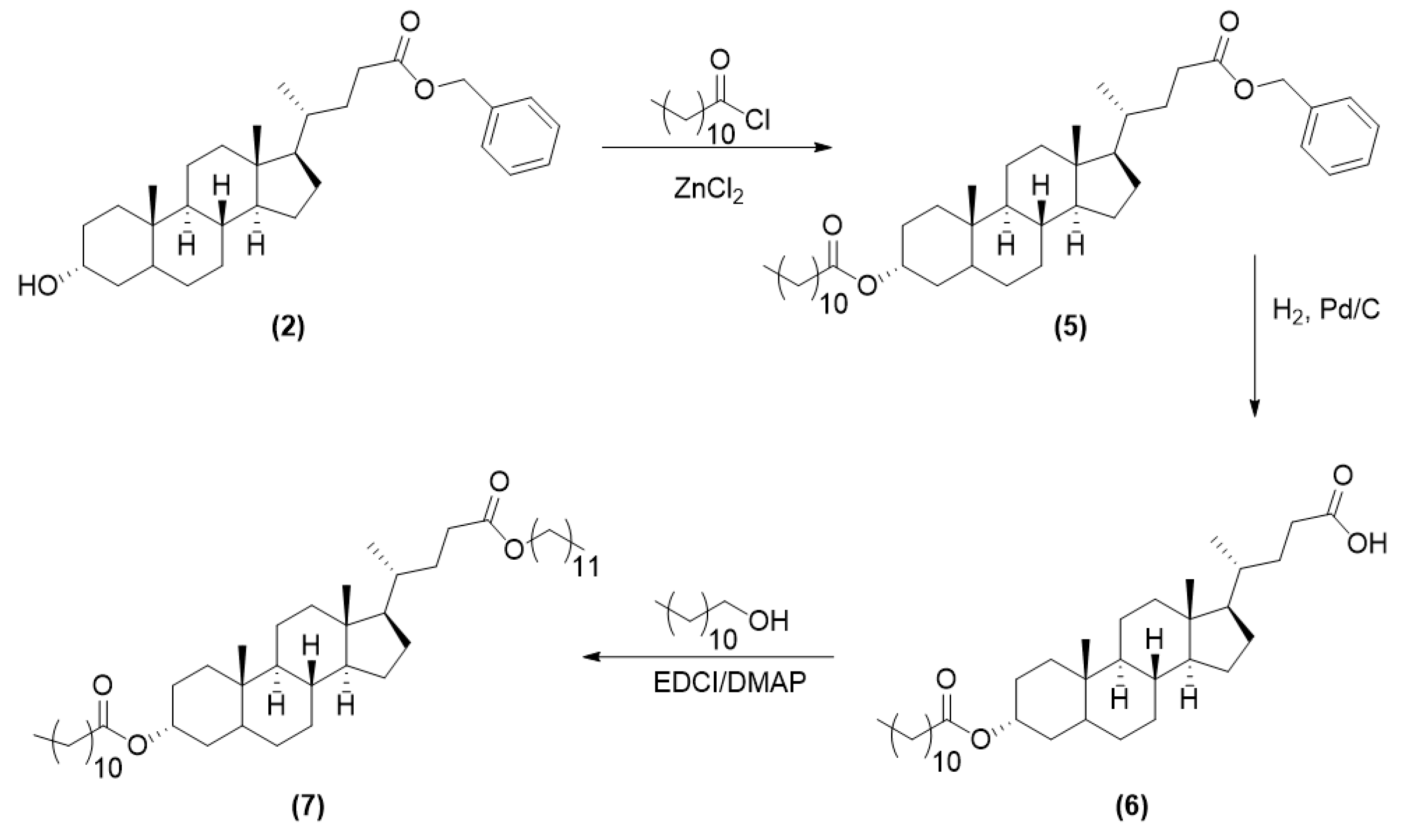
2.3.2. Synthesis of LCA-Based Hydrophobe (7)
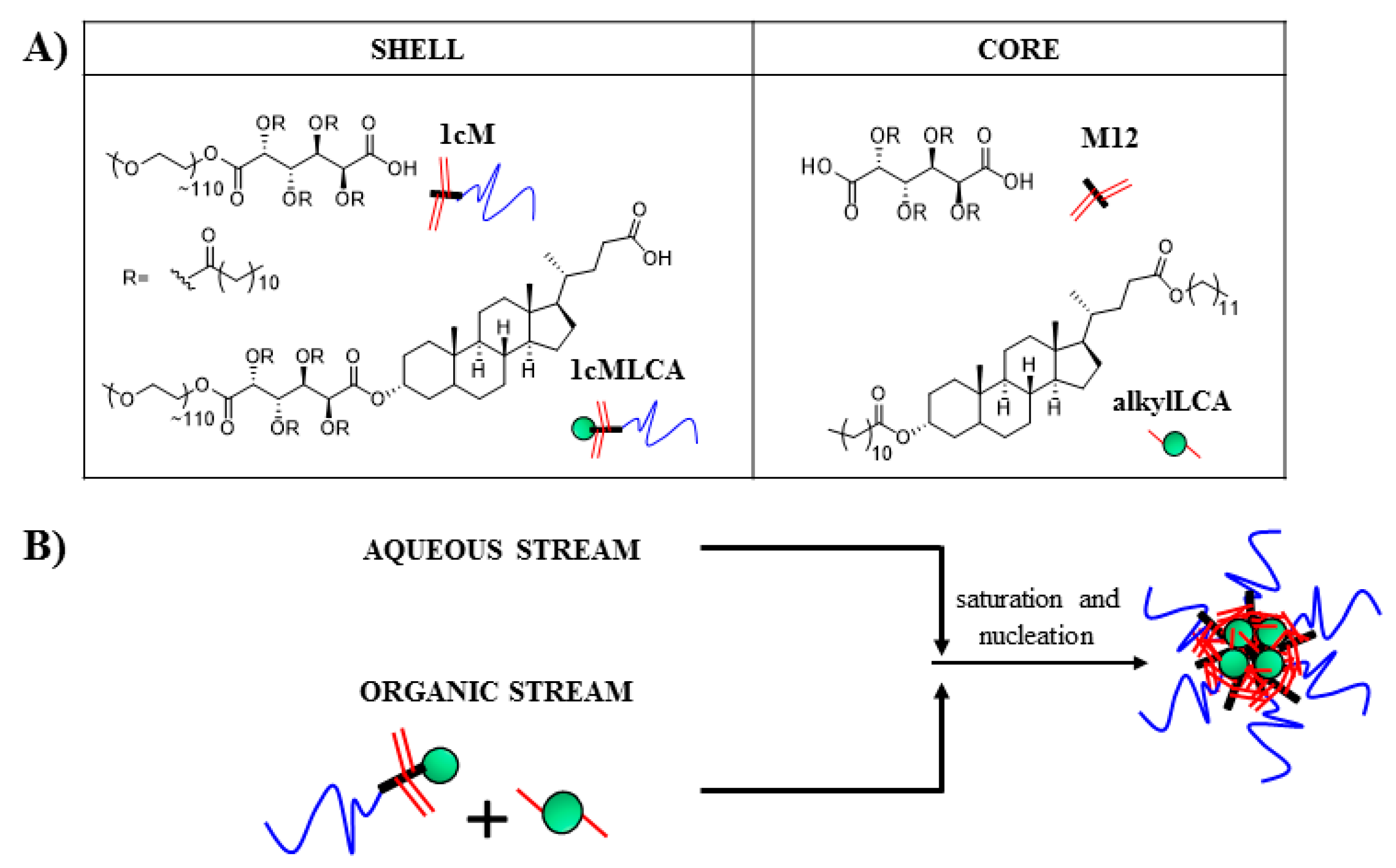
2.4. Nanoparticle Fabrication
2.5. Nanoparticle Characterization
2.6. Isolation of Human Monocyte Derived Macrophages (hMDMs)
2.7. Oxidized Low Density Lipoprotein (oxLDL) Uptake in Macrophages
2.8. Gene Expression in Macrophages
2.9. Confocal Microscopy of oxLDL Uptake in Macrophages
2.10. Statistical Analysis
3. Results and Discussion
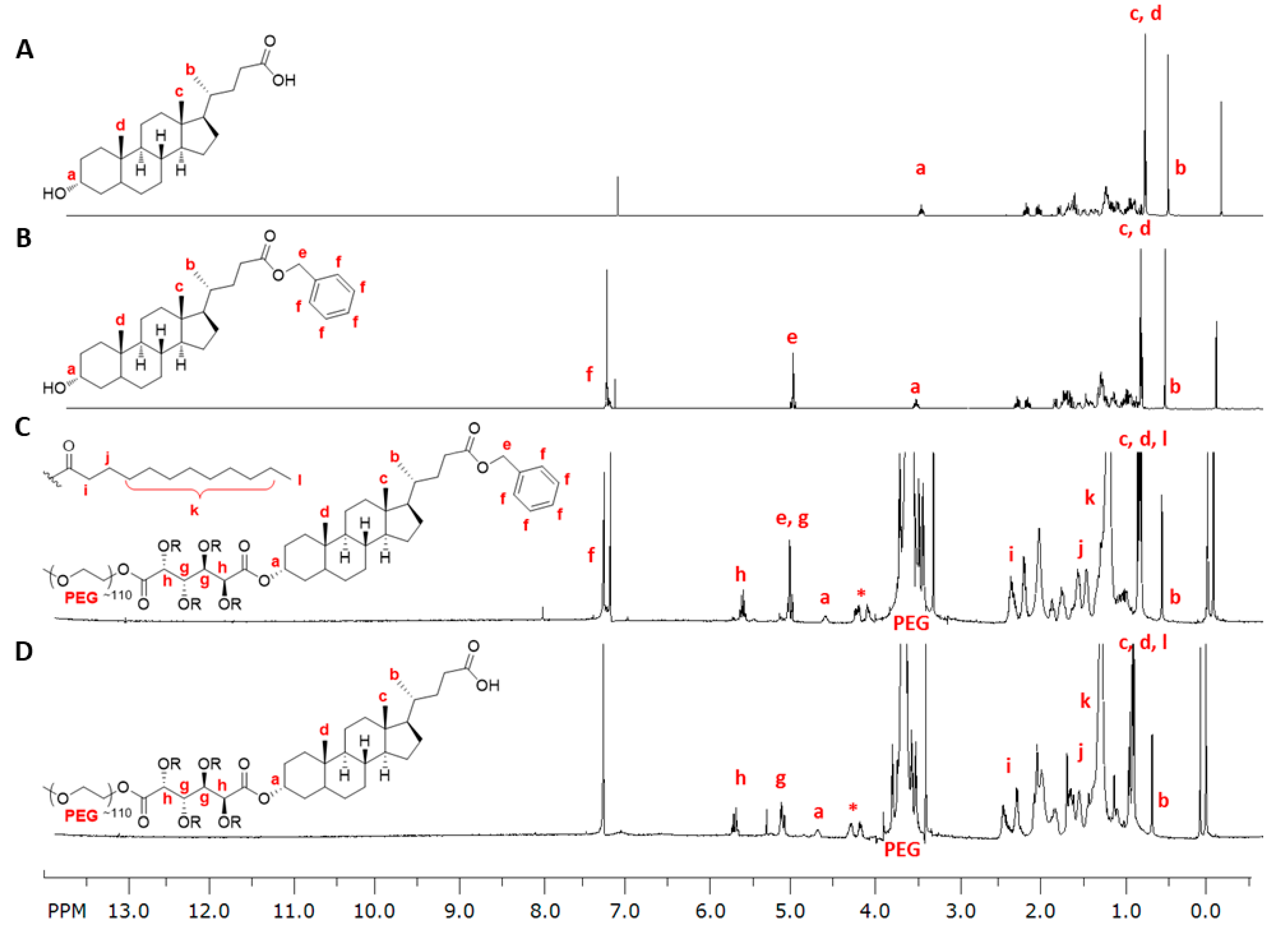
3.1. LCA-Conjugates Successfully Synthesized via Multi-Step Reactions
3.2. Stable Mono-Dispersed Nanoparticles Fabricated with LCA-Conjugates Possess Negative Charge and Desirable Size Distributions
3.3. AScM NPs Inhibit oxLDL Uptake in Human Macrophages
3.4. AScM NP Composition Markedly Impacts Inflammatory Gene Transcription
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; de Ferranti, S.; Després, J.-P.; Fullerton, H.J.; Howard, V.J.; et al. Heart Disease and Stroke Statistics—2015 Update: A Report From the American Heart Association. Circulation 2015, 131, e29–e322. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K.; Libby, P. The immune response in atherosclerosis: A double-edged sword. Nat. Rev. Immunol. 2006, 6, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Kunjathoor, V.V.; Febbraio, M.; Podrez, E.A.; Moore, K.J.; Andersson, L.; Koehn, S.; Rhee, J.S.; Silverstein, R.; Hoff, H.F.; Freeman, M.W. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified sow density lipoprotein leading to lipid loading in macrophages. J. Biol. Chem. 2002, 277, 49982–49988. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.J.; Tabas, I. Macrophages in the Pathogenesis of Atherosclerosis. Cell 2011, 145, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Istvan, E.S.; Deisenhofer, J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science 2001, 292, 1160–1164. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Aikawa, M. Stabilization of atherosclerotic plaques: New mechanisms and clinical targets. Nat. Med. 2002, 8, 1257–1262. [Google Scholar] [CrossRef] [PubMed]
- Koh, K.K.; Sakuma, I.; Quon, M.J. Differential metabolic effects of distinct statins. Atherosclerosis 2011, 215, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Golomb, B.A.; Evans, M.A. Statin adverse effects: A review of the literature and evidence for a mitochondrial mechanism. Am. J. Cardiovasc. Drug 2008, 8, 373–418. [Google Scholar] [CrossRef] [PubMed]
- Iverson, N.; Sparks, S.M.; Demirdirek, B.; Uhrich, K.E.; Moghe, P.V. Controllable inhibition of cellular uptake of oxidized low density lipoprotein: Structure-function relationships for nanoscale amphiphilic polymers. Acta Biomater. 2010, 6, 3081–3091. [Google Scholar] [CrossRef] [PubMed]
- Chnari, E.; Nikitczuk, J.S.; Uhrich, K.E.; Moghe, P.V. Nanoscale anionic macromolecules can inhibit cellular uptake of differentially oxidized LDL. Biomacromolecules 2006, 7, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Chnari, E.; Nikitczuk, J.S.; Wang, J.; Uhrich, K.E.; Moghe, P.V. Engineered polymeric nanoparticles for receptor-targeted blockage of oxidized low density lipoprotein uptake and atherogenesis in macrophages. Biomacromolecules 2006, 7, 1796–1805. [Google Scholar] [CrossRef] [PubMed]
- Faig, A.; Petersen, L.K.; Moghe, P.V.; Uhrich, K.E. Impact of Hydrophobic Chain Composition on Amphiphilic Macromolecule Antiatherogenic Bioactivity. Biomacromolecules 2014, 15, 3328–3337. [Google Scholar] [CrossRef] [PubMed]
- Hehir, S.; Plourde, N.M.; Gu, L.; Poree, D.E.; Welsh, W.J.; Moghe, P.V.; Uhrich, K.E. Carbohydrate composition of amphiphilic macromolecules influences physicochemical properties and binding to atherogenic scavenger receptor A. Acta Biomater. 2012, 8, 3956–3962. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, D.S.; Zhang, Y.; Lewis, D.R.; Moghe, P.V.; Welsh, W.J.; Uhrich, K.E. Tartaric acid-based amphiphilic macromolecules with ether linkages exhibit enhanced repression of oxidized low density lipoprotein uptake. Biomaterials 2015, 53, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Iverson, N.; Plourde, N.M.; Sparks, S.M.; Wang, J.; Patel, E.; Shah, P.; Lewis, D.R.; Zablocki, K.; Nackman, G.B.; Uhrich, K.E.; et al. Dual use of amphiphilic macromolecules as cholesterol efflux triggers and inhibitors of macrophage athero-inflammation. Biomaterials 2011, 32, 8319–8327. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.W.; Lewis, D.R.; Petersen, L.K.; Moghe, P.V.; Uhrich, K.E. Amphiphilic macromolecule nanoassemblies suppress smooth muscle cell proliferation and platelet adhesion. Biomaterials 2016, 84, 219–229. [Google Scholar] [CrossRef] [PubMed]
- York, A.W.; Zablocki, K.R.; Lewis, D.R.; Gu, L.; Uhrich, K.E.; Prud’homme, R.K.; Moghe, P.V. Kinetically assembled nanoparticles of bioactive macromolecules exhibit enhanced stability and cell-targeted biological efficacy. Adv. Mater. 2012, 24, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.R.; Petersen, L.K.; York, A.W.; Ahuja, S.; Chae, H.; Joseph, L.; Rahimi, S.; Uhrich, K.E.; Haser, P.B.; Moghe, P.V. Nanotherapeutics for inhibition of atherogenesis and modulation of inflammation in atherosclerotic plaques. Cardiovasc. Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.R.; Petersen, L.K.; York, A.W.; Zablocki, K.R.; Joseph, L.B.; Kholodovych, V.; Prud’homme, R.K.; Uhrich, K.E.; Moghe, P.V. Sugar-based amphiphilic nanoparticles arrest atherosclerosis in vivo. Proc. Natl. Acad. Sci. USA 2015, 112, 2693–2698. [Google Scholar] [CrossRef] [PubMed]
- Petersen, L.K.; York, A.W.; Lewis, D.R.; Ahuja, S.; Uhrich, K.E.; Prud’homme, R.K.; Moghe, P.V. Amphiphilic nanoparticles repress macrophage atherogenesis: Novel core/shell designs for scavenger receptor targeting and down-regulation. Mol. Pharm. 2014, 11, 2815–2824. [Google Scholar] [CrossRef] [PubMed]
- Pols, T.W.H.; Noriega, L.G.; Nomura, M.; Auwerx, J.; Schoonjans, K. The bile acid membrane receptor TGR5 as an emerging target in metabolism and inflammation. J. Hepatol. 2011, 54, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Pols, T.W.; Nomura, M.; Harach, T.; Sasso, G.L.; Oosterveer, M.H.; Thomas, C.; Rizzo, G.; Gioiello, A.; Adorini, L.; Pellicciari, R. TGR5 activation inhibits atherosclerosis by reducing macrophage inflammation and lipid loading. Cell Metab. 2011, 14, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Kida, T.; Tsubosaka, Y.; Hori, M.; Ozaki, H.; Murata, T. Bile acid receptor TGR5 agonism induces NO production and reduces monocyte adhesion in vascular endothelial cells. Arterioscler. Thromb. Vasc. 2013, 33, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Högenauer, K.; Arista, L.; Schmiedeberg, N.; Werner, G.; Jaksche, H.; Bouhelal, R.; Nguyen, D.G.; Bhat, B.G.; Raad, L.; Rauld, C. G-protein-coupled bile acid receptor 1 (GPBAR1, TGR5) agonists reduce the production of proinflammatory cytokines and stabilize the alternative macrophage phenotype. J. Med. Chem. 2014, 57, 10343–10354. [Google Scholar] [CrossRef] [PubMed]
- Kawamata, Y.; Fujii, R.; Hosoya, M.; Harada, M.; Yoshida, H.; Miwa, M.; Fukusumi, S.; Habata, Y.; Itoh, T.; Shintani, Y.; et al. A G protein-coupled receptor responsive to bile acids. J. Biol. Chem. 2003, 278, 9435–9440. [Google Scholar] [CrossRef] [PubMed]
- Jurček, O.; Bonakdarzadeh, P.; Kalenius, E.; Linnanto, J.M.; Groessl, M.; Knochenmuss, R.; Ihalainen, J.A.; Rissanen, K. Superchiral Pd3L6 coordination complex and its reversible structural conversion into Pd3L3Cl6 metallocycles. Angew. Chem. Int. Edit. 2015, 54, 15462–15467. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Yam, L.; Zhou, N.; Tat, H.; Uhrich, K.E. Amphiphilic scorpion-like macromolecules: Design, Synthesis, and Characterization. Macromolecules 2004, 37, 538–543. [Google Scholar] [CrossRef]
- Menck, K.; Behme, D.; Pantke, M.; Reiling, N.; Binder, C.; Pukrop, T.; Klemm, F. Isolation of human monocytes by double gradient centrifugation and their differentiation to macrophages in teflon-coated cell culture bags. JoVE-J. Vis. Exp. 2014, e51554. [Google Scholar] [CrossRef] [PubMed]
- Levitan, I.; Volkov, S.; Subbaiah, P.V. Oxidized LDL: Diversity, Patterns of Recognition, and Pathophysiology. Antioxid. Redox Signal. 2010, 13, 39–75. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.K.; Prud’homme, R.K. Chemical processing and micromixing in confined impinging jets. AIChE J. 2003, 49, 2264–2282. [Google Scholar] [CrossRef]
- Johnson, B.K.; Prud’homme, R.K. Flash nanoprecipitation of organic actives and block copolymers using a confined impinging jets mixer. Aust. J. Chem. 2003, 56, 1021–1024. [Google Scholar] [CrossRef]
- Ansell, S.M.; Johnstone, S.A.; Tardi, P.G.; Lo, L.; Xie, S.; Shu, Y.; Harasym, T.O.; Harasym, N.L.; Williams, L.; Bermudes, D.; et al. Modulating the therapeutic activity of nanoparticle delivered paclitaxel by manipulating the hydrophobicity of prodrug conjugates. J. Med. Chem. 2008, 51, 3288–3296. [Google Scholar] [CrossRef] [PubMed]
- Win, K.Y.; Feng, S.-S. Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials 2005, 26, 2713–2722. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Q.; Welsh, W.J.; Moghe, P.V.; Uhrich, K.E. Micellar and structural stability of nanoscale amphiphilic polymers: Implications for anti-atherosclerotic bioactivity. Biomaterials 2016, 84, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Perino, A.; Pols, T.W.H.; Nomura, M.; Stein, S.; Pellicciari, R.; Schoonjans, K. TGR5 reduces macrophage migration through mTOR-induced C/EBPβ differential translation. J. Clin. Investig. 2014, 124, 5424–5436. [Google Scholar] [CrossRef] [PubMed]
- Keitel, V.; Donner, M.; Winandy, S.; Kubitz, R.; Häussinger, D. Expression and function of the bile acid receptor TGR5 in Kupffer cells. Biochem. Biophy. Res. Commun. 2008, 372, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Arango Duque, G.; Descoteaux, A. Macrophage Cytokines: Involvement in Immunity and Infectious Diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef] [PubMed]
- Von der Thusen, J.H.; Kuiper, J.; van Berkel, T.J.; Biessen, E.A. Interleukins in atherosclerosis: Molecular pathways and therapeutic potential. Pharmacol. Rev. 2003, 55, 133–166. [Google Scholar] [CrossRef] [PubMed]
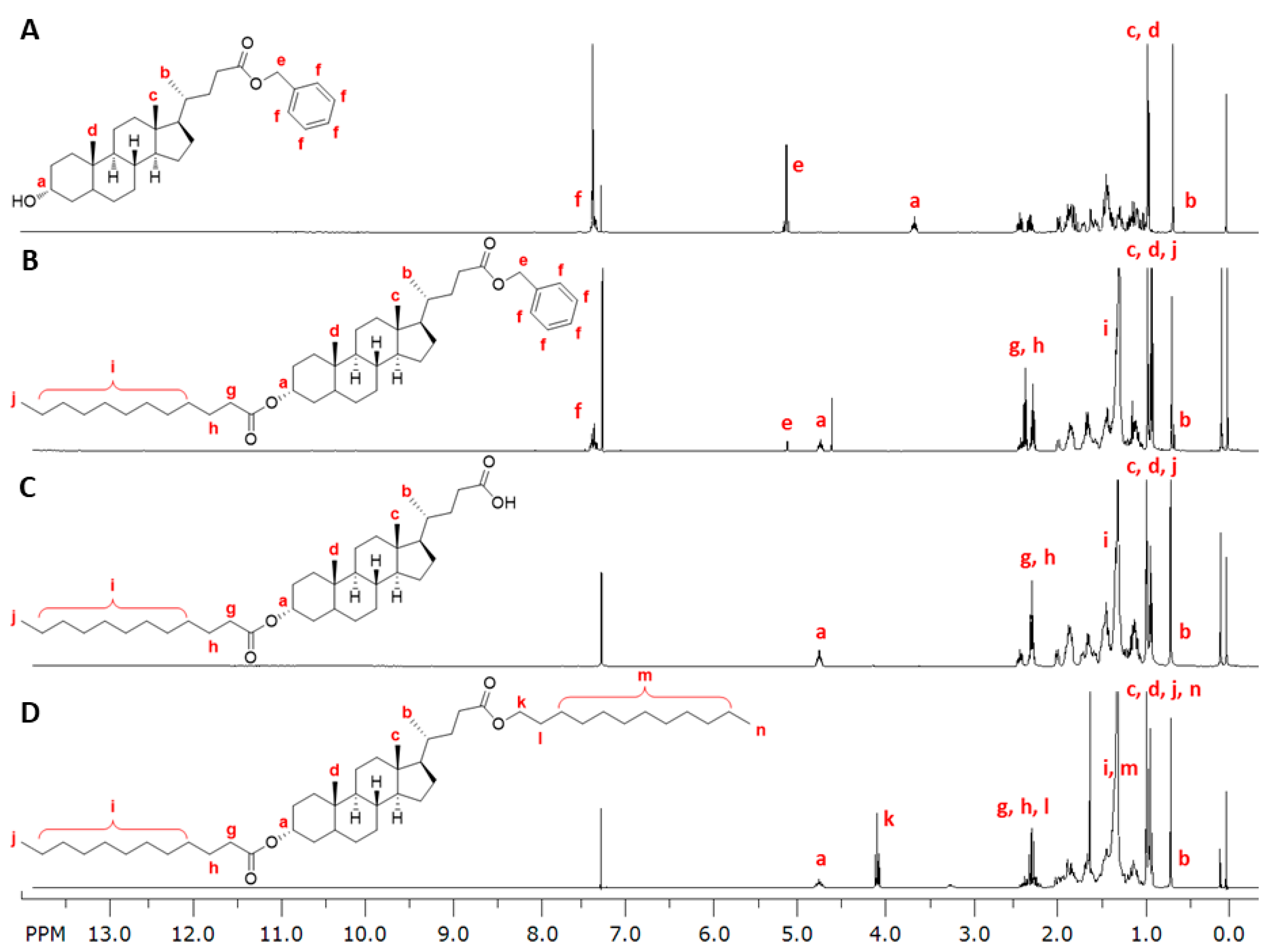
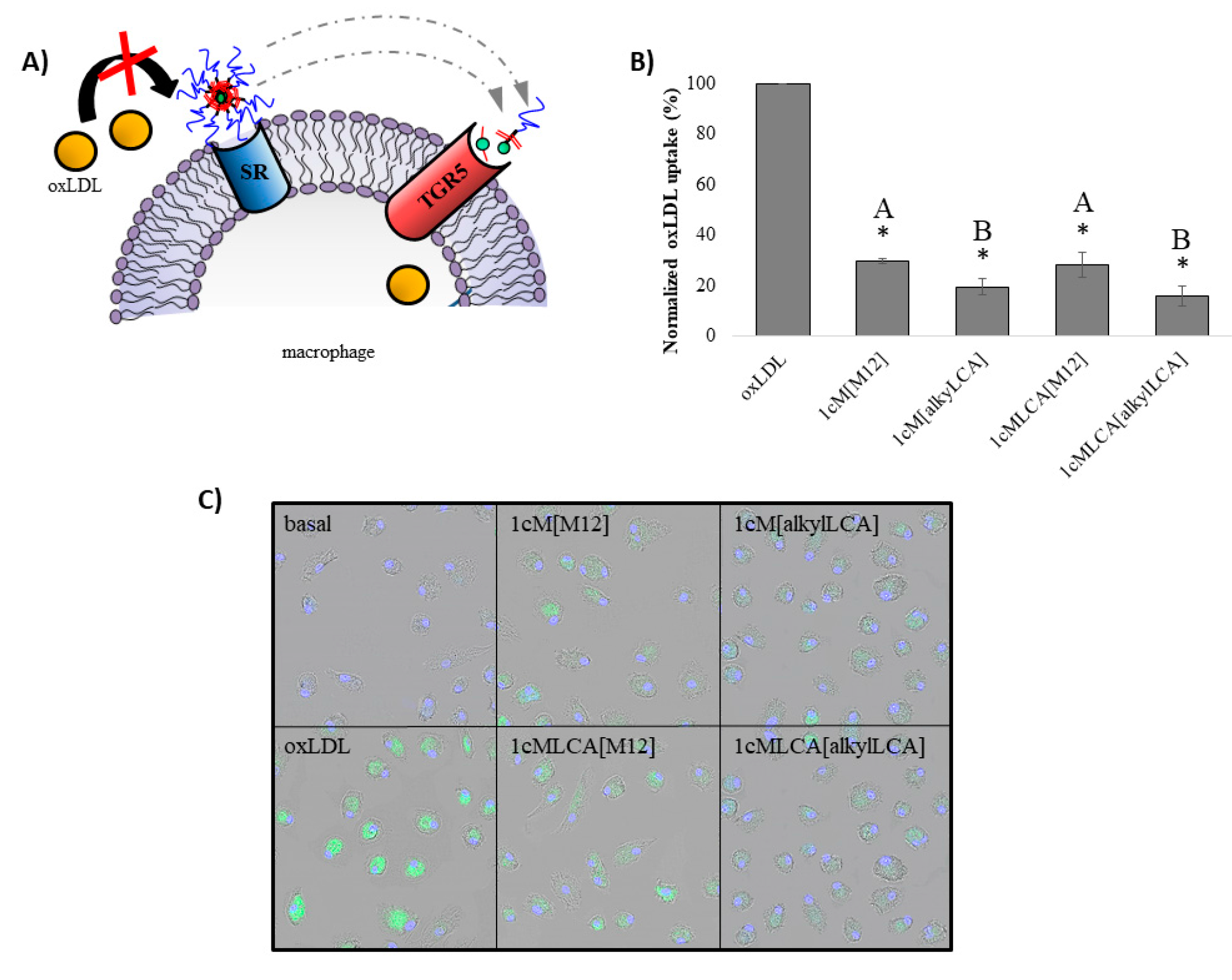
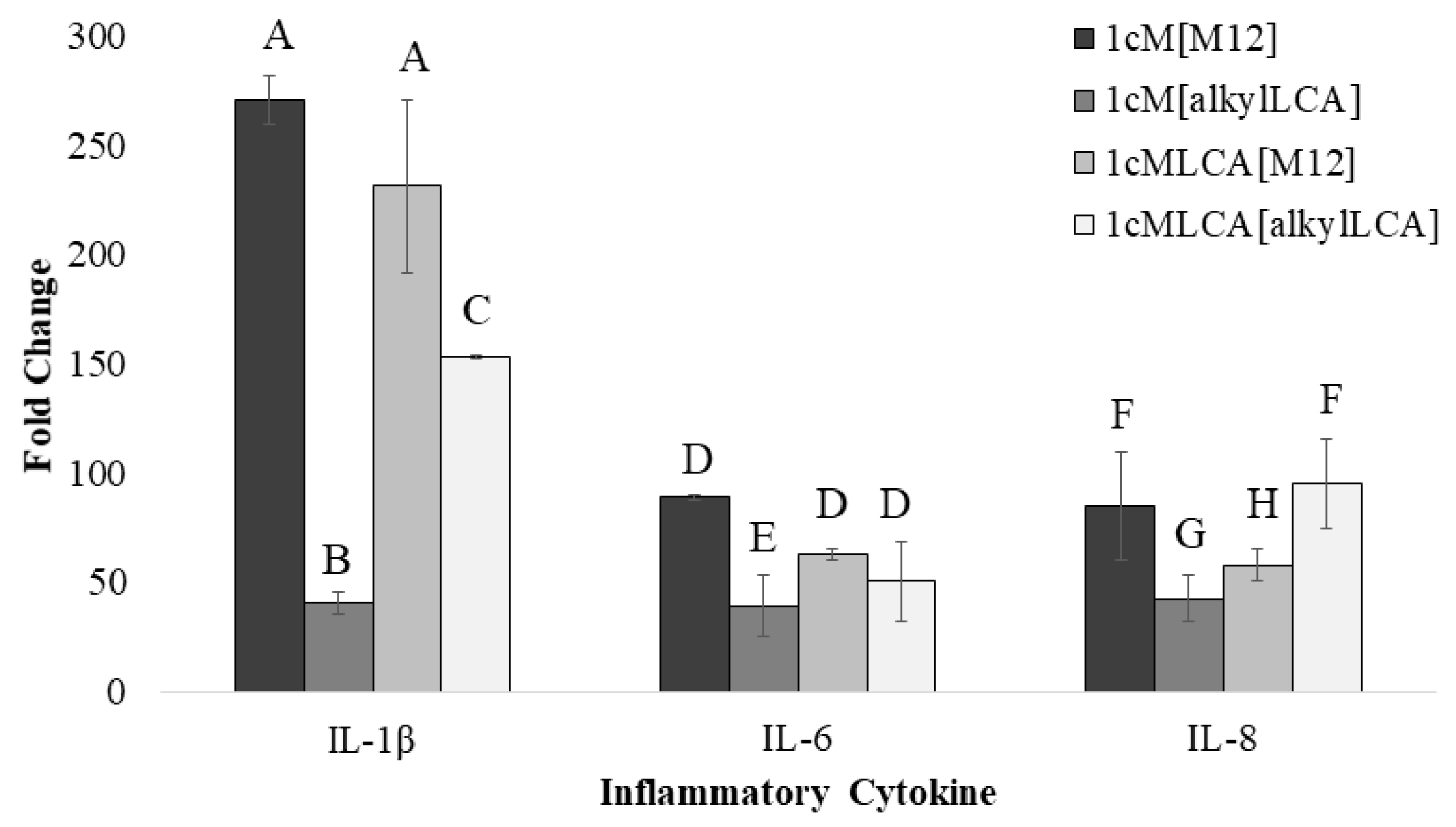
| Formulation | Hydrodynamic Diameter (nm) | PDI | ζ Potential (mV) |
|---|---|---|---|
| 1cM[M12] | 213 + 4 * | 0.11 | −30 |
| 1cM[alkylLCA] | 206 + 16 * | 0.09 | −31 |
| 1cMLCA[M12] | 176 + 14 ǂ | 0.16 | −33 |
| 1cMLCA[alkylLCA] | 173 + 2 ǂ | 0.11 | −31 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moretti, A.; Li, Q.; Chmielowski, R.; Joseph, L.B.; Moghe, P.V.; Uhrich, K.E. Nanotherapeutics Containing Lithocholic Acid-Based Amphiphilic Scorpion-Like Macromolecules Reduce In Vitro Inflammation in Macrophages: Implications for Atherosclerosis. Nanomaterials 2018, 8, 84. https://doi.org/10.3390/nano8020084
Moretti A, Li Q, Chmielowski R, Joseph LB, Moghe PV, Uhrich KE. Nanotherapeutics Containing Lithocholic Acid-Based Amphiphilic Scorpion-Like Macromolecules Reduce In Vitro Inflammation in Macrophages: Implications for Atherosclerosis. Nanomaterials. 2018; 8(2):84. https://doi.org/10.3390/nano8020084
Chicago/Turabian StyleMoretti, Alysha, Qi Li, Rebecca Chmielowski, Laurie B. Joseph, Prabhas V. Moghe, and Kathryn E. Uhrich. 2018. "Nanotherapeutics Containing Lithocholic Acid-Based Amphiphilic Scorpion-Like Macromolecules Reduce In Vitro Inflammation in Macrophages: Implications for Atherosclerosis" Nanomaterials 8, no. 2: 84. https://doi.org/10.3390/nano8020084





