Antimicrobial Membranes of Bio-Based PA 11 and HNTs Filled with Lysozyme Obtained by an Electrospinning Process
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Electrospinning Procedure
2.3. Methods of Analysis
3. Results and Discussion
4. Concluding Remarks
- SEM analysis revealed that, with the used processing conditions, both PA11 membrane and the composites show a narrow average fiber diameter (0.3–0.5 μm).
- The FTIR analysis revealed a shift of the N–H stretching of amide I, indicative of a good interaction between the PA11 and lysozyme molecules.
- The mechanical properties, in terms of elastic modulus, increase with filler content for the reinforcing effect of the HNTs.
- The release kinetics of composites’ membranes were found to be dependent on the nano-hybrid loading and were well fitted with a modified Gallagher–Corrigan model. It was demonstrated that varying the filler loading it is possible to tune the lysozyme release for desired applications.
- The membranes were used as antimicrobial pads for chicken meat storage. The membrane filled with 5.0 wt % of HNTs–lysozyme was tested against Pseudomonas growth for up to 13 days and compared with the unfilled PA11. A reduction in bacterial growth was found for the membrane filled with the antimicrobial compound.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Barros-Velazquez, J. Antimicrobial Food Packaging, 1st ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Han, J.H. Antimicrobial packaging systems. In Plastic Films in Food Packaging; Ebnesajjad, S., Ed.; Elsevier William Andrew: Waltham, MA, USA, 2013; pp. 151–180. [Google Scholar]
- Wanga, G.; Yua, D.; Kelkar, A.D.; Zhang, L. Electrospun nanofiber: Emerging reinforcing filler in polymer matrix composite materials. Prog. Polym. Sci. 2017, 75, 73–107. [Google Scholar] [CrossRef]
- Lim, C.T. Nanofiber technology: Current status and emerging developments. Prog. Polym. Sci. 2017, 70, 1–17. [Google Scholar]
- Cai, J.; Lei, M.; Zhang, Q.; He, J.; Chen, T.; Liu, S.; Fu, S.; Li, T.; Liu, G.; Fei, P. Electrospun composite nanofiber mats of Cellulose@Organically modified montmorillonite for heavy metal ion removal: Design, characterization, evaluation of absorption performance. Compos. Part A 2017, 92, 10–16. [Google Scholar] [CrossRef]
- Essalhi, M.; Khayet, M. Self-sustained webs of polyvinylidene fluoride electrospun nanofibers at different electrospinning times: 1. Desalination by direct contact membrane distillation. J. Membr. Sci. 2013, 433, 167–179. [Google Scholar] [CrossRef]
- Azarniya, A.; Eslahi, N.; Mahmoudi, N.; Simchi, A. Effect of graphene oxide nanosheets on the physico-mechanical properties of chitosan/bacterial cellulose nanofibrous composites. Compos. Part A 2016, 85, 113–122. [Google Scholar] [CrossRef]
- Dhineshbabu, N.R.; Karunakaran, G.; Suriyaprabha, R.; Manivasakan, P.; Rajen-dran, V. Electrospun MgO/Nylon 6 Hybrid Nanofibers for Protective Clothing. Nano-Micro Lett. 2004, 6, 46–54. [Google Scholar] [CrossRef]
- Kadir, R.A.; Li, Z.; Sadek, A.Z.; Rani, R.A.; Zoolfakar, A.S.; Field, M.R.; Ou, J.Z.; Chrimes, A.F.; Kalantar-Zadeh, K. Electrospun granular hollow SnO2 nanofibers hydrogen gas sensors operating at low temperatures. J. Phys. Chem. C 2014, 118, 3129–3139. [Google Scholar] [CrossRef]
- Lai, K.; Jiang, W.; Tang, J.Z.; Wu, Y.; He, B.; Wang, G.; Gu, Z. Superparamagnetic nano-composite scaffolds for promoting bone cell proliferation and defect reparation without a magnetic field. RSC Adv. 2012, 2, 13007–13017. [Google Scholar] [CrossRef]
- He, L.; Shi, Y.; Han, Q.; Zuo, Q.; Ramakrishna, S.; Zhou, L. Surface modification of electrospun nanofibrous scaffolds via polysaccharide–protein assembly multilayer for neurite outgrowth. J. Mater. Chem. 2012, 26, 13187–13196. [Google Scholar] [CrossRef]
- Yu, D.; Chian, W.; Wang, X.; Li, X.; Li, Y.; Liao, Y. Linear drug release membrane prepared by a modified coaxial electrospinning process. J. Membr. Sci. 2013, 428, 150–156. [Google Scholar] [CrossRef]
- Gao, J.; Zhu, J.; Luo, J.; Xiong, J. Investigation of microporous composite scaffolds fabricated by embedding sacrificial polyethylene glycol microspheres in nanofibrous membrane. Compos. Part A 2016, 91, 20–29. [Google Scholar] [CrossRef]
- Anu Bhushani, J.; Anandharamakrishnan, C. Electrospinning and electrospraying techniques: Potential food based applications. Trends Food Sci. Technol. 2014, 38, 21–33. [Google Scholar] [CrossRef]
- Dìez-Pascual, A.M.; Dìez-Vicente, A.L. Antimicrobial and sustainable food packaging based on poly(butylene adipate-co-terephthalate) and electrospun chitosan nanofibers. RSC Adv. 2015, 5, 93095–93107. [Google Scholar] [CrossRef]
- Wen, P.; Zhu, D.; Wu, H.; Zong, M.; Jing, Y.; Han, S. Encapsulation of cinnamon essential oil in electrospun nanofibrous film for active food packaging. Food Control 2016, 59, 366–376. [Google Scholar] [CrossRef]
- Kolesov, I.; Androsch, R.; Mileva, D.; Lebek, W.; Benhamida, A.; Kaci, M.; Focke, W. Crystallization of a polyamide 11/organo-modified montmorillonite nanocomposite at rapid cooling. Colloid Polym. Sci. 2013, 291, 2541–2549. [Google Scholar] [CrossRef]
- Filippone, G.; Carroccio, S.C.; Mendichi, R.; Gioiella, L.; Dintcheva, N.T.; Gambarotti, C. Time-resolved rheology as a tool to monitor the progress of polymer degradation in the melt state—Part I: Thermal and thermo-oxidative degradation of polyamide 11. Polymer 2015, 72, 134–141. [Google Scholar] [CrossRef]
- Filippone, G.; Carroccio, S.C.; Curcuruto, G.; Passaglia, E.; Gambarotti, C.; Dintcheva, N.T. Time-resolved rheology as a tool to monitor the progress of polymer degradation in the melt state—Part II: Thermal and thermo-oxidative degradation of polyamide 11/organo-clay nanocomposites. Polymer 2015, 73, 102–110. [Google Scholar] [CrossRef]
- Kolesov, I.; Androsch, R.; Mileva, D.; Lebek, W.; Benhamida, A.; Kaci, M.; Jariyavidyanont, K.; Focke, W.; Androsch, R. Crystallization kinetics of polyamide 11 in the presence of sepiolite and montmorillonite nanofillers. Colloid Polym. Sci. 2016, 294, 1143–1151. [Google Scholar]
- Risite, H.; El Mabrouk, K.; Bousmina, M.; Fassi-Fehri, O. Role of polyamide 11 interaction with clay and modifier on thermal, rheological and mechanical properties in polymer clay nanocomposites. J. Nanosci. Nanotechnol. 2016, 16, 7584–7593. [Google Scholar] [CrossRef]
- Dintcheva, N.T.; Al-Malaika, S.A.; Morici, E. Novel organo-modifier for thermally-stable polymer-layered silicate nanocomposites. Polym. Degrad. Stab. 2015, 122, 88–101. [Google Scholar] [CrossRef]
- Prashantha, K.; Lacrampe, M.; Krawczak, P. Highly Dispersed Polyamide-11/Halloysite Nanocomposites: Thermal, Rheological, Optical, Dielectric, and Mechanical Properties. J. Appl. Polym. Sci. 2013, 130, 313–321. [Google Scholar] [CrossRef]
- Carponcin, D.; Dantras, E.; Aridon, G.; Levallois, F.; Cadiergues, L.; Lacabanne, C. Evolution of dispersion of carbon nanotubes in Polyamide 11 matrix composites as determined by DC conductivity. Compos. Sci. Technol. 2012, 72, 515–520. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.; Rafiq, R.; Gill, Y.Q.; Song, M. Preparation and characterization of high performance of graphene/nylon nanocomposites. Eur. Polym. J. 2013, 49, 2617–2626. [Google Scholar] [CrossRef]
- Carponcin, D.; Dantras, E.; Dandurand, J.; Aridon, G.; Levallois, F.; Cadiergues, L.; Lacabanne, C. Discontinuity of physical properties of carbon nanotube/polymer composites at the percolation threshold. J. Non-Cryst. Solids 2014, 392, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Rashmi, B.J.; Prashantha, K.; Lacrampe, M.-F.; Krawczak, P. Scalable Production of Multifunctional Bio-Based Polyamide 11/Graphene Nanocomposites by Melt Extrusion Processes via Masterbatch Approach. Adv. Polym. Technol. 2016. [Google Scholar] [CrossRef]
- David, C.; Capsal, J.; Laffont, L.; Dantras, E.; Lacabanne, C. Piezoelectric properties of polyamide 11/NaNbO3 nanowire composites. J. Phys. D Appl. Phys. 2012, 45, 415305. [Google Scholar] [CrossRef]
- Naffakh, M.; Shuttleworth, P.S.; Ellis, G. Bio-based polymer nanocomposites based on nylon 11 and WS2 inorganic nanotubes. RSC Adv. 2015, 5, 17879–17887. [Google Scholar] [CrossRef] [Green Version]
- Yuan, P.; Tan, D.; Annabi-Bergaya, F. Properties and applications of halloysite nanotubes: Recent research advances and future prospects. Appl. Clay Sci. 2015, 112, 75–93. [Google Scholar] [CrossRef]
- Lvov, Y.; Wang, W.; Zhang, L.; Fakhrullin, R. Halloysite Clay Nanotubes for Loading and Sustained Release of Functional Compounds. Adv. Mater. 2016, 28, 1227–1250. [Google Scholar] [CrossRef] [PubMed]
- Lvov, Y.; Abdullayev, E. Green and functional polymer-clay nanotube composites with sustained release of chemical agents. Prog. Polym. Sci. 2013, 38, 1690–1719. [Google Scholar] [CrossRef]
- Hillier, S.; Brydson, R.; Delbos, E.; Fraser, T.; Gray, N.; Pendloeski, H.; Phillips, I.; Robertson, J.; Wilson, I. Correlations among the mineralogical and physical properties of halloysite nanotubes (HNTs). Clay Miner. 2016, 51, 325–350. [Google Scholar] [CrossRef]
- Lvov, Y.; Shchukin, D.; Möhwald, H.; Price, R. Clay Nanotubes for Controlled Release of Protective Agents–Perspectives. ACS Nano 2008, 2, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Arcudi, F.; Cavallaro, G.; Lazzara, G.; Massaro, M.; Milioto, S.; Noto, R.; Riela, S. Selective Functionalization of Halloysite Cavity by Click Reaction: Structured Filler for Enhancing Mechanical Properties of Bionanocomposite Films. J. Phys. Chem. C 2014, 118, 15095–15101. [Google Scholar] [CrossRef] [Green Version]
- Gorrasi, G.; Pantani, R.; Murariu, M.; Dubois, P. PLA/Halloysite Nanocomposite Films: Water Vapor Barrier Properties and Specific Key Characteristics. Macromol. Mater. Eng. 2014, 299, 104–115. [Google Scholar] [CrossRef]
- Liu, M.; Jia, Z.; Jia, D.; Zhou, C. Recent advance in research on halloysite-nanotubes polymer nanocomposites. Prog. Polym. Sci. 2014, 39, 1498–1525. [Google Scholar] [CrossRef]
- Abdullayev, E.; Lvov, Y. Clay Nanotubes for Corrosion Inhibitor Encapsulation: Release Control with End Stoppers. J. Mater. Chem. 2010, 20, 6681–6687. [Google Scholar] [CrossRef]
- Abdullayev, E.; Lvov, Y. Clay Nanotubes for Controlled Release of Protective Agents—A Review. J. Nanosci. Nanotechnol. 2011, 11, 10007–10026. [Google Scholar] [CrossRef] [PubMed]
- Scarfato, P.; Avallone, E.; Incarnato, L.; Di Maio, L. Development and evaluation of halloysite nanotube-based carrier for biocide activity in construction materials protection. Appl. Clay Sci. 2016, 132, 336–342. [Google Scholar] [CrossRef]
- Abdullayev, E.; Shchukin, D.; Lvov, Y. Halloysite Clay Nanotubes as a Reservoir for Corrosion Inhibitors and Template for Layer-by-Layer Encapsulation. Mater. Sci. Eng. 2008, 99, 331–332. [Google Scholar]
- Gorrasi, G. Dispersion of halloysite loaded with natural antimicrobials into pectins: Characterization and controlled release analysis. Carbohydr. Polym. 2015, 127, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Gorrasi, G.; Vertuccio, L. Evaluation of zein/halloysite nano-containers as reservoirs of active molecules for packaging applications: Preparation and analysis of physical properties. J. Cereal Sci. 2016, 70, 66–71. [Google Scholar] [CrossRef]
- Gorrasi, G.; Attanasio, G.; Izzo, L.; Sorrentino, A. Controlled release mechanisms of sodium benzoate from a biodegradable polymer and halloysite nanotube composite. Polym. Int. 2017, 66, 690–698. [Google Scholar] [CrossRef]
- Tully, J.; Yendluri, R.; Lvov, Y. Halloysite Clay Nanotubes for Enzyme Immobilization. Biomacromolecules 2016, 17, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Zhai, R.; Zhang, B.; Liu, L.; Xie, Y.; Zhang, H.; Liu, J. Immobilization of enzyme biocatalyst on natural halloysite nanotubes. Catal. Commun. 2010, 12, 259–263. [Google Scholar] [CrossRef]
- Bugatti, V.; Viscusi, G.; Naddeo, C.; Gorrasi, G. Nanocomposites Based on PCL and Halloysite Nanotubes Filled with Lysozyme: Effect of Draw Ratio on the Physical Properties and Release Analysis. Nanomaterials 2017, 7, 213. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yendluri, R.; Liu, K.; Guo, Y.; Lvov, Y.; Yan, X. Enzyme-immobilized clay nanotube-chitosan membranes with sustainable biocatalytic activities. Phys. Chem. 2017, 19, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Bugatti, V.; Sorrentino, A.; Gorrasi, G. Encapsulation of Lysozyme into halloysite nanotubes and dispersion in PLA: Structural and physical properties and controlled release analysis. Eur. Polym. J. 2017, 93, 495–506. [Google Scholar] [CrossRef]
- Gallagher, K.M.; Corrigan, O.I. Mechanistic aspects of the release of levamisole hydrochloride from biodegradable polymers. J. Control. Release 2000, 69, 261–272. [Google Scholar] [CrossRef]
- Qian, M.; Sun, Y.; Xu, X.; Liu, L.; Song, P.; Yu, Y.; Wangd, H.; Qian, J. 2D-Alumina platelets enhance mechanical and abrasion properties of PA612 via interfacial hydrogen-bond interactions. Chem. Eng. J. 2017, 308, 760–771. [Google Scholar] [CrossRef]
- Nychas, G.J.E.; Skandamis, P.N.; Tassou, C.C.; Koutsoumanis, K.P. Meat spoilage during distribution. Meat Sci. 2008, 78, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Gill, C.O.; Newton, K.G. The ecology of bacterial spoilage of fresh meat at chill temperatures. Meat Sci. 1978, 14, 43–60. [Google Scholar] [CrossRef]
- Jay, J.M.; Loessner, M.J.; Golden, D.A. Modern Food Microbiology; Springer: Berlin, Germany, 2005; Chapter 4; pp. 63–91. [Google Scholar]
- Gorrasi, G.; Milone, C.; Piperopoulos, E.; Lanza, M.; Sorrentino, A. Hybrid clay mineral-carbon nanotube-PLA nanocomposite films. Preparation and photodegradation effect on their mechanical, thermal and electrical properties. Appl. Clay Sci. 2013, 71, 49–54. [Google Scholar] [CrossRef]
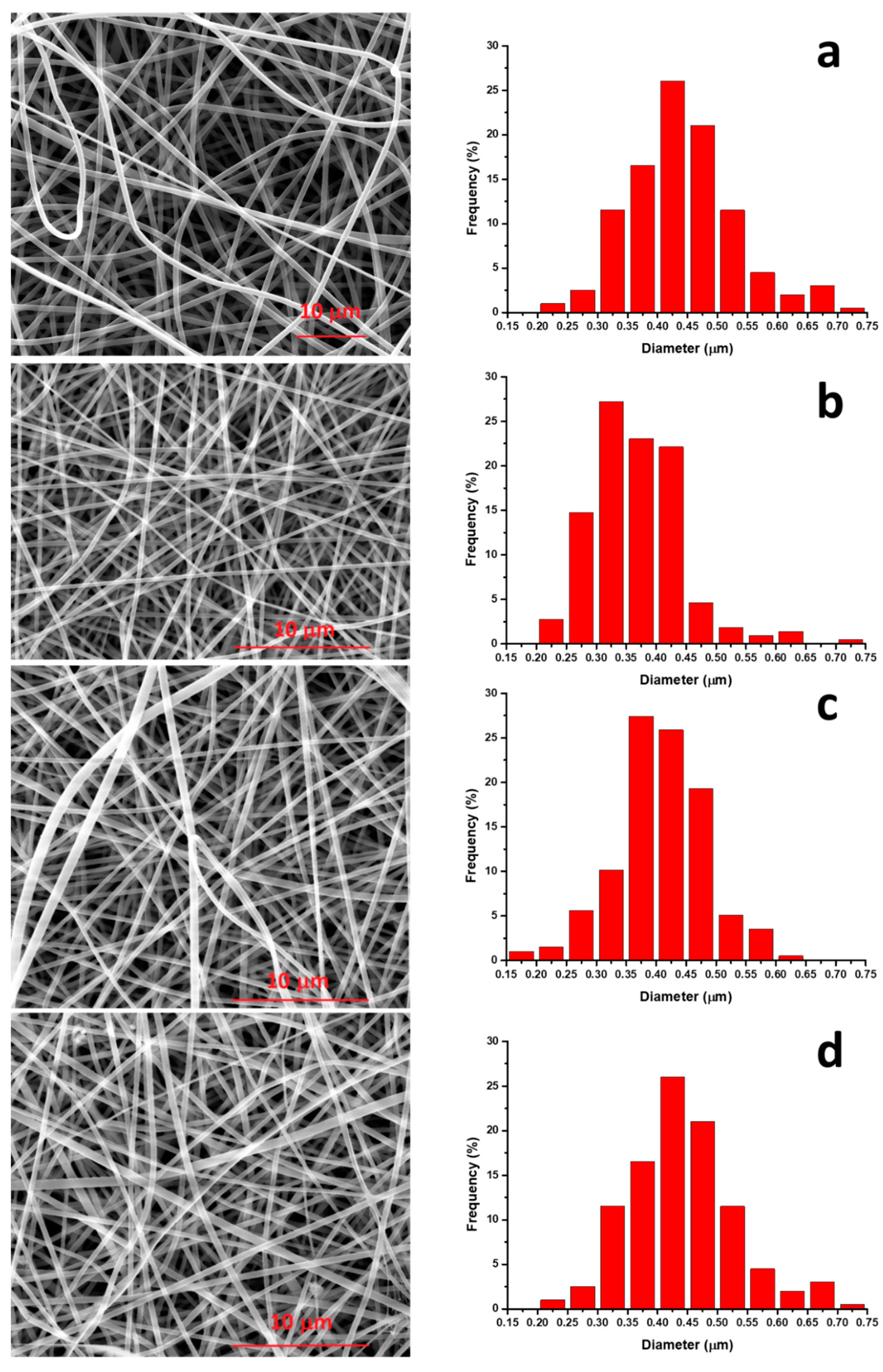
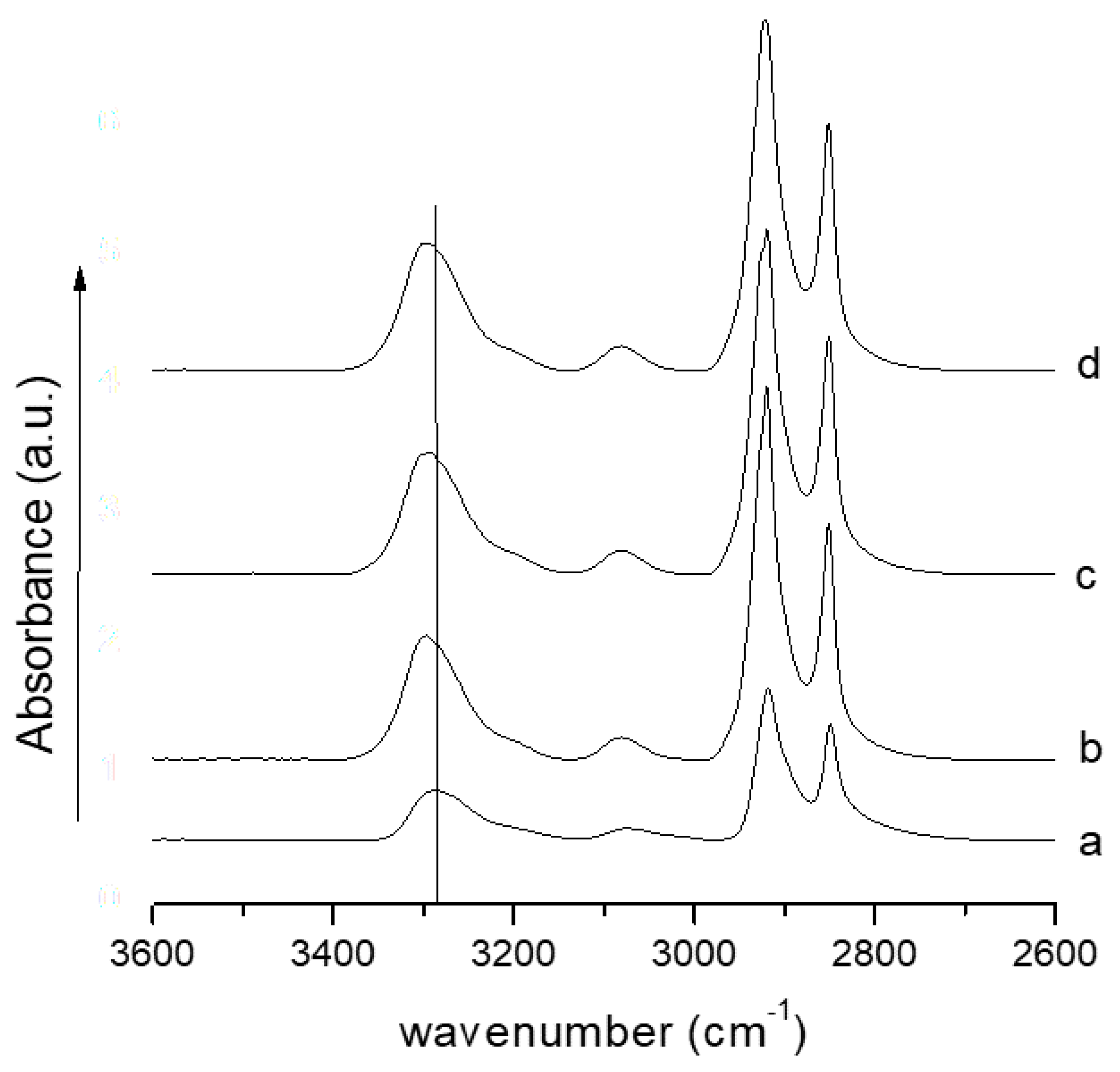

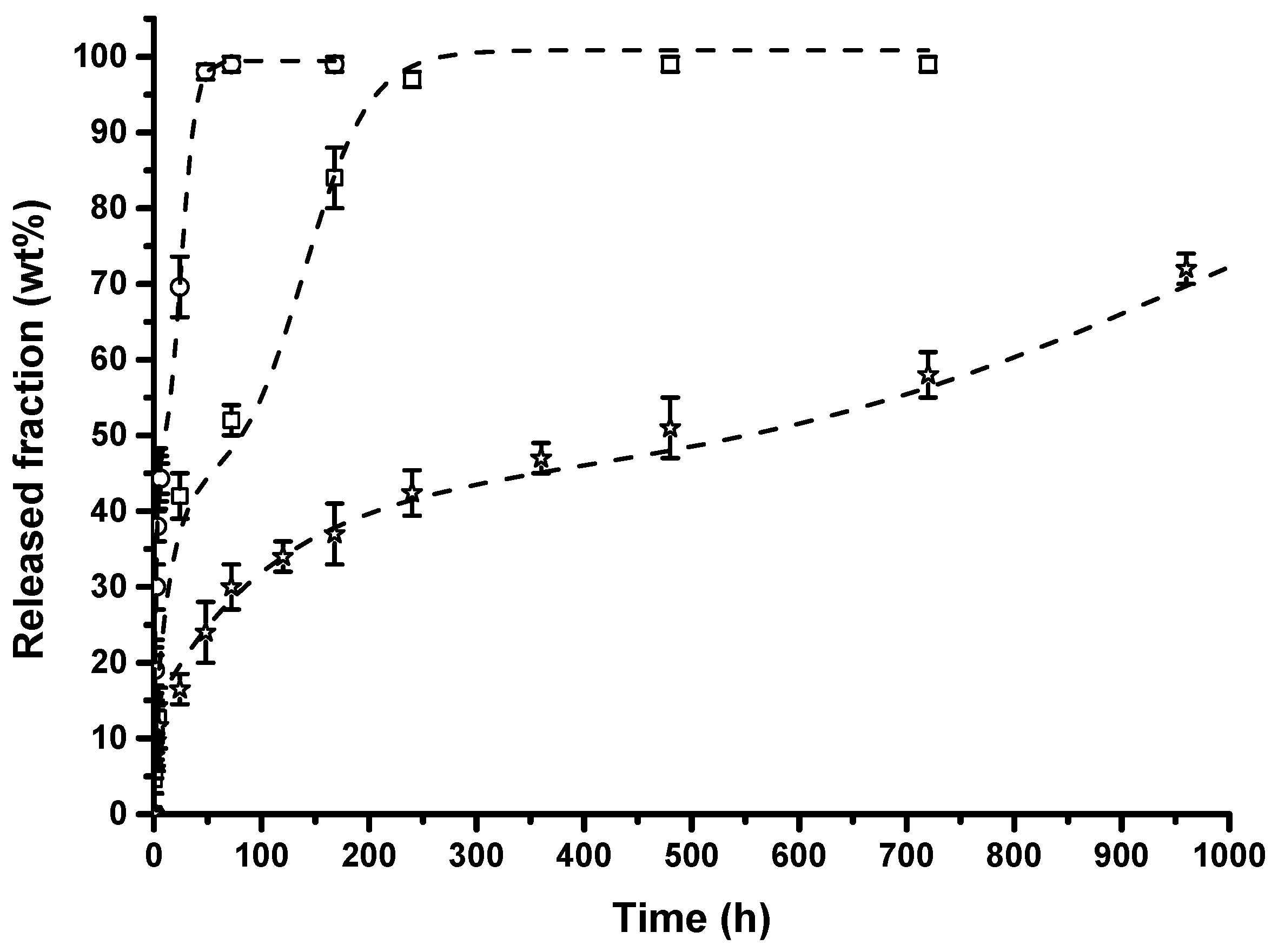
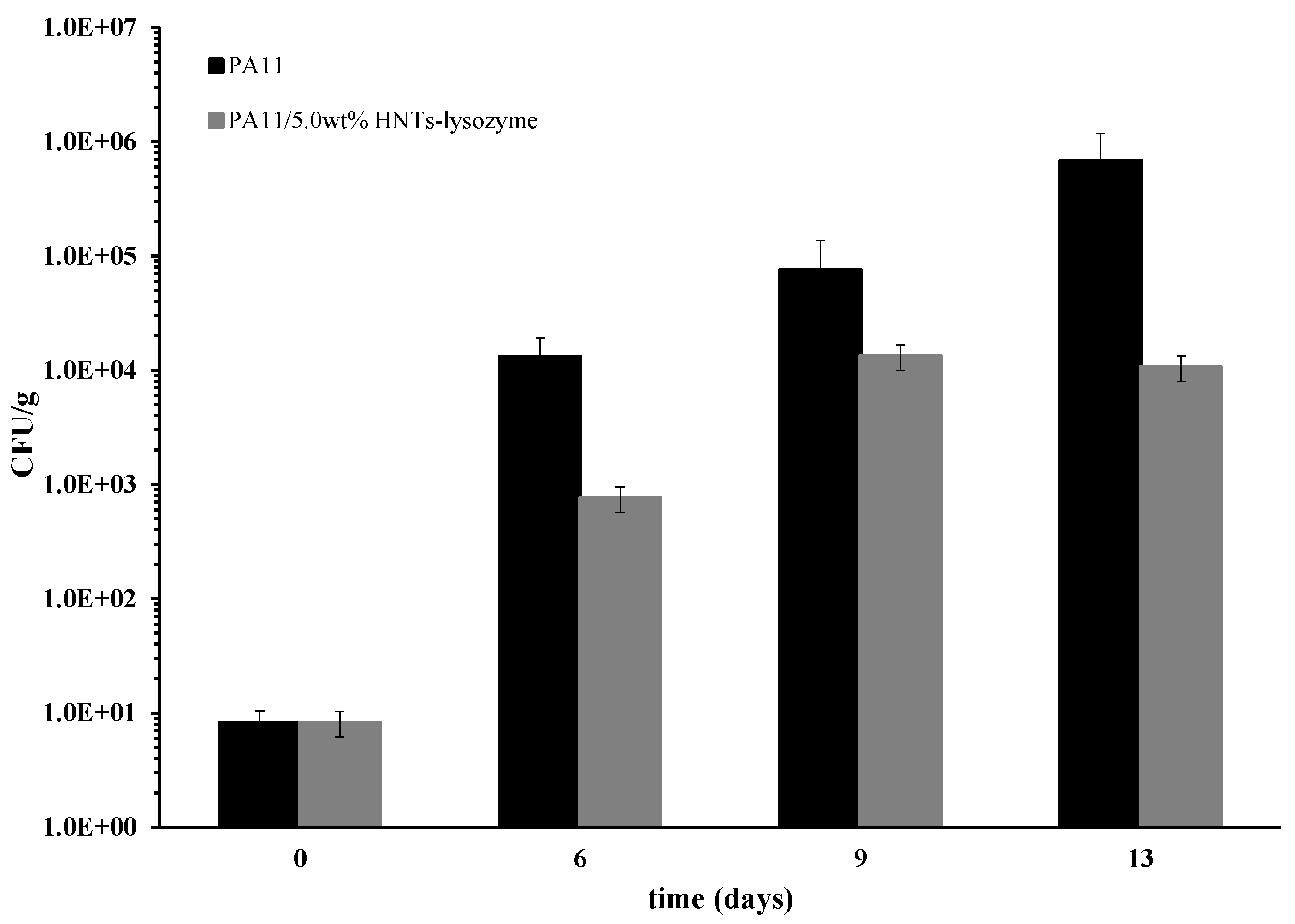
| Filler Loading (wt %) | E (MPa) |
|---|---|
| 0 | 23 ± 8 |
| 1.0 | 27 ± 16 |
| 2.5 | 58 ± 12 |
| 5.0 | 80 ± 18 |
| Sample | A | X1 (%) | X2 (%) | tmax (h) | C1 (h−1) | C2 (h−1) | R2 |
|---|---|---|---|---|---|---|---|
| PA11/1.0 wt % HNTs–lysozyme | 6 | 39 | 55 | 25 | 4.91 × 10−1 | 1.46 × 10−1 | 0.985 |
| PA11/2.5 wt % HNTs–lysozyme | 9 | 34 | 58 | 140 | 6.57 × 10−2 | 3.30 × 10−2 | 0.987 |
| PA11/5.0 wt % HNTs–lysozyme | 13 | 30 | 57 | 984 | 9.07 × 10−3 | 4.37 × 10−3 | 0.985 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bugatti, V.; Vertuccio, L.; Viscusi, G.; Gorrasi, G. Antimicrobial Membranes of Bio-Based PA 11 and HNTs Filled with Lysozyme Obtained by an Electrospinning Process. Nanomaterials 2018, 8, 139. https://doi.org/10.3390/nano8030139
Bugatti V, Vertuccio L, Viscusi G, Gorrasi G. Antimicrobial Membranes of Bio-Based PA 11 and HNTs Filled with Lysozyme Obtained by an Electrospinning Process. Nanomaterials. 2018; 8(3):139. https://doi.org/10.3390/nano8030139
Chicago/Turabian StyleBugatti, Valeria, Luigi Vertuccio, Gianluca Viscusi, and Giuliana Gorrasi. 2018. "Antimicrobial Membranes of Bio-Based PA 11 and HNTs Filled with Lysozyme Obtained by an Electrospinning Process" Nanomaterials 8, no. 3: 139. https://doi.org/10.3390/nano8030139





