Palladium Supported on Titanium Carbide: A Highly Efficient, Durable, and Recyclable Bifunctional Catalyst for the Transformation of 4-Chlorophenol and 4-Nitrophenol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
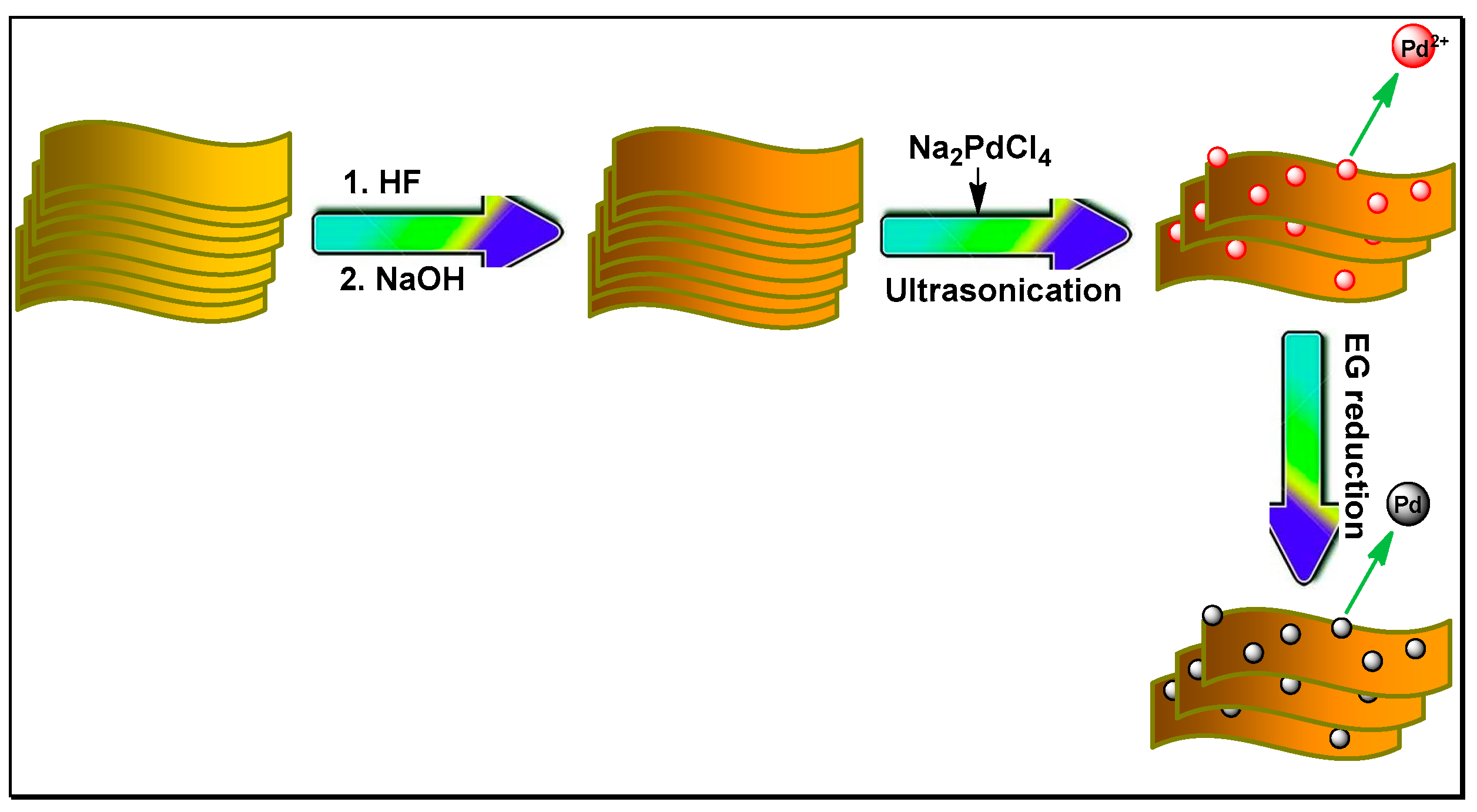
2.3. Synthesis of Ti3C2X2, alk-Ti3C2X2, and Pd/alk-Ti3C2X2
2.4. Activity Tests
3. Results and Discussion
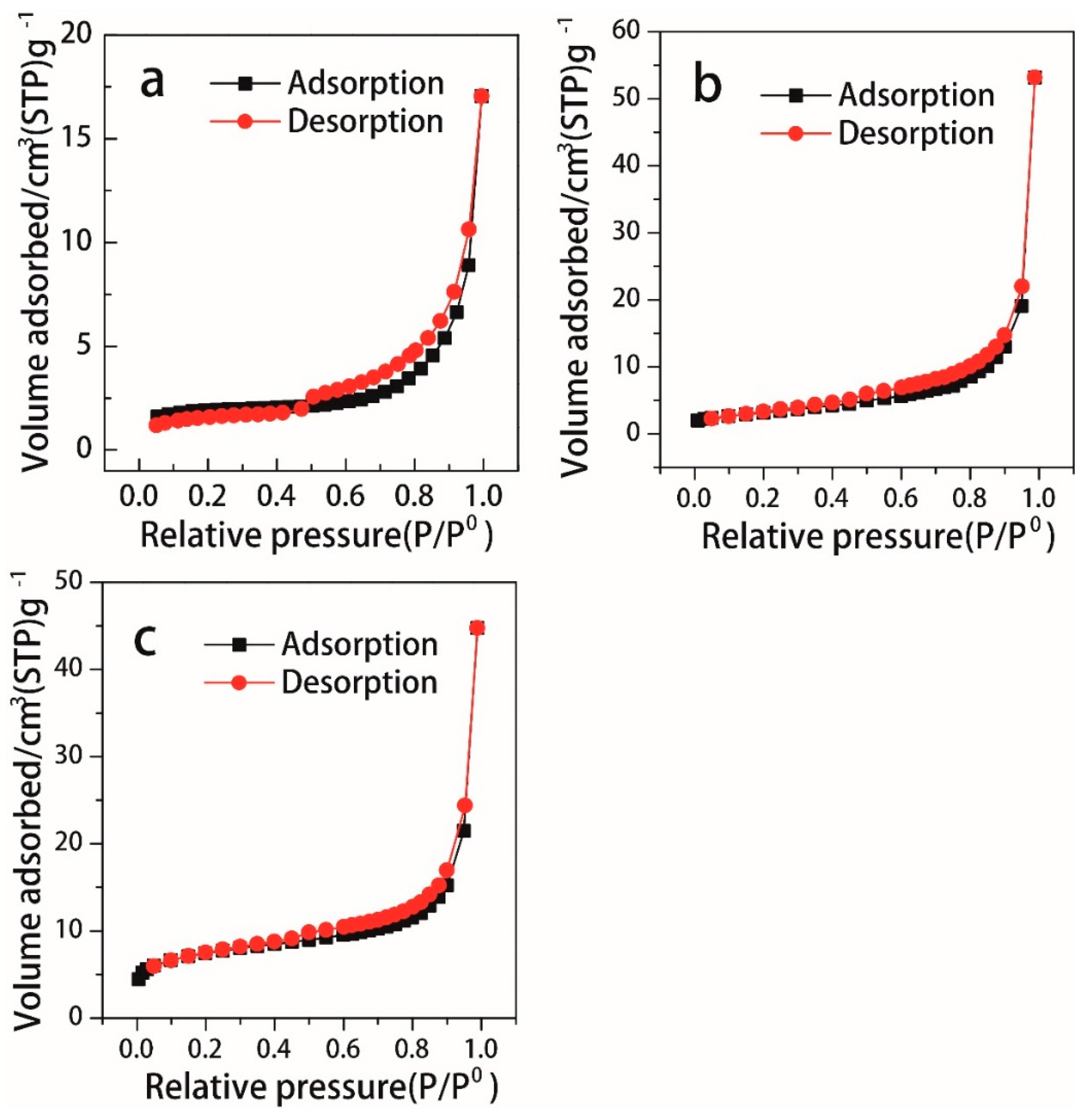
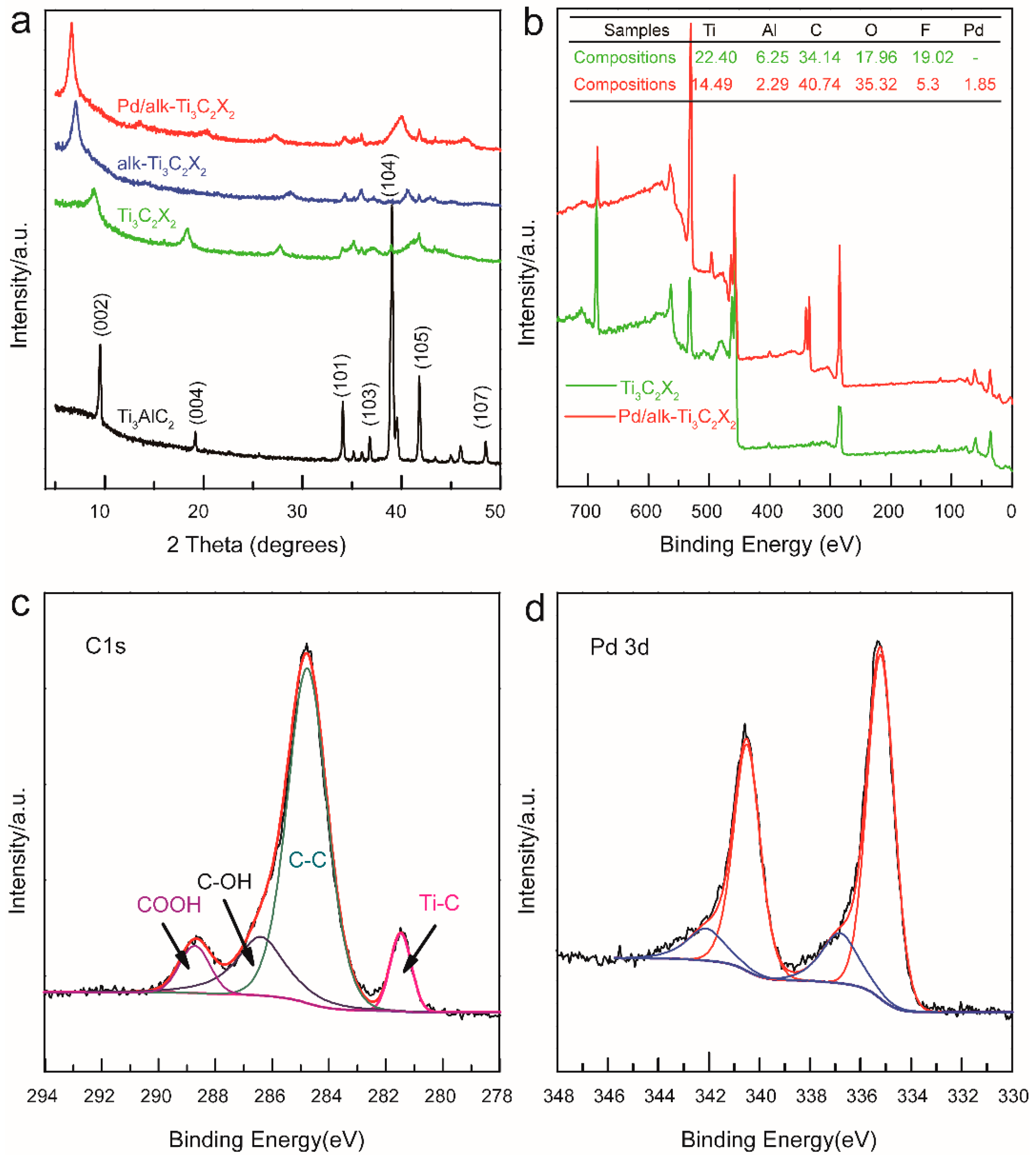
3.1. Characterization of the Catalyst
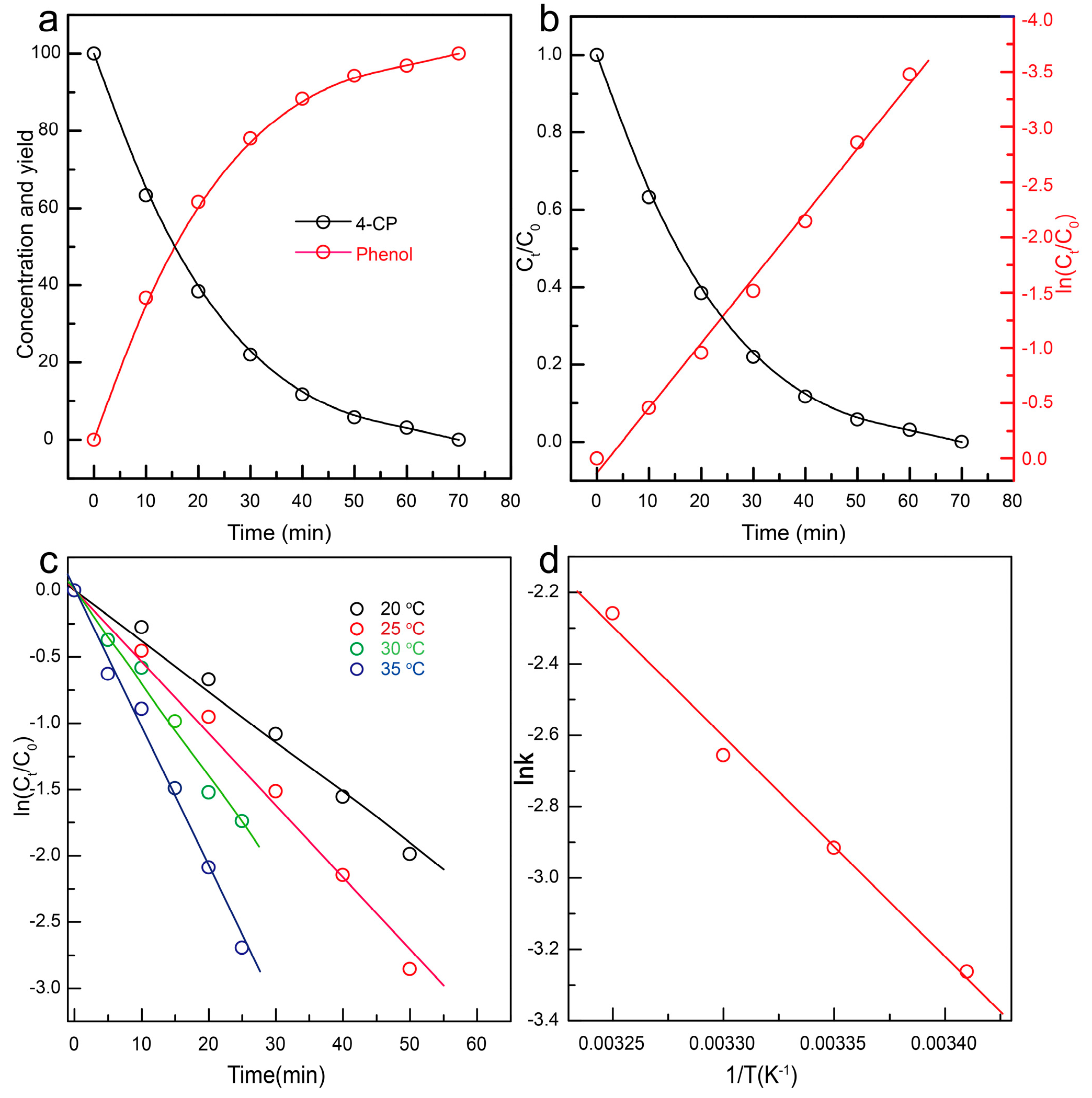
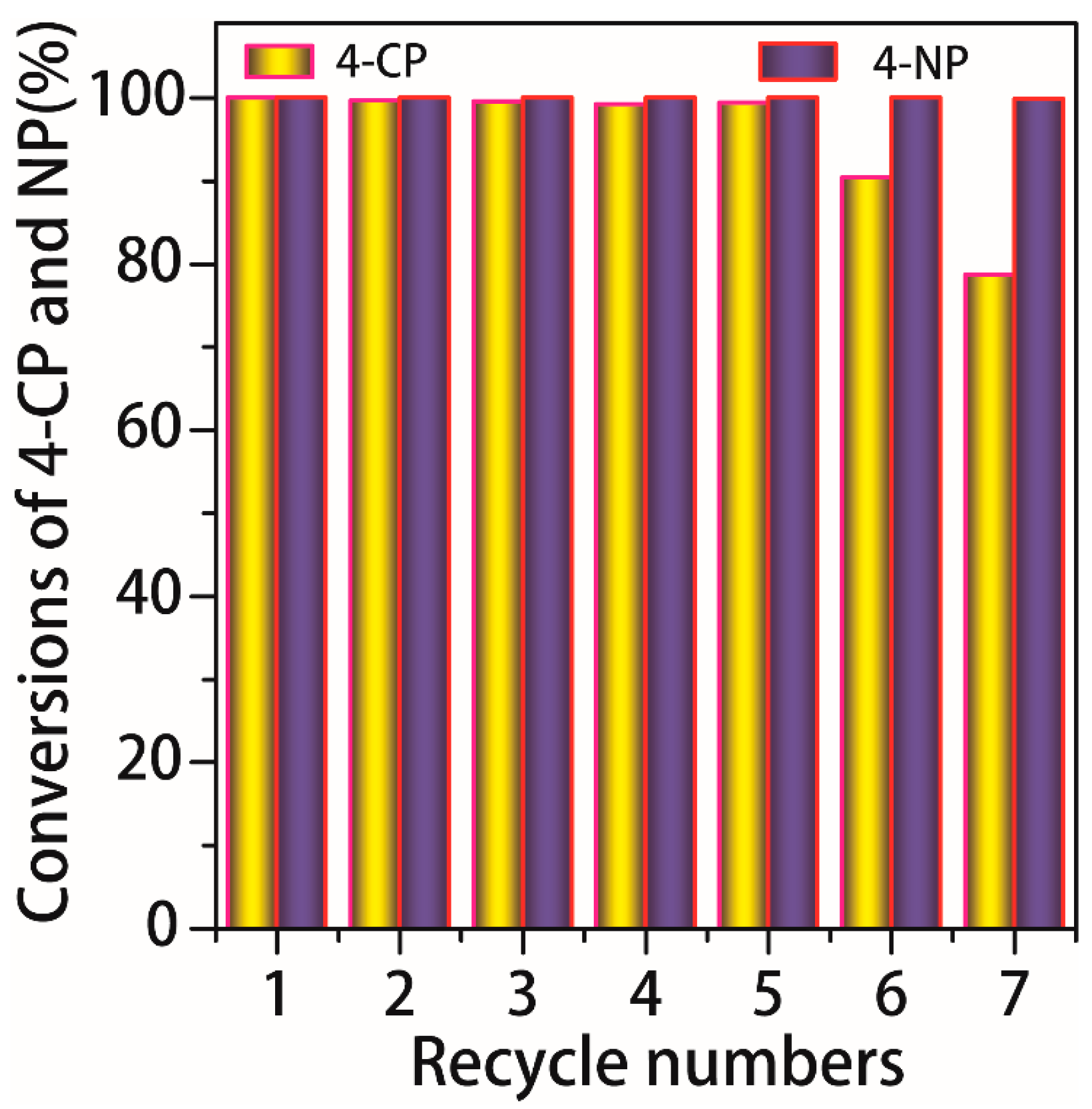
3.2. Catalytic HDC of 4-CP
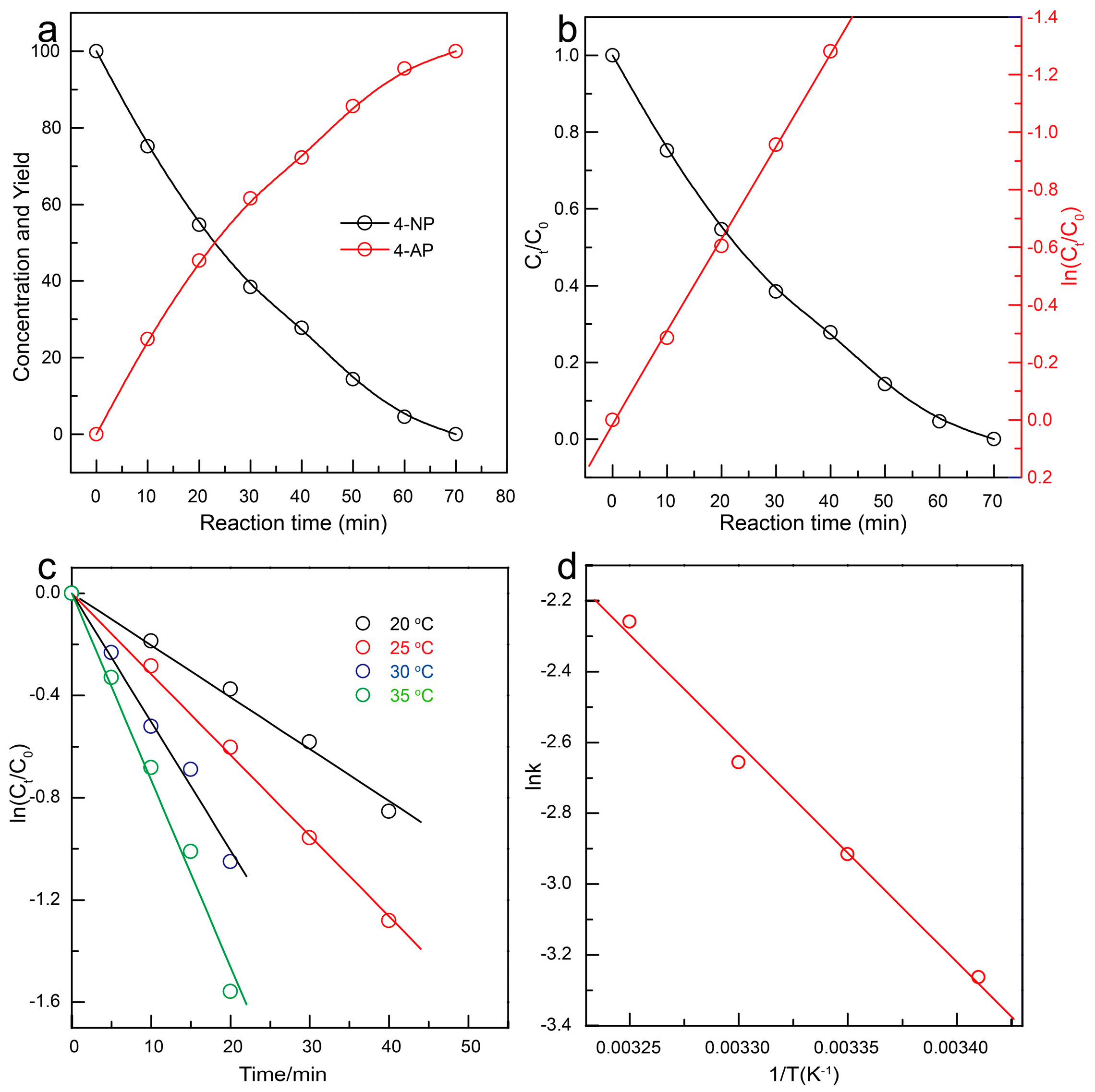
3.3. Catalytic Hydrogenation of 4-NP
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ren, Y.; Fan, G.; Wang, C. Aqueous hydrodechlorination of 4-chlorophenol over an Rh/reduced graphene oxide synthesized by a facile one-pot solvothermal process under mild conditions. J. Hazard. Mater. 2014, 274, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Fan, G.; Wang, C.; Zhang, L. Aqueous phase catalytic hydrodechlorination of 4-chlorophenol over palladium deposited on reduced graphene oxide. Catal. Commun. 2014, 46, 219–223. [Google Scholar] [CrossRef]
- Nie, R.; Wang, J.; Wang, L.; Qin, Y.; Chen, P.; Hou, Z. Platinum supported on reduced graphene oxide as a catalyst for hydrogenation of nitroarenes. Carbon 2012, 50, 586–596. [Google Scholar] [CrossRef]
- Fan, G.; Huang, W.; Wang, C. In situ synthesis of Ru/RGO nanocomposites as a highly efficient catalyst for selective hydrogenation of halonitroaromatics. Nanoscale 2013, 5, 6819–6825. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Guo, J.; Zhang, Q.; Xiang, J.; Liu, B.; Zhou, A.; Liu, R.; Tian, Y. Unique lead adsorption behavior of activated hydroxyl group in two-dimensional titanium carbide. J. Am. Chem. Soc. 2014, 136, 4113–4116. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Chen, S.; Ding, W.; Nie, Y.; Wei, Z. An extraordinarily stable catalyst: Pt NPs supported on two-dimensional Ti3C2X2 (X = OH, F) nanosheets for oxygen reduction reaction. Chem. Commun. 2013, 49, 10112–10114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, H.; Zou, G.; Fernandez, C.; Liu, B.; Zhang, Q.; Hu, J.; Peng, Q. Self-reduction synthesis of new MXene/Ag composites with unexpected electrocatalytic activity. ACS Sustain. Chem. Eng. 2016, 4, 6763–6771. [Google Scholar] [CrossRef]
- Satheeshkumar, E.; Makaryan, T.; Melikyan, A.; Minassian, H.; Gogotsi, Y.; Yoshimura, M. One-step solution processing of Ag, Au and Pd@MXene hybrids for SERS. Sci. Rep. 2016, 6, 32049. [Google Scholar] [CrossRef] [PubMed]
- Zou, G.; Zhang, Z.; Guo, J.; Liu, B.; Zhang, Q.; Fernandez, C.; Peng, Q. Synthesis of MXene/Ag composites for extraordinary long cycle lifetime lithium storage at high rates. ACS Appl. Mater. Interfaces 2016, 8, 22280–22286. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zeng, C.; Fan, G. Magnetic RuCo nanoparticles supported on two-dimensional titanium carbide as highly active catalysts for the hydrolysis of ammonia borane. Int. J. Hydrogen Energy 2015, 40, 9217–9224. [Google Scholar] [CrossRef]
- Li, X.; Zeng, C.; Fan, G. Ultrafast hydrogen generation from the hydrolysis of ammonia borane catalyzed by highly efficient bimetallic RuNi nanoparticles stabilized on Ti3C2X2 (X = OH and/or F). Int. J. Hydrogen Energy 2015, 40, 3883–3891. [Google Scholar] [CrossRef]
- Fan, G.; Li, X.; Ma, Y.; Zhang, Y.; Wu, J.; Xu, B.; Sun, T.; Gao, D.; Bi, J. Magnetic, recyclable PtyCo1−y/Ti3C2X2 (X = O, F) catalyst: a facile synthesis and enhanced catalytic activity for hydrogen generation from the hydrolysis of ammonia borane. New J. Chem. 2017, 41, 2793–2799. [Google Scholar] [CrossRef]
- Ming, M.; Ren, Y.; Hu, M.; Zhang, Y.; Sun, T.; Ma, Y.; Li, X.; Jiang, W.; Gao, D.; Bi, J.; et al. Promoted effect of alkalization on the catalytic performance of Rh/alk-Ti3C2X2 (X = O, F) for the hydrodechlorination of chlorophenols in base-free aqueous medium. Appl. Catal. B 2017, 210, 462–469. [Google Scholar] [CrossRef]
- Molina, C.B.; Pizarro, A.H.; Casas, J.A.; Rodriguez, J.J. Aqueous-phase hydrodechlorination of chlorophenols with pillared clays-supported Pt, Pd and Rh catalysts. Appl. Catal. B 2014, 148–149, 330–338. [Google Scholar] [CrossRef]
- Dong, Z.; Le, X.; Liu, Y.; Dong, C.; Ma, J. Metal organic framework derived magnetic porous carbon composite supported gold and palladium nanoparticles as highly efficient and recyclable catalysts for reduction of 4-nitrophenol and hydrodechlorination of 4-chlorophenol. J. Mater. Chem. A 2014, 2, 18775–18785. [Google Scholar] [CrossRef]
- Dong, Z.; Le, X.; Dong, C.; Zhang, W.; Li, X.; Ma, J. Ni@Pd core–shell nanoparticles modified fibrous silica nanospheres as highly efficient and recoverable catalyst for reduction of 4-nitrophenol and hydrodechlorination of 4-chlorophenol. Appl. Catal. B 2015, 162, 372–380. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Le, X.; Zhang, W.; Gu, H.; Xue, R.; Ma, J. Catalysis of the hydro-dechlorination of 4-chlorophenol by Pd(0)-modified MCM-48 mesoporous microspheres with an ultra-high surface area. New J. Chem. 2015, 39, 4519–4525. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, J.; Wang, D.; Turhong, M.; Zhang, M. Recyclable and effective Pd/poly(N-isopropylacrylamide) catalyst for hydrodechlorination of 4-chlorophenol in aqueous solution. Chem. Eng. J. 2015, 280, 158–164. [Google Scholar] [CrossRef]
- Baeza, J.A.; Calvo, L.; Rodriguez, J.J.; Carbó-Argibay, E.; Rivas, J.M.; Gilarranz, A. Activity enhancement and selectivity tuneability in aqueous phase hydrodechlorination by use of controlled growth Pd-Rh nanoparticles. Appl. Catal. B 2015, 168–169, 283–292. [Google Scholar] [CrossRef]
- Dong, Z.; Dong, C.; Liu, Y.; Le, X.; Jin, Z.; Ma, J. Hydrodechlorination and further hydrogenation of 4-chlorophenol to cyclohexanone in water over Pd nanoparticles modified N-doped mesoporous carbon microspheres. Chem. Eng. J. 2015, 270, 215–222. [Google Scholar] [CrossRef]
- Baeza, J.A.; Calvo, L.; Rodriguez, J.J.; Gilarranz, M.A. Catalysts based on large size-controlled Pd nanoparticles for aqueous-phase hydrodechlorination. Chem. Eng. J. 2016, 294, 40–48. [Google Scholar] [CrossRef]
- Munoz, M.; de Pedro, Z.M.; Casas, J.A.; Rodriguez, J.J. Improved γ-alumina-supported Pd and Rh catalysts for hydrodechlorination of chlorophenols. Appl. Catal. A 2014, 488, 78–85. [Google Scholar] [CrossRef]
- Yang, B.; Deng, S.; Yu, G.; Lu, Y.; Zhang, H.; Xiao, J.; Chen, G.; Cheng, X.; Shi, L. Pd/Al bimetallic nanoparticles for complete hydrodechlorination of 3-chlorophenol in aqueous solution. Chem. Eng. J. 2013, 219, 492–498. [Google Scholar] [CrossRef]
- Fang, D.; Li, W.; Zhao, J.; Liu, S.; Ma, X.; Xu, J.; Xia, C. Catalytic hydrodechlorination of 4-chlorophenol over a series of Pd-Cu/γ-Al2O3 bimetallic catalysts. RSC Adv. 2014, 4, 59204–59210. [Google Scholar] [CrossRef]
- Jiang, W.; Xiang, Z.; Xu, B.; Li, X.; Liu, F.; Fan, G. Convenient preparation of Pd/RGO catalyst for the efficient hydrodechlorination of various chlorophenols. New J. Chem. 2016, 40, 372–376. [Google Scholar] [CrossRef]
- Ren, Y.; Fan, G.; Jiang, W.; Xu, B.; Liu, F. Effective hydrodechlorination of 4-chlorophenol catalysed by magnetic palladium/reduced graphene oxide under mild conditions. RSC Adv. 2014, 4, 25440–25446. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, K.; Wang, W.; Xu, Z.; Wan, H.; Zheng, S. Pd supported on boron-doped mesoporous carbon as highly active catalyst for liquid phase catalytic hydrodechlorination of 2,4-dichlorophenol. Appl. Catal. A 2014, 470, 336–343. [Google Scholar] [CrossRef]
- Xia, C.; Liu, Y.; Xu, J.; Yu, J.; Qin, W.; Liang, X. Catalytic hydrodechlorination reactivity of monochlorophenols in aqueous solutions over palladium/carbon catalyst. Catal. Commun. 2009, 10, 456–458. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, X.; Wang, S.; Li, D.; Lu, G. The effect of triethylamine on the hydrodechlorination of chlorophenols on Pd/C at low temperature. Catal. Commun. 2009, 10, 2027–2030. [Google Scholar] [CrossRef]
- Jin, Z.; Yu, C.; Wang, X.; Wan, Y.; Li, D.; Lu, G. Liquid phase hydrodechlorination of chlorophenols at lower temperature on a novel Pd catalyst. J. Hazard. Mater. 2011, 186, 1726–1732. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Yu, C.; Wang, X.; Wan, Y.; Li, D.; Lu, G. Hydrodechlorination of chlorophenols at low temperature on a novel Pd catalyst. Chem. Commun. 2009, 4438–4440. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, Y.; Gong, Y.; Zhang, P.; Li, H.; Wang, Y. Synthesis of palladium nanoparticles supported on mesoporous N-doped carbon and their catalytic ability for biofuel upgrade. J. Am. Chem. Soc. 2012, 134, 16987–16990. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, Y.; Zhang, J.; Li, G.; Huang, H.; Zhang, X.; Jiang, Q. Enhancement of the electrical properties of MXene Ti3C2 nanosheets by post-treatments of alkalization and calcination. Mater. Lett. 2015, 160, 537–540. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Halim, J.; Dyatkin, B.; Naguib, M.; Buranova, Y.S.; Barsoum, M.W.; Gogotsi, Y. Room-temperature carbide-ferived carbon dynthesis by electrochemical etching of MAX phases. Angew. Chem. Int. Ed. 2014, 53, 4877–4880. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Zuo, W.; Tian, M.; Dong, Z.; Ma, J. Highly efficient and recyclable Ni MOF-derived N-doped magnetic mesoporous carbon-supported palladium catalysts for the hydrodechlorination of chlorophenols. J. Mol. Catal. A Chem. 2016, 423, 386–392. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, Z.; Li, X.; Le, X.; Zhang, W.; Ma, J. Aqueous-phase hydrodechlorination and further hydrogenation of chlorophenols to cyclohexanone in water over palladium nanoparticles modified dendritic mesoporous silica nanospheres catalyst. RSC Adv. 2015, 5, 20716–20723. [Google Scholar] [CrossRef]
- Díaz, E.; Casas, J.A.; Mohedano, Á.F.; Calvo, L.; Gilarranz, M.A.; Rodríguez, J.J. Kinetics of the hydrodechlorination of 4-chlorophenol in water using Pd, Pt, and Rh/Al2O3 catalysts. Ind. Eng. Chem. Res. 2008, 47, 3840–3846. [Google Scholar] [CrossRef]
- Díaz, E.; Casas, J.A.; Mohedano, Á.F.; Calvo, L.; Gilarranz, M.A.; Rodríguez, J.J. Kinetics of 4-chlorophenol hydrodechlorination with alumina and activated carbon-supported Pd and Rh catalysts. Ind. Eng. Chem. Res. 2009, 48, 3351–3358. [Google Scholar] [CrossRef]
- Molina, C.B.; Calvo, L.; Gilarranz, M.A.; Casas, J.A.; Rodriguez, J.J. Pd–Al pillared clays as catalysts for the hydrodechlorination of 4-chlorophenol in aqueous phase. J. Hazard. Mater. 2009, 172, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Sainero, L.M.; Seoane, X.L.; Fierro, J.L.G.; Arcoya, A. Liquid-phase hydrodechlorination of CCl4 to CHCl3 on Pd/carbon catalysts: nature and role of Pd active species. J. Catal. 2002, 209, 279–288. [Google Scholar] [CrossRef]
- Baeza, J.A.; Calvo, L.; Gilarranz, M.A.; Mohedano, A.F.; Casas, J.A.; Rodriguez, J.J. Catalytic behavior of size-controlled palladium nanoparticles in the hydrodechlorination of 4-chlorophenol in aqueous phase. J. Catal. 2012, 293, 85–93. [Google Scholar] [CrossRef]
- Álvarez-Montero, M.A.; Gómez-Sainero, L.M.; Martín-Martínez, M.; Heras, F.; Rodriguez, J.J. Hydrodechlorination of chloromethanes with Pd on activated carbon catalysts for the treatment of residual gas streams. Appl. Catal. B 2010, 96, 148–156. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, Q.; Han, Y.; Zheng, S. Enhanced catalytic hydrodechlorination of 2,4-dichlorophenol over Pd catalysts supported on nitrogen-doped graphene. RSC Adv. 2015, 5, 91363–91371. [Google Scholar] [CrossRef]

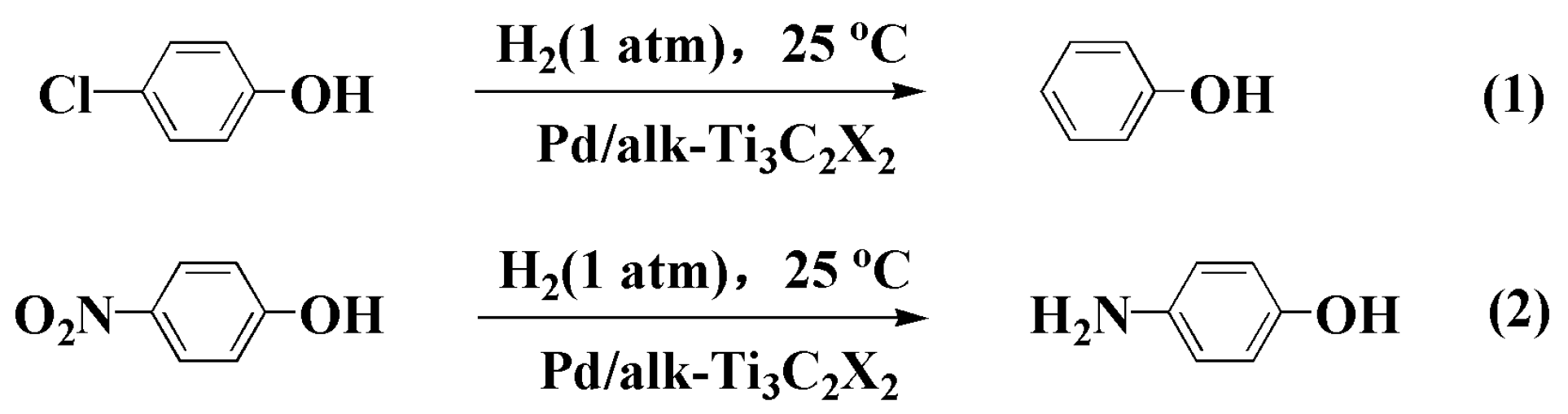
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, G.; Li, X.; Xu, C.; Jiang, W.; Zhang, Y.; Gao, D.; Bi, J.; Wang, Y. Palladium Supported on Titanium Carbide: A Highly Efficient, Durable, and Recyclable Bifunctional Catalyst for the Transformation of 4-Chlorophenol and 4-Nitrophenol. Nanomaterials 2018, 8, 141. https://doi.org/10.3390/nano8030141
Fan G, Li X, Xu C, Jiang W, Zhang Y, Gao D, Bi J, Wang Y. Palladium Supported on Titanium Carbide: A Highly Efficient, Durable, and Recyclable Bifunctional Catalyst for the Transformation of 4-Chlorophenol and 4-Nitrophenol. Nanomaterials. 2018; 8(3):141. https://doi.org/10.3390/nano8030141
Chicago/Turabian StyleFan, Guangyin, Xiaojing Li, Caili Xu, Weidong Jiang, Yun Zhang, Daojiang Gao, Jian Bi, and Yi Wang. 2018. "Palladium Supported on Titanium Carbide: A Highly Efficient, Durable, and Recyclable Bifunctional Catalyst for the Transformation of 4-Chlorophenol and 4-Nitrophenol" Nanomaterials 8, no. 3: 141. https://doi.org/10.3390/nano8030141