3.1. TEM Observations on Freeze-Fracture Replicas
Consider the UFB replicas. In the replicas, we found spherical or elliptical holes with a diameter of several hundred nm in every replica film. For example,
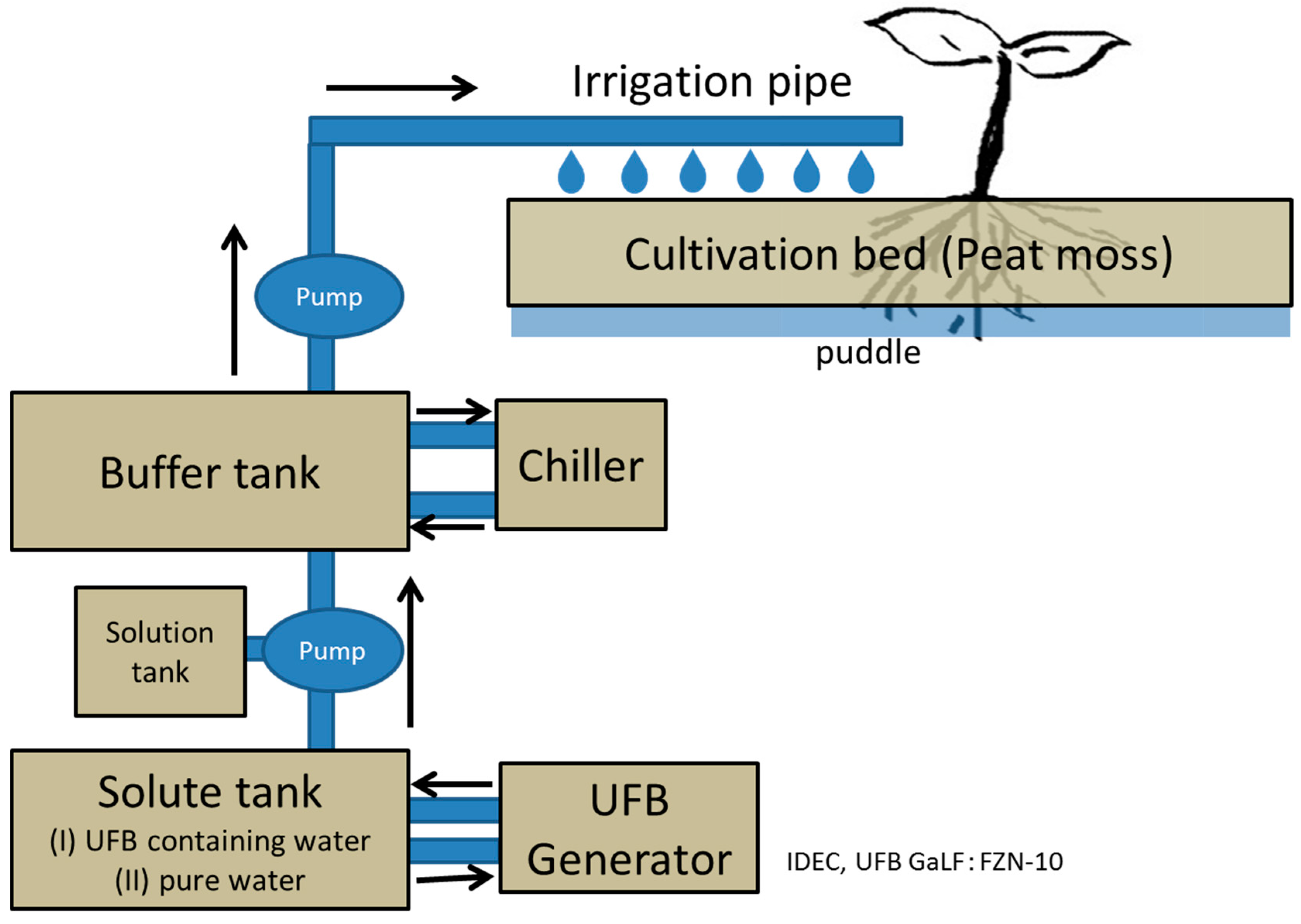
Figure 1 shows TEM images of typical UFBs observed in seven solution samples, including samples from the UFB-added solution (I) and the control solution without added UFBs (II) (see Figure 8 for sample locations). The samples from the buffer tank are called
I-BT and
II-BT as initial conditions, from the irrigation pipe are
I-P and
II-P as transporting conditions, and from the puddle near the root are
I-R and
II-R as near-plant conditions. The tap water sample used for the UFB solution preparation is called
I-W, which is also referred to as the initial condition. We found that enough UFBs, at least 10
6 mL
−1 in number concentration, were present in each solution sample to determine the size distributions with the freeze-fracture replica method.
Except for the pure-water case, the images show a thin layer, about 10-nm thick for most samples, on the UFB surface (e.g.,
Figure 1b). This layer closely resembles that observed on UFBs dispersed in wastewater (Figure 5 in [
6]) or that observed in NaCl solution (Figure 8 in [
7]). Thus, we consider that this thin layer is a concentrated layer of impurities, likely coming from solutes in the nutrient solution.
The thickness of this “impurity layer” was often much greater in the rhizosphere
I-R and
II-R samples (e.g.,
Figure 1f). In such a UFB, impurity masses are sometimes found clustered into islands on the surface (
Figure 1h). Moreover,
Figure 1i shows that impurity masses can also occur away from UFBs. Regarding the distinction between these impurity masses and UFBs, we examined the roughness (concave or convex) of the objects using the direction of shadow when platinum was deposited. As the “impurity layers” and impurity deposits are thicker below the peat moss (i.e., in the
I-R samples), we consider that the thick layer as seen in
Figure 1h likely formed when the UFBs passed through the soil.
3.2. Size Distribution of UFBs
To obtain quantitative data on UFBs in the solution, we prepared five replica samples from each type of solution and each sample location. Then, for each sample, we examined the bubble forms in at least five areas with the TEM. Assuming that the bubble shapes are elliptical, we measured the long and short diameters in the images, and then calculated the equivalent diameter d of the circle having the same cross-sectional area. These diameters were put into 1 of 13 size classes (100 nm bin-size). As the number of observed UFBs is different in each area of a given sample, the number in each size class is normalized by the total observed number n of UFBs in all five (or more) areas of the sample (n is about 100). We then determined the total number density N in the sample solution (estimated from n, described later), and multiplied the above fraction for each size class to determine their number density. Then, we obtained the diameter histogram and calculated the average bubble diameter <d>.
First, we consider the size distribution and state of the UFBs in nutrient solution before feeding. We find that the UFB distributions peak at diameters
d below 300 nm in all solutions, but some MBs have
d > 1 μm. For example,
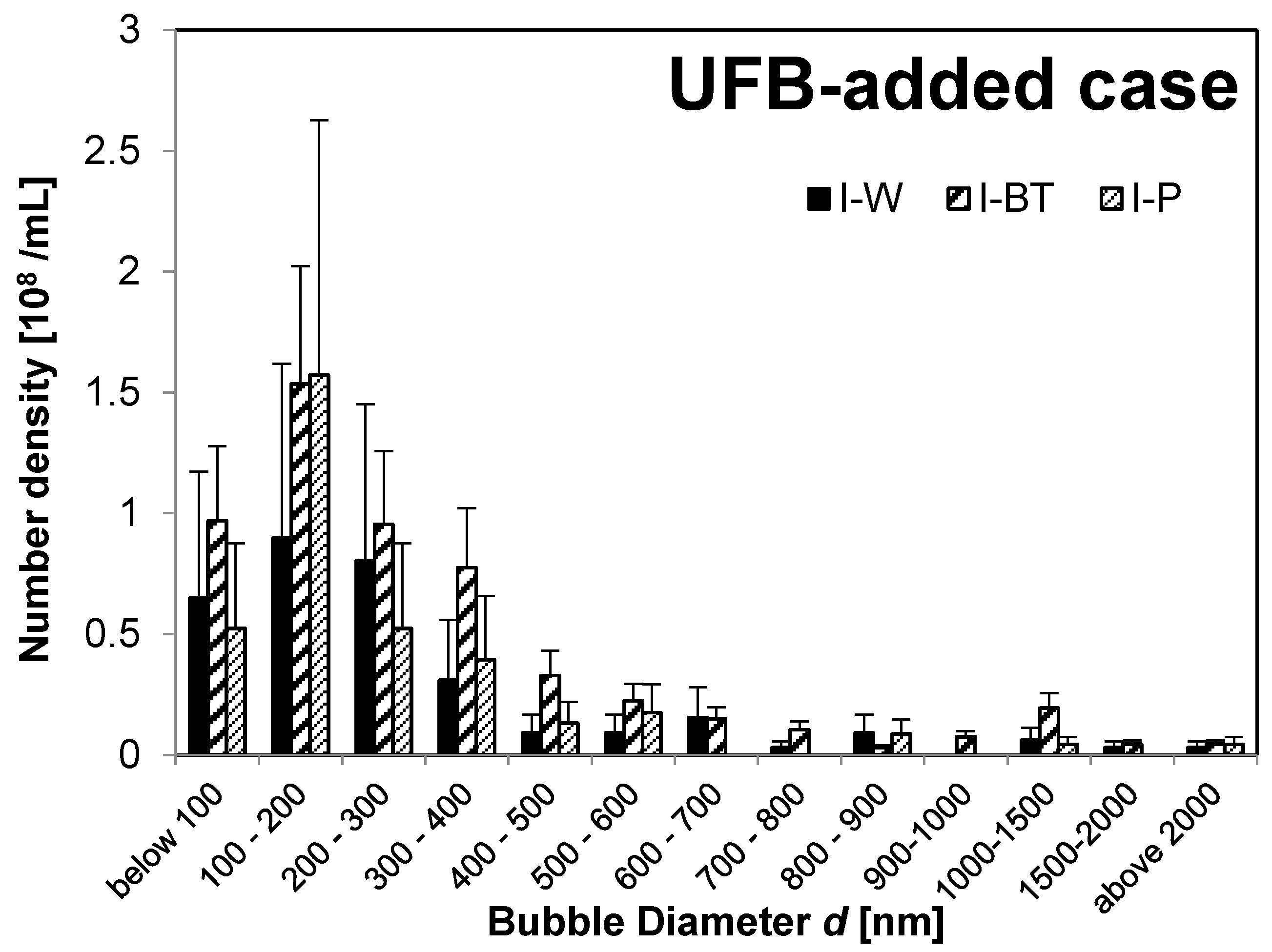
Figure 2 shows the bubble-diameter histograms of the UFB-added case solutions
(I-W solution,
I-BT solution, and
I-P solution), whereas
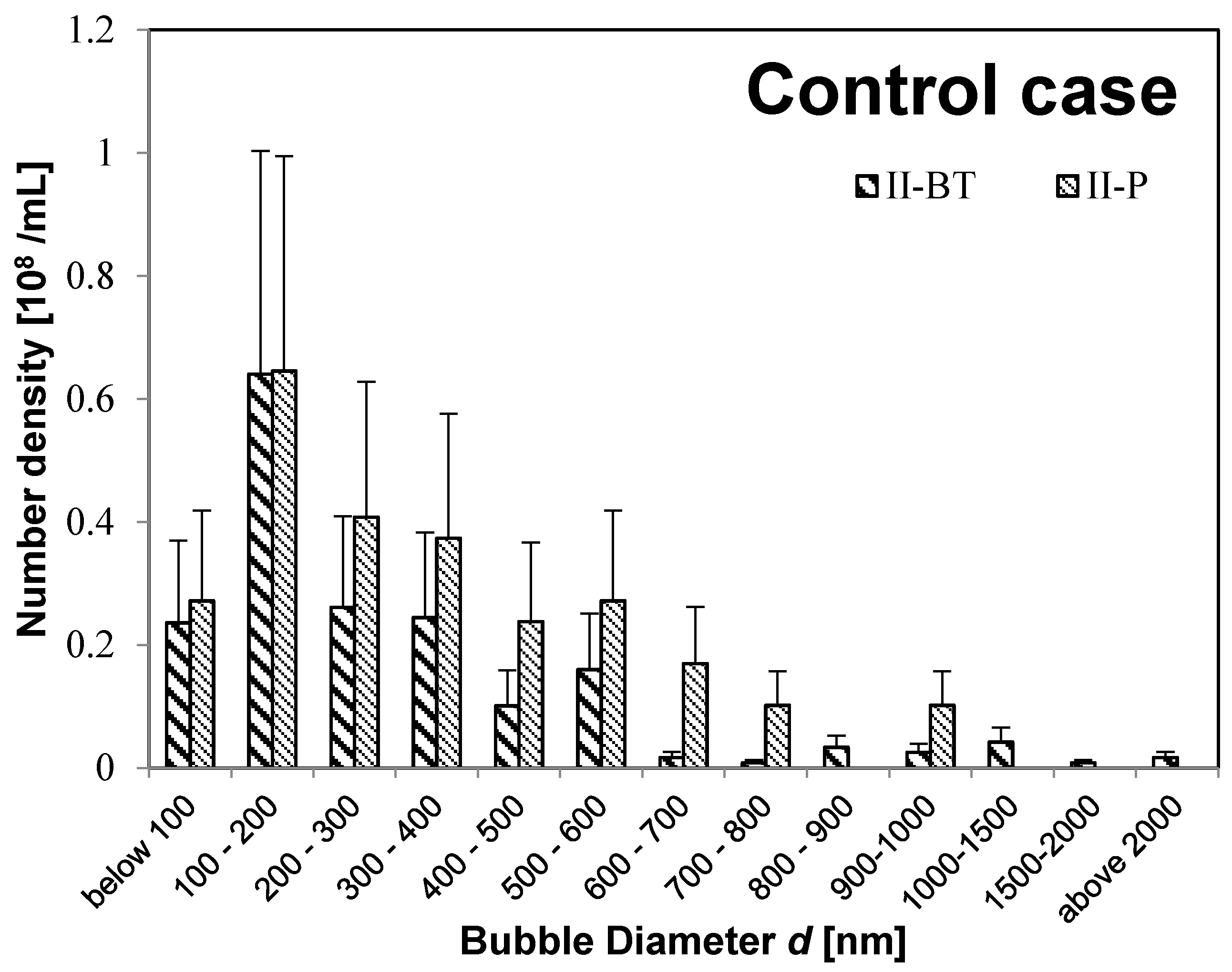
Figure 3 shows the results of the control case (
II-BT solution and
II-P solution).
The
I-W and
I-BT solutions have nearly the same UFB size distributions, meaning that the addition of the nutrient solution (about 0.3%) did not change the size distribution. However, the TEM images in
Figure 1b, and d show that an impurity layer forms on the surface of UFBs in the nutrient solution. So, if the amount of nutrient solution were to increase, the size distribution of UFBs may begin to be affected by the accumulation of impurities on the UFBs.
Moreover, the buffer-tank UFB-distribution is nearly the same as that for the irrigation pipe for both the UFB-added case (
I-BT and
I-P in
Figure 2) and the control case (
II-BT and
II-P in
Figure 3). Therefore, the UFB-distribution in the nutrient solution in the buffer tank was supplied to the plant without change in the UFB-distribution. However, the UFB-supplied water (
I-P) has significantly more fine bubbles compared to that in the control zone (
II-P).
Although the control solution had no added bubbles, it nevertheless had UFBs after the freeze-fracture observations. Since the minimum number density of UFBs for the freeze-fracture replica method is the order 10
6/mL, the observed UFBs in control cases are significant. We will discuss the possible origin of these UFBs in
Section 3.4.
Next, we examine how the distribution of UFBs change after reaching the plants. For the UFB-added case,
Figure 4 shows that the size distribution of UFBs shifts to larger sizes upon reaching the rhizosphere. The same shift occurs for the control case (
Figure 5). Thus, almost the same number of UFBs remain in the solution even after the solution passes through the soil layer.
Figure 1 shows that the surface of UFBs in
I-R and
II-R solutions tends to have impurity. Thus, we speculate that the UFB size will increase as small UFBs either coalesce or undergo the Ostwald-ripening process.
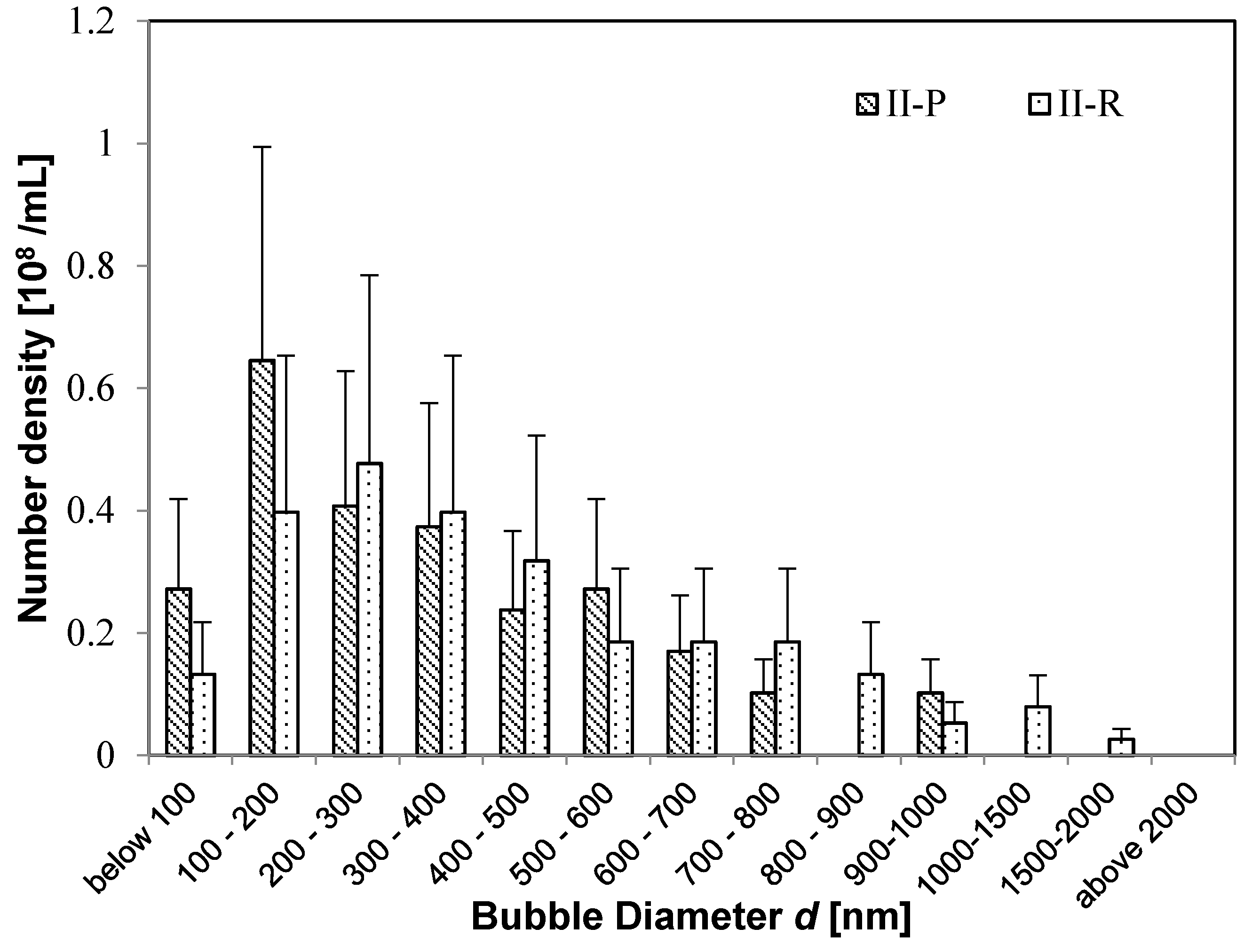
When the size distributions of
I-R solution and
II-R solution are compared, they are very similar even though their distributions differ in the irrigation pipe (
Figure 4 and
Figure 5). This result shows that UFBs tend to stabilize upon reaching several hundred nm, and that the size distribution of UFBs tends to a more nearly uniform distribution. It is interesting that this size distribution resembles the UFB distribution in a 100-mM NaCl solution, from a study in which the UFBs were more stable when a solution contained a small amount of NaCl ([
7]
Figure 3b).
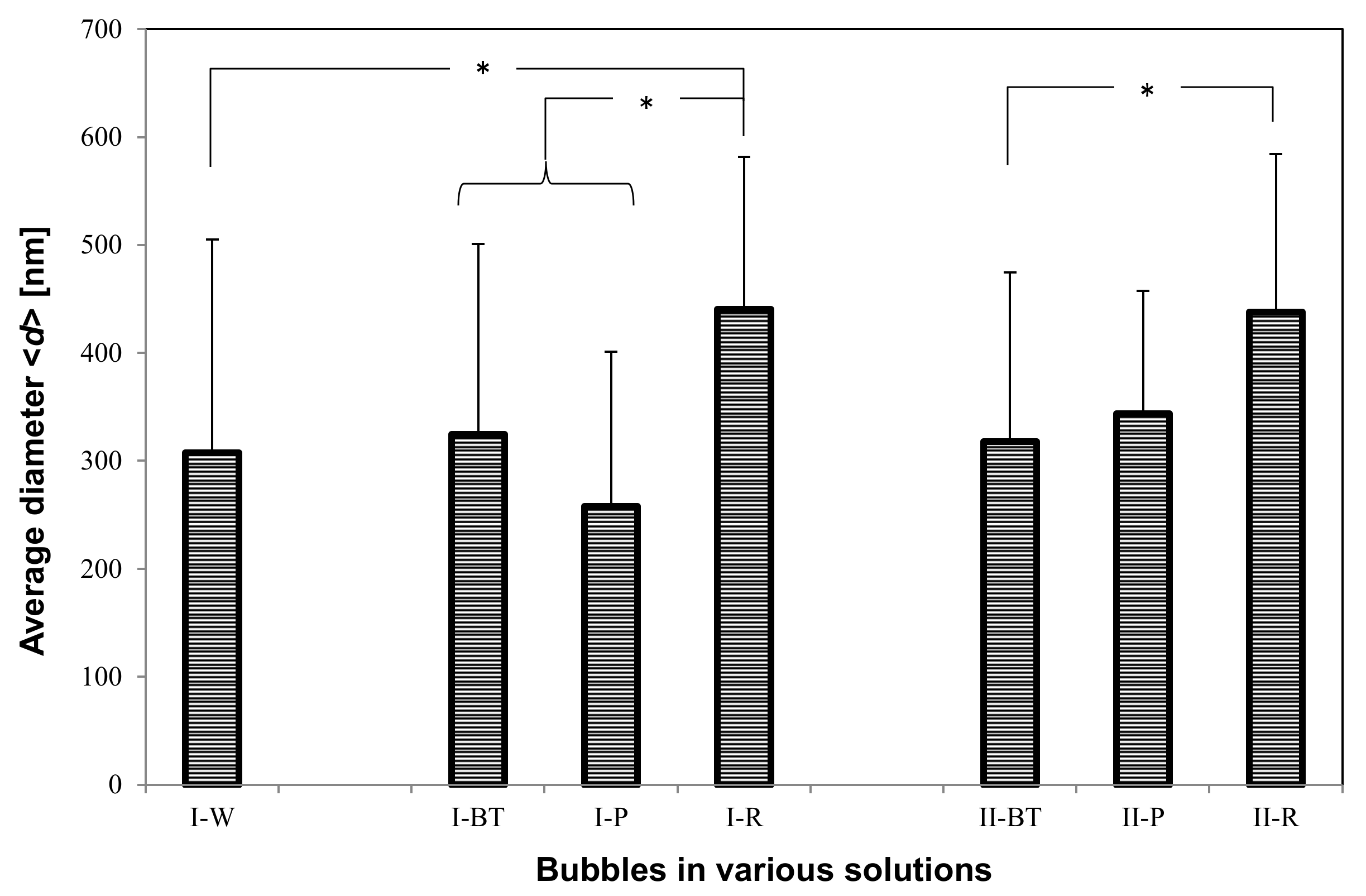
3.3. UFB Number Concentration and Average Diameter
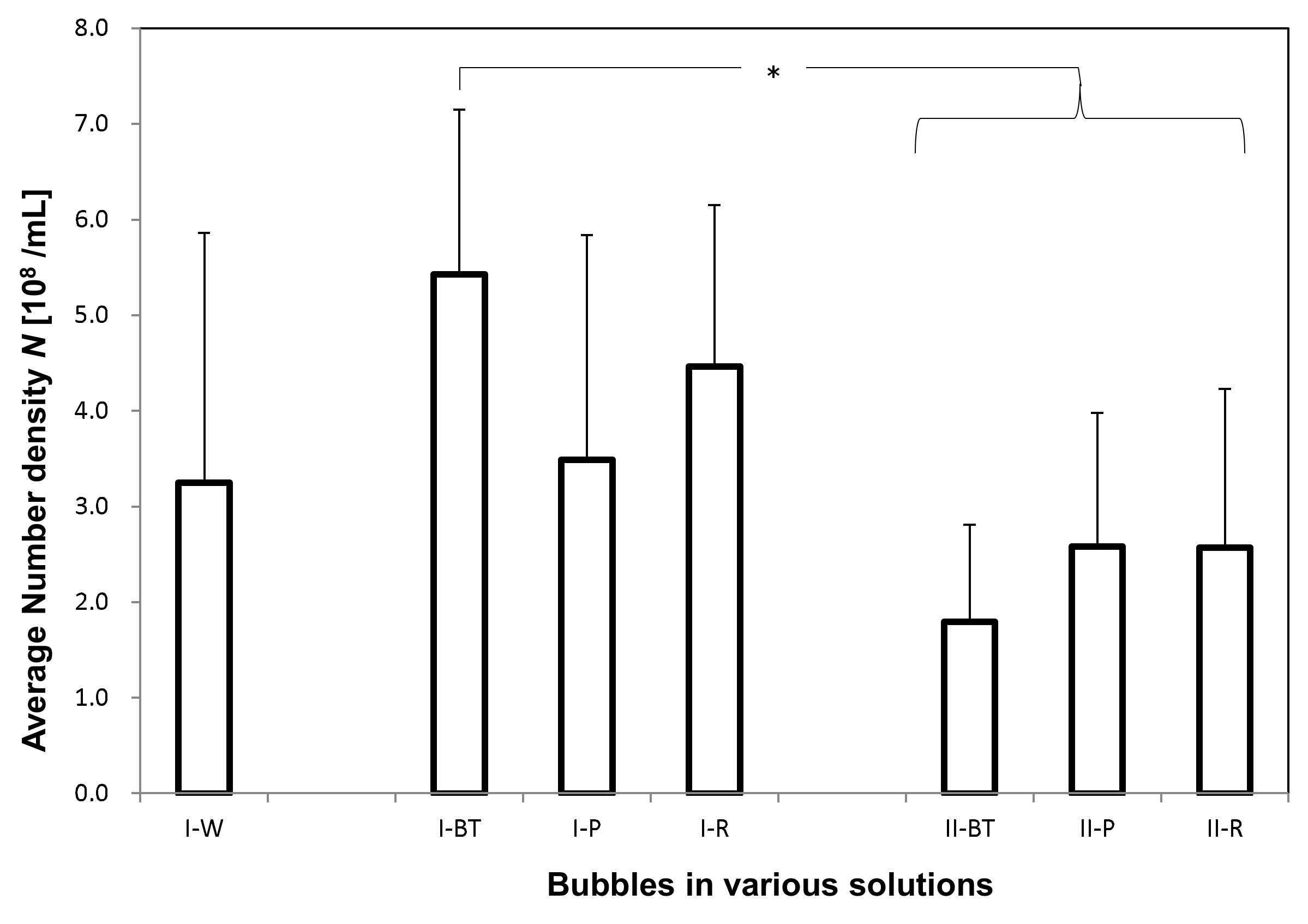
The total number concentration
N (
Figure 6) and the arithmetic-averaged UFB diameter <
d> (
Figure 7) were determined by averaging all UFBs observed in each solution sample. For the
I-W solution,
N = 3.3 ± 2.6 × 10
8/mL and <
d> = 3.1 ± 2.0 × 10
2 nm, which roughly agrees with the set value of the UFB generator. On the other hand, the value of
N in the
I-BT solution is 5.4 ± 1.7 × 10
8/mL, slightly larger but not significantly different from that in the
I-W solution (
Figure 6). The measurement uncertainty (standard deviation: SD) of the
I-W solution is particularly large due to the observed large concentration variations of the UFBs. Concerning the diameters, their values also were consistent, with <
d> = 3.2 ± 1.8 × 10
2 nm in the
I-BT solution (
Figure 7). As the histograms of the solutions are also similar (see
Figure 2), it appears that the addition of the nutrients does not significantly affect the stability of the UFBs.
Figure 6 and
Figure 7 also show that both
N and <
d> in
I-BT solution and
I-P solution are consistent within their uncertainties. Thus, it appears that the supply line from the buffer tank preserves the UFBs. We find the same conclusion holds for the control case.
However, the value of <
d> in the solutions slightly increases between the buffer tank and the plant roots (
Figure 7). This reflects the results observed in the histograms (
Figure 4 and
Figure 5), in which the number of fine UFBs decreased while the number of submicron bubbles increased. Yet the total value
N (
Figure 6) shows no significant change. This finding indicates that transport through the peat moss does not significantly affect the number of UFBs in the UFB-added solution. In addition, the increase in size (together with the TEM images showing a thicker UFB–water interface) suggest that the passage through the soil causes impurities to accumulate on the UFB surface, increasing the UFB size.
Comparing the control and UFB-added cases, the average UFB diameters <
d> are nearly the same (
Figure 7), whereas the value of
N is significantly smaller in each location for the control case (
Figure 6). That is, the solutions in the UFB-added case contain significantly more UFBs due to the artificially generated UFBs.
We conclude that the number of UFBs observed in the UFB-added solutions roughly equals the set level of the UFB generator that is being manufactured at the plant factory, in which N is about 4 × 108/mL and <d> is about 300 nm. The number of UFBs is roughly conserved through to the rhizosphere, but the average diameter increases about 50%. Although there were also UFBs in the solutions of the control zone, they were likely generated mostly during the nutrient solution preparation and freeze-fracture replication. The reproducibility of these results was confirmed by repeating these measurements at different times (June 2015 and October 2016).
3.4. Source of UFBs Observed in Control-Case Solutions
We now consider why UFBs are observed in the control-case solution. Two possible sources of UFBs are: (1) being formed during replica preparation as ‘secondary bubbles’ and (2) having existed in the solution as ‘primary bubbles’.
Secondary bubbles can form in the solution from dissolved air at the freezing front because the solubility of air in ice is negligible compared to that in water. For example, Lipp et al. [
12] studied secondary bubble formation under different freezing speeds and found that the bubbles were smaller and greater in number under a higher freezing speed.
To estimate the amount of secondary bubbles, we first estimate the amount of air in the water that will form bubbles during freezing. This amount depends on the freezing speed
Vf and the diffusion coefficient of air in water D
air. In our study, the cooling rate of the solution is about 10
3 K/min [
7], which corresponds to a freezing speed
Vf of about 10
5 μm/s. From this value, the diffusion boundary layer in front of the ice-water interface is calculated as D
air/
Vf. Not knowing the precise D
air value at freezing temperature, we assume it equals that of oxygen in water at atmospheric pressure and room temperature, which is 2 × 10
−9 m
2/s [
13]. The resulting thickness D
air/
Vf is about 10
−8 m. Within this region, we consider that the air molecules cannot diffuse away during freezing, meaning that they instead collect into bubbles that are frozen immediately in ice.
Now consider how many secondary bubbles can be formed from the dissolved air. The amount of air molecules in pure water is assumed to be about 0.8 times that in saturated conditions. This is caused by the ratio of the dissolved oxygen (DO) value between that in pure water (5.5) and that in UFB-added water (6.9), in which the dissolved gas is considered to be saturated [
7]. If all dissolved air converted to UFBs having its diameter <
d> = 300 nm (about the average diameter of UFBs in the control-case solution, in
Figure 7) and its inner pressure ∆
P is calculated from Equation (2), the number concentration of the secondary bubbles
N* would be
where
s = 0.019 cm
3/mL [
14] is the solubility of air in water at 293.2 K, R is the molar gas constant,
T is temperature,
P0 is atmospheric pressure,
Vbub is the volume of a UFB with <
d> = 300 nm, and
vN is the volume of 1 mol of a gas at standard temperature and pressure. The resulting
N* is about 1.1 × 10
11/mL. Uchida et al. [
7] observed UFBs of order 10
7/mL from the replica of pure water at room temperature by the same freeze-fracture replica method. If these UFBs were secondary bubbles, this means that the nucleation probability of a secondary bubble in pure water is about 10
−4. However, the nutrient solution used in the present study included various solutes that would concentrate at the ice-water interface during freezing. Such a concentration of solute would increase the nucleation probability. A ten-fold increase would explain the number of UFBs in the control case here. This estimation contains several assumptions about the freezing process, bubble formation, and physical parameters, and thus should be considered as a very rough estimate. However, it suggests a plausible explanation for the observed UFBs.
Some of these UFBs may instead be primary bubbles. One possible source of these additional UFBs is a size-reduction of MBs and/or milli-bubbles produced during the nutrient solution preparation process. For example, when comparing the size distribution of UFBs before being supplied to the plant body (
Figure 2 and
Figure 3), the
II-P solution has more UFBs above 500-nm diameter than the
I-P solution.
Figure 3 also shows that, when compared in the buffer tank and in the irrigation pipe in the control zone, the number of MBs of 1 μm or more decreases while the UFBs of submicron order increases. In the replica observation method, most MBs larger than 5 μm are difficult to measure because they are usually located at the edge of the observed area. So, the distribution of fine bubbles larger than 5 μm would be slightly underestimated. To evaluate this possibility quantitatively, we should know the whole range of fine bubbles, ranging from 10
−4 to 10
−8 m. However, we do not yet have a technique to measure such a wide range. For example, the freeze-fracture replica method covers diameters of 10
−6 to 10
−8 m, whereas the particle-tracking analysis method covers only 10
−7 to 10
−8 m [
15].
3.5. Possible Plant-Growth Promotion Mechanisms by UFBs in Nutrient Solution
UFBs are thought to promote the growth of plants [
1,
2]. However, its mechanism has not been determined yet. One proposed mechanism is in the germination process. Liu et al. [
4] revealed that the presence of UFBs induced a constant development of radicals in nutrient solutions, thereby promoting germination. However, the mechanism of promoting the growth of growing plants and the magnitude of their effect are still under investigation.
The mechanism may involve either the UFB air content or the interface. Due to the air/water interfacial tension, the pressure inside a UFB should be relatively high according to Equation (2). However, various substances readily accumulate at the interface [
7], as shown by the impurity deposits in
Figure 1, and such a change at the interface should decrease the air/water interfacial tension. As a result, the actual internal pressure would be lower than that predicted by use of the air/water interfacial tension of bulk water [
16]. Therefore, the bubble internal pressure in an actual solution should be smaller than that estimated from Equation (2). We also consider that the UFB may be stabilized by a diffusion barrier [
17].
Takahashi [
9] pointed out one of the main mechanisms by which UFBs act lies in their action as a gas buffer in the liquid phase. As UFBs provide a large gas-liquid interface in the liquid and their internal pressure is high, gas is constantly supplied from UFBs into the nutrient solution for as long as UFBs are present. Thus, the nutrient solution can be considered to maintain a saturation state of the gas. Uchida et al. [
7] measured the time change of the dissolved oxygen (DO) value of the O
2-UFB-added solution and confirmed that the aqueous solution was in the O
2 supersaturated state during the UFB-existence period. In the present study, the DO value of each nutrient solution was measured to be a constant value of 6.4 mg/L (at 298 K) in the solution from the buffer tank to just before reaching the plant. In other words, due to the UFBs, the nutrient solution in this plant factory is expected to be supersaturated in O
2, and thus O
2 should be efficiently supplied to the plant body.
Consider now the interface. The surface of a UFB is negatively charged, and thus cations in the nutrient solution might concentrate at the UFB interface. A concentration of solutes (such as calcium, magnesium, potassium, and ammonia) may explain the thin membrane-like structure that appears on the UFB in the nutrient solution supplied to the plant body. Moreover, as the UFB-added solution passes through the peat moss, the peat moss should add other impurities to the solution that may deposit on the surface of the UFBs (
Figure 1h). Therefore, it is possible that the UFBs help transport concentrated nutrients to the rhizosphere, and then into the plant body. As the surface of the UFB is also a gas-liquid interface, the UFBs also may help transport hydrophobic materials and solid solutes.
On the other hand, the effect of UFBs on growth is unlikely to come from pH. The pH of the nutrient solution was approximately constant at about 5.8 within the range from the buffer tank to the irrigation pipe. This pH value is also about 5.8 in the control nutrient solution, so if a growth-promotion effect is verified, then it cannot be due to a difference in pH value. Also as the influence of pH on the ζ potential value is small if pH is around 5.8 [
7,
9], we assume that there is no influence of pH on the UFB distribution.
Therefore, the results of this research indicate that UFBs continue to exist stably even while moving through a supply pipe from a buffer tank and through peat moss. Through their transport, UFBs supply gas molecules into the solution and collect impurity on their surface, some of which may benefit the plant. However, as all the observations were on replicas, we could not identify what substances were on the UFB surface. Also, we still need to clarify how UFBs may promote plant growth.